Modified Endoscopic Submucosal Dissection—An Alternative Modality for the Treatment of Sporadic Duodenal Papillary Adenomas
Abstract
Background and Aim: Endoscopic submucosal dissection (ESD) is commonly employed in the treatment of epithelial gastrointestinal tumors, but few studies have explored ESD for treatment of duodenal papillary adenomas (PAs). In this study, we aim to evaluate the feasibility of a modified ESD method as an alternative modality in the resection of PAs.
Methods: We evaluated potential advantages of modified ESD for resection of sporadic duodenal PAs through retrospective analysis of 10 PAs resected via ESD compared to paired lesions undergoing endoscopic papillectomy (EP).
Results: All lesions undergoing ESD were resected en bloc with negative margins, compared to 60% of lesions undergoing EP. Within the experimental group, there was one case each of melena and pancreatitis compared to four bleeds and one case of pancreatitis in the control group. No recurrence was detected within the ESD group after a mean follow-up time of 11.2 months compared to three recurrences within a mean follow-up time of 27.7 months.
Conclusions: From our preliminary experience, ESD is a promising alternative in the treatment of superficial duodenal PAs; however, further investigation is needed.
1. Introduction
Duodenal papillary adenomas (PAs) are premalignant neoplasms of the ampulla of Vater. Surgical resection is essential for the prevention of disease progression [1]. Previously, PAs frequently went undetected until progression to malignancy, but as recognition of the therapeutic value of endoscopy has grown, so too has detection of incidental PAs [2]. Common therapeutic approaches include surgical resection, pancreatoduodenectomy, and endoscopic papillectomy (EP). Although EP circumvents some of the morbidity of surgery, limitations include risk of incomplete removal, local recurrence, and complications including intraoperative and postoperative bleeding [3]. While lesions with intraductal extension > 10 mm, friability, or ulceration should be preferentially considered for surgical resection, there is no consensus regarding a size threshold above which EP should not be attempted [1]. As freehand dissection techniques accommodate irregular lesions, endoscopic submucosal dissection (ESD) is increasingly utilized for gastrointestinal epithelial tumors due to high en bloc resection rates. Nevertheless, few studies have explored application of ESD to PAs. Here, we examine the feasibility of a modified ESD method as an alternative modality in the resection of PAs.
2. Materials and Methods
This study was a single-center retrospective observational study. We retrospectively collected data from 10 patients with superficial PAs who underwent modified ESD from July 2019 to May 2021 at Nanfang Hospital, Guangzhou, China. Data from 10 paired lesions undergoing EP from December 2013 to March 2021 was also collected retrospectively to further evaluate the potential advantages and disadvantages of ESD compared to EP. The procedures were performed by two experienced endoscopists (ESD > 200 cases). The study protocol (NFEC-2017-164) was approved by the Institutional Review Board at Nanfang Hospital.
Endoscopic assessment with biopsy, endoscopic ultrasonography (EUS), and upper abdominal CT or MRI were performed prior to ESD and EP. Lesions with macroscopic signs of ulceration, friability, or evidence of intraductal extension or muscularis propria involvement were excluded. Patients in both study groups underwent routine endoscopic surveillance.
2.1. ESD Procedure (Supporting Information 1 and 2: Video Clips S1 and S2)
Single-channel esophagogastroduodenoscopes (EGD, GIF-Q260J, Olympus, Japan) were fitted with transparent caps for the operation. Procedure steps are illustrated in Figure 1. Submucosal dissection was performed using “EndoCut I” modality (effect 3, cutting duration 3) (ERBE, Tubingen, Germany). Soft coagulation (80 W on effect 4) was used for hemostasis.
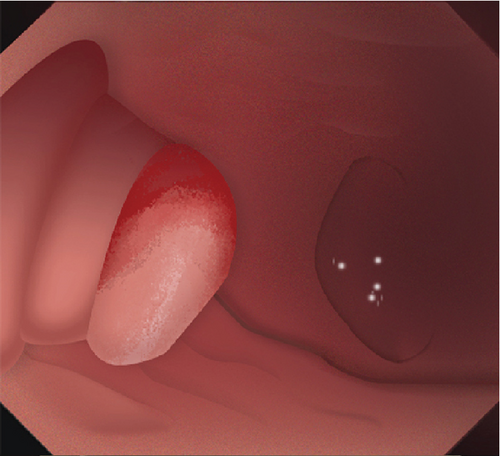
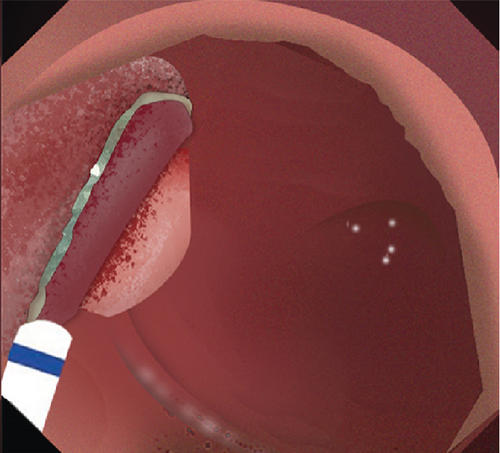
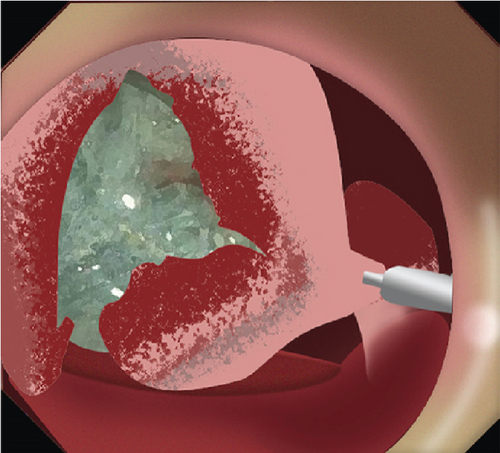
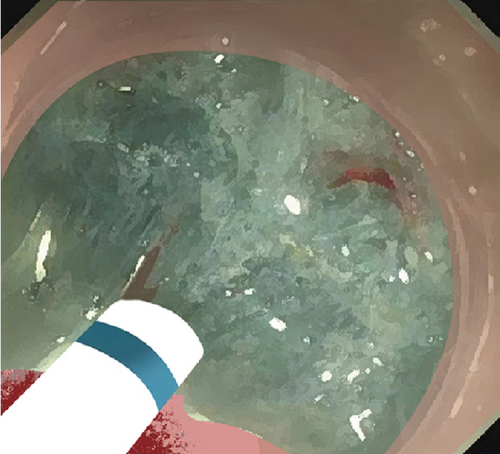
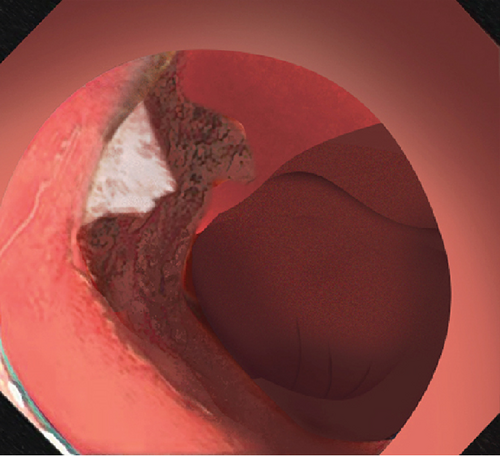
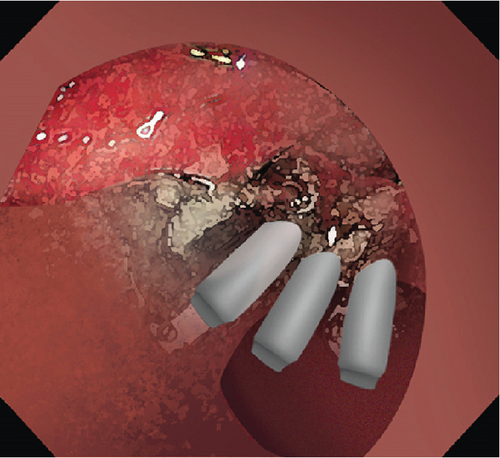
2.2. Mucosal Incision
Submucosal injection of saline solution and indigo carmine was performed proximal to the lesion. A semicircular mucosal incision was made before submucosal dissection with a DualKnife J (KD655L, Olympus, Japan) or a HybridKnife (I type, ERBE, Germany). Buscopan was administered for intensive intestine peristalsis.
2.3. Submucosal Dissection
Dissected mucosal flaps were anchored to the contralateral intestinal wall using endoclips. Internal traction enabled adequate submucosal exposure in the duodenal lumen and facilitated visualization of submucosal vessels. Large submucosal vessels were coagulated to minimize bleeding. During the dissection, the sphincter of Oddi is visualized as a muscular complex in the submucosal plane. It is essential to dissect this muscular complex carefully, maintaining a dissection line parallel to the exposed submucosal plane. This approach is necessary to avoid shallow dissection and prevent potential perforation. The submucosal layer was dissected towards the distal part of the lesion until complete resection.
2.4. Prophylactic Clip Closure
Exposed vessels on the raw surface were soft-coagulated, and mucosal defects distal to the papilla were closed with endoclips with or without endoloops. Proton pump inhibitors, somatostatin, or rectal NSAIDs were administered perioperatively. Serum amylase and lipase were monitored postoperatively.
3. Results
Ten patients (mean age 51.6 years, SD 16.8 years, 7 males) with PAs underwent ESD. ESD was technically successful in six laterally spreading tumors (LSTs) (mean size 32.5 mm, range 25–45 mm) and four ampullary adenomas (AAs) (mean size 15.5 mm, range 12–23 mm). Characteristics of the patients and lesions are summarized in Table 1. All lesions were resected en bloc (Figure 2, Table 1, and Supporting Information 3: Figure S1) with negative margins. The average specimen size was 25.7 mm (SD 10.78 mm, range 12–45 mm). Pathological examination of the resected specimens revealed low-grade dysplasia (LGD) in five patients, high-grade dysplasia (HGD) in four patients, and HGD with intramucosal adenocarcinoma in one patient. The average operation time was 50 min (SD 15.97 min, range from 25 to 80 min). No major bleeding or any perforation occurred during the operations. One patient experienced an episode of melena following resection of a LST, and one patient displayed elevated amylase and lipase. However, both patients were discharged after conservative treatment without additional procedural intervention. No perforation, stenosis, cholangitis, or deaths occurred, and no recurrence was detected after a mean endoscopic follow-up time of 11.2 (range 3–29) months.
| Patient no. | Gender | Age | Tumor size (mm) | LST | Operating time (min) | Estimated blood loss (mL) | Pathology | En bloc resection | Intraoperative or postoperative perforation | Postoperative bleeding | Postoperative pancreatitis | Follow-up (month) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 70 | 15 × 15 | None | 50 | ≈0 | Low-grade | Yes | None | None | Mild | 29 |
| 2 | Male | 32 | 23 × 15 | None | 60 | 1 | Low-grade | Yes | None | None | None | 18 |
| 3 | Female | 58 | 35 × 25 | Yes | 40 | 2 | High-grade | Yes | None | None | None | 16 |
| 4 | Male | 70 | 12 × 12 | None | 50 | 1 | Low-grade | Yes | None | None | None | 3 |
| 5 | Male | 41 | 25 × 20 | Yes | 25 | ≈0 | High-grade | Yes | None | None | None | 14 |
| 6 | Female | 44 | 40 × 40 | Yes | 60 | ≈0 | High-grade | Yes | None | None | None | 6 |
| 7 | Male | 73 | 12 × 12 | None | 60 | 1 | High-grade | Yes | None | None | None | 6 |
| 8 | Male | 53 | 23 × 25 | Yes | 80 | 1 | High-grade, intramucosal cancer | Yes | None | Yes | None | 9 |
| 9 | Male | 19 | 25 × 20 | Yes | 25 | 1 | Low-grade | Yes | None | None | None | 8 |
| 10 | Female | 56 | 45 × 25 | Yes | 50 | ≈0 | Low-grade | Yes | None | None | None | 3 |
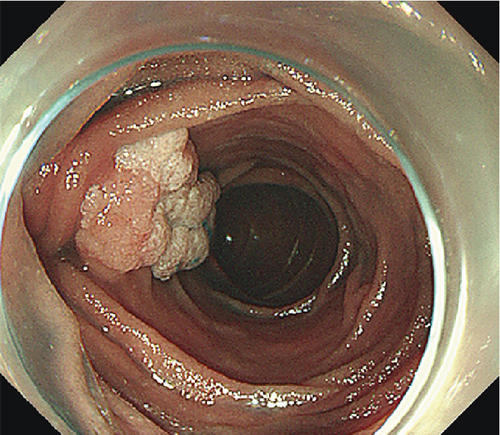
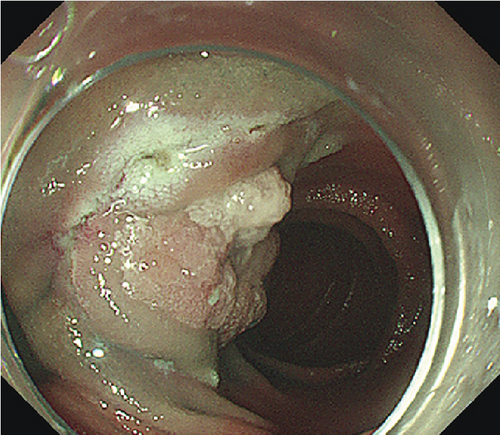
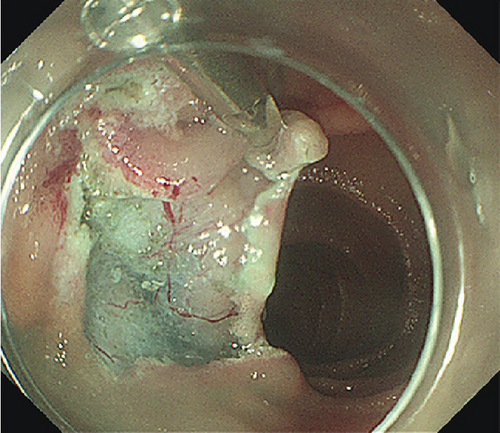
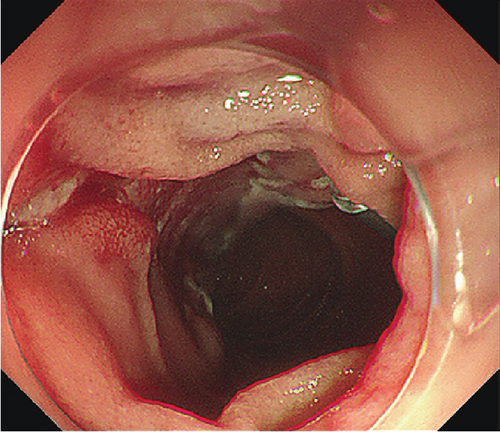
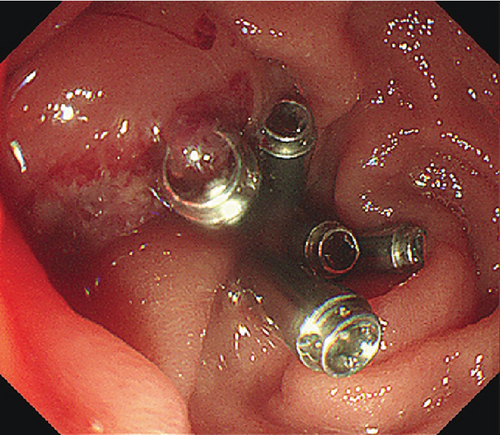
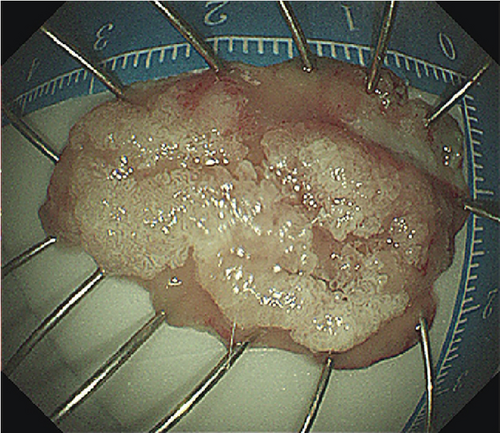
The patients undergoing ESD were paired with ten patients (mean age 53.9 years, SD 12.8 years, 7 males) undergoing EP. Lesions were matched for size and pathology (Table 2). EP was performed on six LSTs (mean size 34.2 mm, range 30–45 mm) and four AAs (mean size 17.25, range 13–20 mm). Four lesions including three LSTs were resected in a piecemeal fashion (Table 2). Three patients experienced postoperative bleeding, and one experienced postoperative pancreatitis. One LST that underwent piecemeal resection demonstrated recurrence 1 month postprocedure, and three lesions displayed recurrence during a mean follow up period of 27.7 (range 1–68) months.
| Patient no. | Gender | Age | Tumor size (mm) | LST | Pathology | En bloc resection | Intraoperative or postoperative perforation | Postoperative bleeding | Postoperative pancreatitis | Follow-up (month) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 69 | 20 × 10 | No | Low-grade | No | None | Yes | None | 68 |
| 2 | Male | 55 | 20 × 15 | No | Low-grade | Yes | None | Yes | None | 25 |
| 3 | Male | 49 | 35 × 25 | Yes | High-grade | No | None | None | None | 57 |
| 4 | Female | 59 | 16 × 8 | No | Low-grade | Yes | None | None | None | 45 |
| 5 | Female | 77 | 30 × 20 | Yes | High-grade | No | None | None | None | 18 |
| 6 | Male | 54 | 35 × 35 | Yes | High-grade | Yes | None | Yes | None | 37 |
| 7 | Male | 51 | 13 × 12 | None | High-grade | Yes | None | None | None | 61 |
| 8 | Female | 47 | 30 × 20 | Yes | High-grade | Yes | None | Yes | None | 3 |
| 9 | Male | 26 | 30 × 20 | Yes | Low-grade | Yes | None | None | Yes | 39 |
| 10 | Female | 52 | 45 × 25 | Yes | Low-grade | No | None | None | None | 1 |
4. Discussion
ESD is well established in the treatment of superficial gastrointestinal neoplasms with higher en bloc resection rates compared to snare resection and endoscopic mucosal resection (EMR). However, ESD has thus far been rarely used in the treatment of PAs due to technical difficulties involved in performing ESD in the descending part of the duodenum (D2) and around the duodenal papilla [4]. In this study, we achieved a 100% success rate in the removal of superficial PAs. ESD procedures were modified to suit the anatomy of the duodenum. We postulate that clip traction optimizes tissue tension within the submucosa and exposure of the dissection line. Direct visualization of the submucosal vessels facilitates pretreatment of submucosal vessels in the ampullary region and minimizes major intraoperative bleeding.
Although some studies suggest prophylactic pancreatic duct (PD) stent placement reduces the risk of postprocedural pancreatitis in EP [5], routine PD stent placement is not always successful and has been questioned in recent case series [6, 7]. The benefit of routine deployment of PD stents following ESD is unclear at this time. In lieu of prophylactic biliary or pancreatic stents, somatostatin or rectal NSAIDs were administered perioperatively. One patient with mild pancreatitis was observed postoperatively, but the patient was asymptomatic, and serum biomarkers decreased to less than 3 times the upper limit of normal after 48 h of conservative treatment. None of the patients undergoing ESD had postoperative complications requiring further procedural intervention, and the rate of postoperative pancreatitis was comparable to that of EP described with PD stent placement (7.2%–20%) [8, 9]. However, given the sample size, further studies with more cases are needed to evaluate ideal postoperative management and adverse events in the removal of PAs using this technique.
Currently, EP is favored over surgical resection for the treatment of superficial PAs due to its minimally invasive approach [7]. Reported rates of en bloc resection and local recurrence using snare resection or EMR for AA and ampullary LST range from 59.1% to 90.3% and 5.0% to 22.9% [9–13], respectively, and the recurrence rate was reported to be 8%–33% [3, 10, 14]. Studies have shown that histologically confirmed recurrence is associated with piecemeal resection (p = 0.02) and number of pieces (p = 0.02) [3]. One of the most important advantages of ESD is that it enables a high rate of en bloc resection and thus significantly reduces the rates of incomplete tumor resection, especially for large, irregular lesions. Intact specimens following ESD facilitate accurate histopathological assessment. A R0 resection rate of 100% was achieved in the ESD group, and no recurrence was detected with a mean of 11.2 months compared to a 30% recurrence rate in lesions that underwent EP with a mean of 27.7 months. Of note, EP was performed prior to ESD, and as such, the mean follow-up period was shorter in the ESD group. Even so, our sample supports the hypothesis that ESD results in a reduction of local recurrence compared to EP.
To our knowledge, this is the first case series exploring the application of ESD techniques in PAs. The high en bloc resection rate and low adverse event rate of the lesions in the study group as compared to the control suggest that this modified ESD procedure provides an alternative endoscopic approach to EP for the treatment of superficial PAs, particularly for LSTs. Nevertheless, as ESD procedures within D2 are more technically challenging than those in the stomach or colon, ESD in PAs should be performed only by endoscopists with extensive ESD experience.
This is a single-center retrospective observational study with limited sample size and follow-up time. Prospective controlled trials with a larger number of cases are needed to examine the advantages of higher en bloc resection rate and decreased recurrence as compared to traditional approaches, to evaluate rates of adverse events throughout time, and to determine ideal postoperative management. Additional comparison of ESD and EP approaches to PA resection is needed to explore the optimal indications for these minimally invasive treatment strategies.
5. Conclusions
Our data supports ESD as a promising alternative in the treatment of superficial duodenal PAs. This study provides a foundation for further investigation supporting the application of ESD techniques towards PAs and suggests that ESD techniques may be particularly helpful in reducing recurrence in LSTs with similar complication rates as EP.
Disclosure
We would like to acknowledge that the content of this article had been previously presented as an oral presentation by Luo et al. in the 2023 Digestive Disease Week (DDW) in the Endoscopic Technology Session Topic Forum. The earlier version of the manuscript has been presented as a poster in the 2023 Digestive Disease Week.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Xiaobei Luo and Zelong Han performed the endoscopic surgery. Ying Huang and Shaohui Huang were the assistants. Aimin Li, Zhenjiang Wang, and Jun Zhong collected and analyzed the data. Xiaobei Luo, Qingyuan Li, and Chanelle Yeh Chua drafted and revised the manuscript. Aimin Li and Side Liu critically reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This work was partially supported by the Basic and Applied Basic Research Foundation of Guangdong Province (2022A1515011477), Science and Technology Planning Project of Guangdong Province (2020A1414010265 and 2017B020209003), and National Natural Science Foundation of China (82303855 and 12026605). This work was also partially supported by the Key Technologies R&D Program of Guangdong Province (2022B0303020003) funding.
Acknowledgments
We would like to express our sincere appreciation to following participating endoscopists and assistants for their efforts with data recording and the organization of patient information: Hongxiang Gu, Dongbo Zhang, Zhen Wang, Panpan Liu, Qihong Cheng, Rong Huang, and Li Xu. We would like to acknowledge Pazhou Lab, Guangzhou, for its support of this research.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information section.