Ability of Different Flavonols and Commercial Mannoproteins to Enhance Wine Colour through Copigmentation
Abstract
Copigmentation is a noncovalent interaction between anthocyanins and colourless copigments that stabilises the anthocyanin-coloured forms by protecting them against water attack. Copigmentation can be responsible for almost half of the colour observed in young red wines. It has been reported that flavonols are the most effective wine copigments, but studies on the influence of the structure of these copigments on the magnitude of copigmentation are scarce. However, flavonol profiles change with grape variety and can condition the colour of the resulting wines. In addition, mannoproteins (MPs) have been reported to interact with flavonoids, which, consequently, might affect the flavonol-anthocyanin interaction. The objective of the present study was to evaluate the ability of five grape flavonols (isorhamnetin, kaempferol, quercetin, syringetin, and myricetin 3-O-glucosides: I-3-glc, K-3-glc, Q-3-glc, S-3-glc, and My-3-glc) by conventional spectrophotometric measurements and CIELAB analysis to copigment with malvidin 3-O-glucoside (mv-3-glc) in the absence or presence of five different MPs (binary and ternary model systems). Among the tested flavonols, I-3-glc was the most effective copigment, whereas K-3-glc was the least one. S-3-glc and Q-3-glc showed intermediate effectiveness, but that of S-3-glc was closer to I-3-glc and that of Q-3-glc to K-3-glc. These results point out that the presence of methoxyl groups in the B-ring of the flavonol increases copigmentation magnitude. The participation of My-3-glc in copigmentation interactions seemed to be hindered by its colloidal instability. Regarding the tested MPs, they did not provoke a direct copigmentation effect, but were able to modify the magnitude of the interaction between the flavonols and the anthocyanin and, in some cases, the colloidal behaviour of coloured aggregates formed over time. Finally, the results of the additional HPLC-DAD analyses point to a greater protection of the anthocyanin against degradation in the model systems where the magnitude of copigmentation was greater.
1. Introduction
Intermolecular copigmentation is a spontaneous and exothermic process consisting of the stacking of copigments (noncoloured organic molecules) on the planar surface of the anthocyanin-coloured forms (flavylium ion or quinonoid forms), which partially prevents the formation of the anthocyanin colourless form (hemiketal) by water attack [1, 2]. In red wine, the existence of this supramolecular association can explain why at its usual pH values (3.2–4), and the colour expressed by the pigments is greater than it would be expected (hyperchromic effect), accounting for up to half of the observed colour in young wines [1, 2] and also contributing to their bluish hues (bathochromic effect). Furthermore, other types of copigmentation can occur in wines, such as self-association, if the copigment is another anthocyanin, and intramolecular copigmentation, if the interaction occurs between the anthocyanin chromophore and an aromatic acyl residue linked to the sugar of that anthocyanin [1, 2].
The magnitude of copigmentation depends on several factors, such as anthocyanin structure [3], copigment type [1, 2, 4], and anthocyanin:copigment molar ratio [5]. Flavonols have been reported to be powerful wine copigments [1, 4] probably due to their more planar structure compared to other wine compounds. In grapes, flavonols are synthesised mainly in response to light, accumulating in skins, where they act as UV screens [6]. Grape flavonols derive from six aglycones (kaempferol, quercetin, myricetin, isorhamnetin, laricitrin, and syringetin), which differ on the substitution pattern of B-ring. In grapes, they accumulate only as 3-O-substituted compounds (3-O-glucosides, 3-O-glucuronides, 3-O-galactosides, and even as 3-O-acetylglucosides) [7]. However, during winemaking, they can be extracted to the must and then undergo hydrolysis reactions, thus explaining the presence of free aglycones in wines. Wine flavonol quantitative and qualitative compositions depend on several factors related to grapes (variety, sunlight exposure, and maturity) but also to winemaking practices, ageing, and storage conditions [7, 8]. Thus, a large variety of flavanols could exist in wine and participate in copigmentation interactions. Most of the studies on copigmentation that involve flavonols are performed with quercetin 3-O-glucoside, assuming that all the flavonols behave similarly [3, 9, 10]. However, in the case of anthocyanins, which show similar structures to flavonols, the substitution pattern of B-ring has been reported to be relevant in terms of copigmentation intensity [3]. Similarly, a relevant influence of the substitution of B-ring of the different flavonols in that phenomenon might be also expected and, to our knowledge, this issue has not been investigated up to date. From the results of such novel research, the effectiveness of the different flavonols as copigments would be established. Flavonol profile changes from one grape variety to another and from grapes to wine and, in some cases, other flavonols are present in greater percentages than quercetin 3-O-glucoside [7]. For instance, in Vitis vinifera cv. Tempranillo grapes, the red grape variety mostly grown in Spain, and in wines made from it, myricetin 3-O-glucoside is usually the major flavonol. Thus, knowing the effectiveness of the different flavonols present in the grape would be useful to estimate the potential of a given grape variety to yield wines with increased colour through copigmentation or to explain differences in copigmentation between wines that might be due to not only to differences in the total flavonol content but also to the qualitative profile.
In contrast to flavonols, other wine constituents, such as mannoproteins (MP), were reported to reduce wine colour intensity after their addition [11]. MPs are glycoproteins from the cell wall of Saccharomyces cerevisiae that can be released into the wine during alcoholic fermentation or during yeast autolysis [12, 13]. Furthermore, they can be added to the wine as commercial oenological products for different purposes [14]. MPs can prevent tannin precipitation in wines by reducing tannin aggregation and aggregate growth [14, 15] through their adsorption on the tannin molecule [16]. Additionally, some MPs seem to reduce the anthocyanin and flavonol losses occurring by their trapping in these tannin aggregates and subsequent precipitation [17], whereas other MPs have shown the ability to improve the colloidal stability of anthocyanins and anthocyanin-derived pigments in wines [18, 19]. MPs can interact with phenolic compounds through their glucidic part, where the sugar composition, presence of phosphorylation, and branching can condition the interactions. Furthermore, they can also interact through their protein part through hydrogen bonds and hydrophobic interaction [20]. Therefore, MPs can interact either with anthocyanins or with wine copigments, such as flavonols and flavanols, and modify their colloidal behaviour, which, in turn, might induce changes in their ability to interact through copigmentation. Despite the influence that MPs might have in the copigmentation interaction between flavonols and anthocyanins and, consequently, in wine colour, this issue has not been studied to date.
Thus, in order to fill the knowledge gaps in the flavanol-anthocyanin copigmentation interaction, the present study firstly aimed to evaluate the effectiveness of five wine flavonols (myricetin, quercetin, kaempferol, isorhamnetin, and syringetin 3-O-glucosides) in the copigmentation with malvidin 3-O-glucoside (mv-3-glc), the main anthocyanin in Vitis vinifera L. red wines. For this purpose, binary model systems containing one type of flavonol and mv-3-glc in wine-like solutions were prepared and measured in a spectrophotometer and the CIELAB and copigmentation parameters were calculated from visible absorbance. The second objective of this study was to assess the role of five different types of MPs in copigmentation, either by its direct interaction with the anthocyanin (binary model systems containing one type of MP and mv-3-glc) or by interfering with the flavonol-anthocyanin copigmentation (ternary model systems containing one type of flavonol and one type of MP and mv-3-glc). The effectiveness of the copigmentation interaction was monitored in all of the model systems during 4 weeks, and, at the end of the study, the model systems were analysed by HPLC-DAD to evaluate the effect of copigmentation in the transformation of the anthocyanin into anthocyanin-derived pigments and/or into degradation products.
2. Material and Methods
2.1. Model Systems
A model system exclusively containing mv-3-glc (A; 0.40 mM) in wine-like solution (ultrapure water with tartaric acid, 5 g/L; ethanol, 14%; ionic strength adjusted with NaCl, 11.65 g/L; pH, 3.6) was prepared to serve as control. Binary model systems (AMy, AQ, AK, AI, and AS for flavonols and A1, AF, AL, AM, and AP for MPs) contained mv-3-glc (A; 0.40 mM) and one flavonol (myricetin, quercetin, kaempferol, isorhamnetin, or syringetin 3-O-glucosides; My, Q, K, I, or S, respectively; 0.40 mM) or one mannoprotein (MP: 1, F, L, M, or P; 400 mg/L) in wine-like solution. Ternary model systems were prepared in wine-like solution at the same concentrations used in binary model systems (A: 0.40 mM; flavonols: 0.40 mM; MPs: 400 mg/L). Codification of these ternary systems first contains “A” for the anthocyanin, “1, F, L, M, or P” to indicate the type of MP, and then “My, Q, K, I, or S” to indicate the type of flavonol. Concentrations of mv-3-glc and the different flavonols were selected on the basis of previous studies on copigmentation [4, 5, 10] in order to obtain: (i) a final concentration of anthocyanin lower than 1 mM to avoid self-association interactions and (ii) a ratio 1 : 1 between pigment and copigment. The concentration chosen for the different MPs was within the ranges of use recommended by the suppliers.
Stock solutions of mv-3-glc, of the different flavonols and of the different MPs were initially prepared and then mixed in different proportions to obtain the final desired concentrations of all of the constituents. To be precise, mv-3-glc stock solution (0.67 mM) was prepared in 100 mL of ultrapure water containing NaCl (1.94 g) and tartaric acid (833 mg) and, after complete dissolution of all of the constituents, the pH was adjusted to 3.6. The stock solutions of the different flavonols (2.86 mM) were prepared in ethanol in order to favour their complete dissolution. The stock solutions of the different MPs were prepared by dissolving 15.4 mg of each in 10 mL of ultrapure water adjusted at pH 3.6. Each type of model system was prepared in triplicate by mixing 0.9 mL of the stock solution of mv-3-glc, 0.21 mL of the stock solution of the flavonol, and 0.39 mL of the stock solution of the MPs. In the model systems containing mv-3-glc, but not containing one flavonol or one MP or any of them, 0.21 mL of ethanol and/or 0.39 mL of ultrapure water at pH 3.6 was added, respectively, to compensate for the constituent(s) not added. All the model systems were kept in darkness at 25°C in a controlled temperature incubator for 22 days and monitored at days 1, 2, 5, 8, and 22. Day 1 corresponded to the samples measured 2 h after their preparation to allow their equilibration.
2.2. Reagents and Standards
Mv-3-glc was isolated from Vitis vinifera L. cv Tempranillo grape skins. To be precise, an extract from grape skins was obtained using acidified methanol (MeOH : HCl 0.5 N 95 : 5), which was evaporated under reduced pressure and redissolved in aqueous HCl 0.1 M before its fractionation. The resulting extract was loaded onto a Sephadex LH-20 (Sigma-Aldrich, St. Louis, MO, USA) column (30 × 300 mm) previously conditioned with aqueous HCl 0.1 M. Fractions were eluted also with aqueous HCl 0.1 M, and their richness in mv-3-glc was monitored by HPLC-DAD [18]. Fractions purer than 95% were gathered, evaporated with a rotary evaporator and, finally, freeze-dried.
Flavonols (myricetin, quercetin, kaempferol, isorhamnetin, and syringetin 3-O-glucosides) were purchased from Sigma-Aldrich Chemie (Steinheim, Germany). These five flavonols were selected because they are all present in Vitis vinifera red grapes and wines and because they show different substitution patterns in B-ring, which could be useful to draw conclusions about the influence of flavonol structure on the magnitude of copigmentation.
Mannoproteins were commercially available and were supplied as a powder either by Lallemand (Lallemand Enología España-Portugal, Rivas-Vaciamadrid, Spain) or by Laffort® España (Rentería, Spain). These five mannoproteins were selected for the present study because previous studies carried out in our laboratory revealed that they were able to interact with flavonoids [18, 19, 21].
All the solvents were HPLC-grade and ultrapure water was supplied by an Autwomatic Plus 1 + 2 equipment (Wasserlab, Barbatáin, Spain).
2.3. Colour and Copigmentation Analyses
The visible absorption spectra (380–770 nm) of all of the model solutions were recorded with an Agilent 8453 UV–vis spectrophotometer (Santa Clara, CA, USA) controlled by Agilent ChemStation software, using 2 mm path length quartz cells. A wine-like solution (ultrapure water with tartaric acid, 5 g/L; ethanol, 14%; ionic strength adjusted with NaCl, 11.65 g/L; pH, 3.6) kept for 22 days in the same conditions as the model system was used as reference at each sampling point. Copigmentation was first evaluated from the shifts in the λmax and from the changes in the absorption at λmax. However, considering that copigmentation can affect all the visible spectra [22], it was also evaluated from the modifications of the CIELAB parameters [4] in relation to model system A (only containing mv-3-glc). CIELAB parameters (L∗, a∗, and b∗ coordinates, and ) were calculated from the absorbances in the visible spectra by using CromaLab software [23], employing, as recommended (CIE, 2004), the 10° Standard Observer as references and Standard Illuminant D65 as recommended (CIE, 2004) [24]. L∗, which corresponds to the lightness of the sample, can take values from 0 (black) to 100 (white). a∗ and b∗ are the chromaticity scalar coordinates and correspond, respectively, to the green(−a∗)-red(+a∗) and to the blue(−b∗)-yellow(+b∗) scales. Hue angle (hab) and chroma () were calculated from a∗ and b∗ values and can be interpreted, respectively, as the colour tone and as the colour purity (difference from a grey colour with the same lightness). In addition, colour difference () was also calculated to evaluate whether colour differences between two samples could be discriminated by human eye ( > 3 for wine samples [25]). This parameter corresponds to the Euclidean distance between two points in the three-dimensional space defined by L∗, a∗, and b∗ and was calculated by the following equation: . The percentage of the total colour that is due to copigmentation (CCI) [5] was also calculated: CCI = ((EMS − EA)/EA) × 100. The variable E (total colour) was calculated for the model solution only containing the anthocyanin (EA) and for the different model systems (EMS) as the colour difference between their L∗, a∗, and b∗ values and those of distilled water (L∗ = 100, a∗ = 0, b∗ = 0) [5].
2.4. HPLC-DAD
Samples were analysed by HPLC-DAD at day 22 to study the possible formation of anthocyanin-derived pigments or mv-3-glc degradation products. They were filtered (0.45 μm Millex syringe-driven filter unit; Millipore Corporation, Bedford, MA, USA) and injected into an Agilent HP1200 series liquid chromatograph (Agilent Technologies, Waldbronn, Germany). UV-vis spectra were recorded from 220 to 600 nm. HPLC-DAD stationary phase, eluents, and conditions, which were previously employed and optimised in our laboratory [19], allowed the monitoring of mv-3-glc (520 nm), flavonols (360 nm), and mv-3-glc degradation compounds (280 nm) in a single run.
2.5. Statistical Analysis
The IBM-SPSS Statistics 26 for Windows software package was used for the statistical analysis. Tukey’s honestly significant difference test (p < 0.05) was performed to evaluate the significance of the differences observed among samples.
3. Results and Discussion
3.1. Influence of the Type of Flavonol in the Copigmentation with mv-3-glc
All the tested flavonols, except myricetin 3-O-glucoside, caused a bathochromic shift (2–6 nm) and a hyperchromic effect (0.03–0.16 AU) in λmax in the visible spectra of the anthocyanin, which was observable in the binary systems with flavonols (Table S1) from day 1 to day 22. Isorhamnetin 3-O-glucoside caused, in general, the lowest bathochromic shift at day 1, although differences among AI, AK, AQ, and AS were lowered over time. AI and AS showed the greatest absorbance at λmax during the whole experiment, but the hyperchromic effect was visually appreciable in all these four model systems even at day 22 (Figure 1(a)). According to these results, it can be concluded that I, K, Q, and S took part in copigmentation interactions with mv-3-glc that lasted all the experiment. In the model system prepared with myricetin 3-O-glucoside (AMy), a pale rose precipitate appeared from day 2 to end of the experiment. This precipitate was easily dispersed in the solution after vortex agitation, causing turbidity of the sample and an increase of the absorbance at λmax that could not be attributable to copigmentation. Despite this behaviour, this model system and all of the model systems containing My continued to be monitored to detect a possible modification of this behaviour by the different tested MPs.
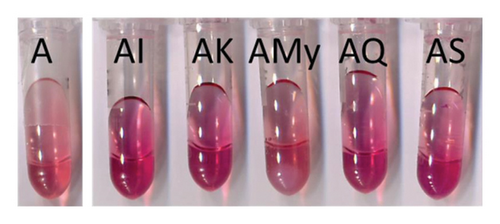
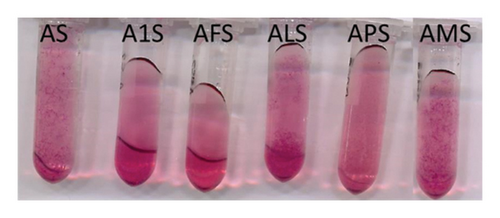
Interestingly, in the model systems prepared with syringetin 3-O-glucoside, the formation of coloured aggregates with more bluish hues than that of the solution of mv-3-glc occurred over time (Figure 2). This might be pointing to the occurrence of copigmentation interactions inside of the aggregates. Previous studies performed by dynamic light scattering and transmission electron microscopy in solutions of phenolic compounds have revealed that flavonoids are more prone than other phenolic compounds to form spherical aggregates in solution [26]. To our knowledge, there are no studies on the predisposition of the different flavonols to form these aggregates, but taking into account the results of the present study, it seems that syringetin 3-O-glucoside can form them more easily than the rest of the tested flavonols in the conditions employed. This compound has been reported to possess a lower astringency threshold in relation to other flavonols [26], which might be also related to this greater tendency to form aggregates, since one of the astringency mechanisms relates to the compound ability to form aggregates with salivary proteins that precipitate in the oral cavity, increasing the friction on the mouth. Bearing in mind this possible behaviour of syringetin 3-O-glucoside, the occurrence of the coloured aggregates in the solutions containing this flavonol and mv-3-glc might be due to two possible types of interactions: On the one hand, mv-3-glc molecules might be interacting with flavonol molecules involved in the formation of the aggregates through intermolecular copigmentation. On the other, mv-3-glc molecules might be trapped inside the aggregates, increasing their relative concentration and making possible the occurrence of self-association.
Copigmentation was also assessed from the modifications of the CIELAB parameters of the mv-3-glc solution after the addition of the different flavonols and from the indexes calculated from them. At day 1, the presence of all of the flavonols made the solutions darker (lower L∗ values), with purer colours (greater values) and bluisher hues (more negative values of ) than the model systems only containing mv-3-glc (A) (Table 1). These changes in the CIELAB parameters, which were visible to human eye (colour differences, > 3) [25], confirmed the occurrence of copigmentation [4] in these binary systems between one flavonol and mv-3-glc. The greatest were observed in My-containing model system (AMy), but, as explained above, these colour changes were more related to turbidity than to copigmentation. Among the rest of the flavonol-mv-3-glc binary solutions, model system AI showed the greatest in relation to A, and the greatest modifications in chroma () and hue (). In addition, the colour modification due to copigmentation (CCI) was also the greatest in that model system: copigmentation increased the colour 66.8% in AI in relation to the total colour of model system A. These results mean that, among the tested flavonols, isorhamnetin 3-O-glucoside was the most efficient copigment. However, as indicated above, this flavonol caused the lowest bathochromic shift at λmax, which highlights the usefulness of indexes that consider the whole visible spectra rather than those that only considers the modifications at a single wavelength to evaluate copigmentation. In contrast to isorhamnetin 3-O-glucoside, kaempferol 3-O-glucoside caused the lowest (3.5) and CCI (23.8%), pointing to a lower effectiveness of this flavonol in copigmentation interactions with mv-3-glc. At day 1, the binary model systems containing quercetin and syringetin 3-O-glucosides (AQ and AS) showed intermediate and CCI values (Table 1). Over time, CCI values of AQ tended to reach those of AK and those of AS tended to reach those of AI (Table 1 and Figure 3(a)) causing a clear separation between AS-AI (greater values) and AK-AQ (lower values). These results indicate, for the first time, that the substitution pattern of the B-ring of the flavonol has a great influence on the magnitude of copigmentation and that the presence of methoxyl groups seems to increase it. These results are in line with those reported in previous studies dealing with the influence of B-ring substitution of the anthocyanin in the copigmentation with quercetin 3-O-glucoside [3] and in self-association interactions [4]. In both cases, the greater interaction magnitude was obtained for the anthocyanins containing methoxyl groups in their B-rings, and more precisely, the most stable copigmentation complex with quercetin 3-O-glucoside was obtained with peonidin 3-O-glucoside, which possesses the same B-ring substitution as isorhamnetin 3-O-glucoside, the most effective copigment in the present study. These first results of the study show that the magnitude of copigmentation in a given wine would depend not only on the total amount of flavonols, but also on the relative percentages of the different flavonols. According to these results, copigmentation due to flavonols might be favoured in wines made from grapes richer in I or S, such as Syrah or Bobal [7], or might be reduced in wines containing great percentages of My, such as those made with Tempranillo grapes [7], due to the colloidal instability of this flavonol.
| Day 1 | L∗ | CCI | Day 22 | L∗ | CCI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Binary systems with flavonols | |||||||||||
| A | 93.1d | 11.2a | −9.5d | — | — | A | 92.8d | 10.5a | −1.3b | — | — |
| AI | 88.0bc | 18.4c | −15.7ab | 8.9c | 66.8c | AI | 88.4c | 16.0c | −9.2a | 7.3b | 56.0b |
| AK | 91.2cd | 13.7b | −16.4a | 3.5a | 23.8a | AK | 90.5cd | 13.2b | −9.0a | 3.9a | 28.4a |
| AMy | 79.2a | 13.5b | 15.4e | 15.0d | 88.6d | AMy | 67.1a | 17.0c | 52.6d | 29.2d | 192.6c |
| AQ | 89.5bcd | 14.7b | −13.2bc | 5.1ab | 37.3ab | AQ | 89.7cd | 12.8b | −5.0ab | 4.0a | 29.3a |
| AS | 86.2b | 13.6b | −10.6 cd | 7.6bc | 49.1bc | AS | 82.5b | 10.8a | 5.2c | 10.5c | 62.6b |
| Binary systems with mannoproteins | |||||||||||
| A | 93.1a | 11.2b | −9.5a | — | — | A | 92.8a | 10.5a | −1.3a | — | — |
| A1 | 93.3a | 10.9ab | −9.7a | 0.4b | −2.6b | A1 | 92.5a | 10.4a | 0.3a | 0.7b | 1.0a |
| AF | 93.1a | 11.2b | −9.2a | 0.2ab | −0.1b | AF | 92.5a | 10.3a | 1.5a | 0.7b | 0.9a |
| AL | 93.5a | 10.6a | −9.2a | 0.7c | −5.5a | AL | 92.5a | 10.1a | 0.6a | 0.6b | −0.9a |
| AP | 93.1a | 11.2b | −8.8a | 0.2ab | 0.0b | AP | 92.6a | 10.3a | −0.6a | 0.3ab | 0.3a |
| AM | 93.3a | 11.0ab | −9.3a | 0.3b | −2.1b | AM | 92.7a | 10.4a | −1.2a | 0.2a | 0.7a |
| Ternary systems of isorhamnetin 3-O-glucoside with mannoproteins | |||||||||||
| A | 93.1d | 11.2a | −9.5c | — | — | A | 92.8d | 10.5a | −1.3c | — | — |
| AI | 88.0a | 18.4d | −15.7a | 8.9c | 66.8c | AI | 88.4a | 16.0d | −9.2a | 7.3c | 56.0c |
| A1I | 89.7c | 14.7bc | −15.6a | 5.0a | 36.3a | A1I | 89.5b | 13.9c | −11.3a | 5.3b | 37.6b |
| AFI | 89.8c | 14.8bc | −15.6a | 5.1a | 36.6a | AFI | 89.3ab | 13.6c | −11.1a | 5.2b | 36.8b |
| ALI | 89.5c | 14.5b | −14.8ab | 5.0a | 36.1a | ALI | 89.5b | 14.0c | −12.4a | 5.4b | 38.1b |
| API | 89.5c | 14.8bc | −14.4b | 5.3a | 38.3a | API | 91.2c | 11.7b | −5.6b | 2.3a | 15.7a |
| AMI | 88.7b | 15.5c | −14.5b | 6.22b | 45.6b | AMI | 89.3ab | 14.1c | −10.2a | 5.4b | 39.6b |
| Ternary systems of kaempferol 3-O-glucoside with mannoproteins | |||||||||||
| A | 93.1c | 11.2a | −9.5d | — | — | A | 92.8c | 10.5a | −1.3b | — | — |
| AK | 91.2b | 13.7b | −16.4a | 3.5a | 23.8a | AK | 90.5b | 13.2bc | −9.0a | 3.9a | 28.4a |
| A1K | 90.9b | 14.0b | −15.8a | 3.8a | 26.8ab | A1K | 90.6b | 13.2bc | −11.0a | 4.1a | 27.9a |
| AFK | 90.7b | 14.3bc | −15.5ab | 4.1a | 29.5ab | AFK | 90.5b | 13.5bc | −10.5a | 4.3ab | 30.2ab |
| ALK | 90.4ab | 14.1b | −14.8bc | 4.0a | 28.9ab | ALK | 90.5b | 13.2bc | −11.1a | 4.2ab | 28.7a |
| APK | 90.3ab | 14.4bc | −14.1c | 4.4a | 31.9b | APK | 90.7b | 13.1b | −9.8a | 3.9a | 27.1a |
| AMK | 89.7a | 15.0c | −14.3c | 5.2b | 38.3c | AMK | 90.1a | 13.8c | −9.7a | 4.7b | 33.9b |
| Ternary systems of myricetin 3-O-glucoside with mannoproteins | |||||||||||
| A | 93.1c | 11.2a | −9.5a | — | — | A | 92.8c | 10.5a | −1.2a | — | — |
| AMy | 79.2b | 13.5b | 15.4bc | 15.0a | 88.6a | AMy | 67.1ab | 17.0d | 52.6d | 29.2b | 192.6b |
| A1My | 77.5b | 13.1b | 20.0bc | 16.9a | 98.1a | A1My | 67.6ab | 16.2cd | 49.9cd | 28.3ab | 186.2ab |
| AFMy | 69.3a | 13.2b | 29.1c | 25.2b | 154.4b | AFMy | 65.3a | 14.1bc | 40.6bc | 29.2b | 195.9b |
| ALMy | 81.7b | 12.8b | 8.6abc | 12.1a | 69.9a | ALMy | 68.9b | 14.5bc | 44.8bcd | 26.2a | 171.0a |
| APMy | 77.7b | 13.3b | 12.9bc | 16.3a | 97.8a | APMy | 67.0ab | 13.2b | 40.5bc | 27.4ab | 180.8ab |
| AMMy | 79.5b | 13.5b | 6.1ab | 14.2a | 86.5a | AMMy | 66.6ab | 13.6b | 37.6b | 27.6ab | 184.6ab |
| Ternary systems of quercetin 3-O-glucoside with mannoproteins | |||||||||||
| A | 93.1c | 11.2a | −9.5c | — | — | A | 92.8b | 10.5a | −1.3b | — | — |
| AQ | 89.5b | 14.7b | −13.2a | 5.1ab | 37.3ab | AQ | 89.7a | 12.8b | −5.0a | 4.0a | 29.3a |
| A1Q | 89.7b | 14.2b | −12.2ab | 4.6a | 33.5a | A1Q | 89.6a | 13.1b | −7.2a | 4.3a | 32.0a |
| AFQ | 89.1ab | 13.9b | −8.8c | 4.9ab | 34.5ab | AFQ | 89.6a | 13.4b | −7.6a | 4.6a | 34.0a |
| ALQ | 89.6b | 13.7b | −11.1abc | 4.3a | 30.9a | ALQ | 89.6a | 13.2b | −7.8a | 4.5a | 32.9a |
| APQ | 89.3ab | 14.2b | −10.3bc | 4.9ab | 35.1ab | APQ | 89.6a | 13.2b | −6.6a | 4.4a | 32.5a |
| AMQ | 88.8a | 14.7b | −10.5bc | 5.6b | 40.6b | AMQ | 89.4a | 13.5b | −6.9a | 4.8a | 35.8a |
| Ternary systems of syringetin 3-O-glucoside with mannoproteins | |||||||||||
| A | 93.1c | 11.2a | −9.5a | — | — | A | 92.8d | 10.5a | −1.3a | — | — |
| AS | 86.2ab | 13.6b | −10.6a | 7.6bc | 49.1bcd | AS | 82.5b | 10.8ab | 5.2ab | 10.5c | 62.6bc |
| A1S | 86.6ab | 13.1b | −9.3a | 6.9bc | 43.1bcd | A1S | 86.5b | 11.8bc | −4.3a | 6.6bc | 42.2abc |
| AFS | 90.5bc | 12.8b | −8.0a | 3.10ab | 21.4ab | AFS | 91.0cd | 12.1c | −6.1a | 2.7ab | 19.2ab |
| ALS | 87.9abc | 13.9b | −10.8a | 5.9bc | 40.bc | ALS | 83.2b | 10.2a | 6.3ab | 9.7c | 55.1bc |
| APS | 82.1a | 13.9b | −7.2a | 11.3c | 71.2d | APS | 81.5b | 11.7bc | −3.0a | 11.4c | 72.7cd |
| AMS | 83.5a | 13.9b | −7.3a | 10.2c | 65.6cd | AMS | 75.5a | 10.3a | 14.5b | 17.7d | 110.0d |
- For each group of model systems (binary and ternary model systems in italics), different letters in the same column indicate statistically significant differences (p < 0.05; n = 3). See Table S2 for the deviation standard values for each sample and variable.
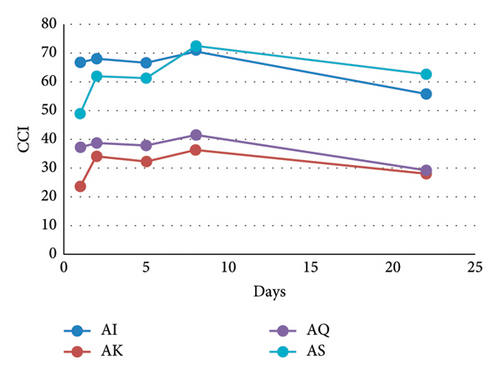
It is important to highlight that the different flavonols not only caused a different magnitude of copigmentation (CCI), but that they also differently affected the different CIELAB parameters from which this index is calculated (Table 1). This fact points to the existence of differences in the interactions between the different flavonols and mv-3-glc. To be precise, syringetin and isorhamnetin 3-O-glucosides significantly affected lightness of the solution, causing the greatest decrease in L∗ (darker colour than A), whereas Q- and K-containing systems showed L∗ values not statistically different from that observed in model system A. Since L∗ evolved similarly in all of the model systems (Figure S1), the lowest values at the end of the experiment (day 22) were still observable in AS and then in AI (Table 1). Regarding colour purity (), I caused the greatest increase and, despite the global decrease observed for this parameter in all of the model systems over time (Figure S1), the greatest value at day 22 still corresponded to system AI. In contrast, S affected this parameter less, and at day 22, its value was not statistically different from that of model system A (Table 1). Kaempferol 3-O-glucoside, in spite of causing the lowest copigmentation effect (lowest CCI) and the lowest colour difference (), was, along with isorhamnetin 3-O-glucoside, the flavonol that provoked the greatest negative increase in hue (), shifting the colour of the solution to more bluish hues. Over time, values of all the model systems tended to be less negative (Table 1 and Figure S1), meaning that the hues of the copigmented solutions were less bluish than the initial ones. This was especially remarkable for AS, which showed, at day 22, higher hue values than that of noncopigmented model system (A), which could be interpreted as a loss of copigmentation effectiveness of this flavonol over time. However, as explained above, the formation of aggregates over time in S-containing model systems can be at the origin of the greater L∗ and and lower values observed in that model system in the last sampling points. Consequently, CIELAB values and copigmentation indexes in the AS model system were only representative of the real colour of the samples and of the copigmentation effect in the first sampling points, where these aggregates were not present yet.
3.2. Influence of MPs in the Colour of mv-3-glc and in the Copigmentation between mv-3-glc and Different Flavonols
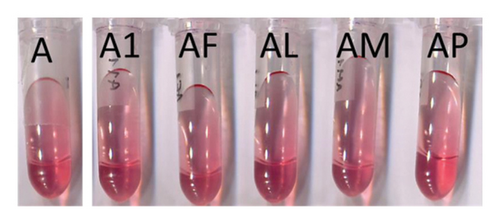
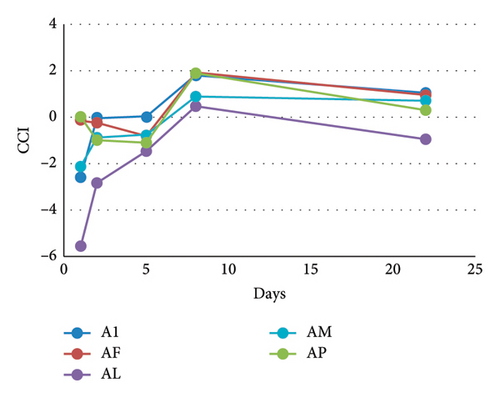
Binary model systems containing mv-3-glc in the presence of five different MPs were prepared to evaluate the possible copigmentation between the anthocyanin and them. Contrarily to flavonols, MPs hardly modified λmax or absorbance at λmax (Table S1). The colours of these binary model systems were visually identical (Figure 1(b)) to that of mv-3-glc solution (model system A). Thus, the preliminary results obtained from conventional measurements indicated that the tested MPs did not copigment with mv-3-glc. This observation was further corroborated from the CIELAB analysis of the samples: the low values of (<1) between these binary model systems and model system A confirmed that the presence of MPs hardly affected the colour of the anthocyanin and that the small changes were not detectable by human eye. At day 1, only slight reductions in were observed in the case of the model system containing MP-L (AL) (Table 1). Over time, differences in L∗ and tended to increase (Figure S1), but at the end of the study, they were still not significant (Table 1). In previous studies [18, 19] carried out in wines treated with some of the MPs tested in the present study (MP-M and/or MP-F), it could be observed that the moment of the addition of the MP (during or after alcoholic fermentation) and the moment of analysis (few days after the addition or after malolactic fermentation) can influence the magnitude of the colour differences between treated and nontreated samples, which can move from not significant to ≥3 and, consequently, visible by human eye. The results of the present study agree with those studies in the fact that the effect of the MPs in anthocyanin colour tended to be more noticeable as the time from the treatment increased. Differences in the magnitude of the modifications can be related to the fact that previous studies were performed in wines, where colour depends on multiple interactions and on wine colloidal stability, whereas in the present study, only interactions between MPs and mv-3-glc were possible.
The use of CIELAB parameters and indexes calculated from them to study copigmentation supplied additional information in relation to conventional measurements. It was very interesting to discover that all of the MPs, except MP-P, seemed to exert a slight anticopigment effect, since at day 1, CCI of these binary model systems displayed negative values (Table 1). These CCI values were still negative at day 5 reaching values next to 0 from then onwards (Figure 3(b) and Table 1). As previously indicated, the experimental conditions were set to avoid self-association (mv-3-glc concentration <1 mM and presence of ethanol), and consequently, the loss of colour observed in the presence of MPs should not be due to the disruption of self-association complexes by MPs. However, in previous studies in wine-like solutions containing pure anthocyanins [27], an increase in the pK values of mv-3-glc could be observed as the concentration of the anthocyanin raised from 0.02 mM to 1.2 mM, which could be indicative of the occurrence of self-association in the solution even at concentrations lower than 1 mM. In addition, other studies [28] reported that at pH’s 2.5–4.5, copigmentation complexes can be formed between the flavylium and the colourless Z-chalcone forms of mv-3-glc. Thus, a possible explanation for the anticopigment effect observed for MPs might be related to the disruption of these two types of self-association complexes, releasing molecules of mv-3-glc in flavylium form that can further undergo hydration and be converted into colourless hemiketal forms.
Ternary model systems were prepared to evaluate the influence of different MPs in the copigmentation between flavonols and mv-3-glc. The effects on λmax or absorbance at λmax (Table S1) were assessed by comparison of the values measured in the binary system of a given flavonol to those measured in the corresponding ternary model systems with different MPs. In the ternary model systems containing isorhamnetin 3-O-glucoside (A1I, AFI, ALI, API, and AMI), the presence of all of the MPs caused a bathochromic shift (2 nm) and reduced the absorbance at λmax in relation to binary model system (AI), but did not reach the values observed in model system A (only mv-3-glc). This means that all the MPs reduced the copigmentation between I and mv-3-glc, but they did not completely hinder it. In the model systems containing kaempferol and quercetin 3-O-glucosides, MPs hardly modified λmax, but it increased at some sample points the hyperchromic effect caused by the copigmentation between the flavonol and mv-3-glc. This effect was noticeable, above all, in the model systems containing MP-M and highlights the different effects of MPs depending on the flavonol. Concerning the ternary model systems containing syringetin 3-O-glucoside, it was interesting to observe that the bluish aggregates occurring in binary model system AS were also detectable in the presence of MP-L, MP-P, and MP-M, but not in the presence of MP-1 and MP-F (Figure 2). The final colour of all of these ternary solutions was more intense and bluish than that of mv-3-glc, although in some cases, the copigmented colour was visible mostly in the aggregates (APS and AMS), or in the solution (A1S and AFS) or in both (ALS). The presence of MP-1 and MP-F seemed to revert the formation of the aggregates, obtaining, as a result, a “classic” copigmented solution and similar to those obtained in the copigmentation with I, Q, and K. The effect of these two MPs reminds that of a nonionic detergent (Triton X-100), which was able to revert the formation of flavonol aggregates when added to the solution [29]. In the rest of the ternary model systems where aggregates were formed (ALS, APS, and AMS), the visual aspect (size and colour, above all) of these aggregates was also different (Figure 2). These results highlight that the different MPs interact differently with mv-3-glc and syringetin 3-O-glucoside in wine-like solution and they are in line with those of previous studies on the interactions between some of these MPs, flavanols, and salivary proteins [21], which reported different types of interactions depending on the structure of the flavanol and on the glucidic and protein composition of MPs. Regarding the MPs tested in the present study [21], MP-1 and MP-F could be considered MPs with low percentages of protein (around 10%) and high percentages of mannose (>75%) in the glucidic fraction. In contrast, MP-L contains a relatively high protein content (31%) with a low mannose proportion (45%) and a large variety of other sugars (arabinose, galactose, glucose, and glucuronic acid). MP-P showed an intermediate composition (proteins around 17%, mannose (67%), and glucose (31%) prevailing in the glucidic fraction). According to these results, it seems that MPs with low protein content and high mannose content can revert the formation of flavonol aggregates and favour the copigmentation between syringetin 3-O-glucoside and mv-3-glc in solution. However, in the case of MP-M, factors other than glycosidic composition and protein percentage should be influencing its behaviour towards the copigmentation interaction, since this MP was not able to revert the formation of flavonol aggregates despite its similar composition to MP-1 and MP-F (10.3% of proteins, 82% of mannose, and 17% of glucose). Differences might involve the distribution and branching of the saccharide residues and the phosphorylation degree in the glucidic part of the molecule or the composition and/or structure of the protein fraction [20]. The different behaviours of the different MPs in S ternary systems were not only visually observable but also detectable from the modifications of λmax and absorbance at λmax (Table S1) caused by their presence.
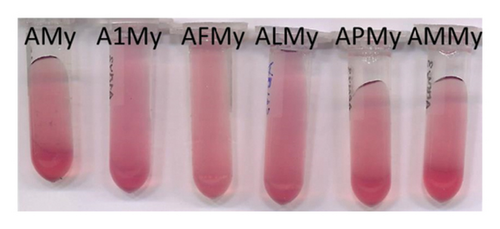
As indicated above, the binary model system containing myricetin 3-O-glucoside (AMy) developed a slightly coloured precipitate over time, which caused a relatively stable turbidity in the model system following shaking and responsible for its greater absorbance at λmax in relation to the rest of binary model systems. In this case, no coloured aggregates were observed. None of the MPs added in the ternary model systems were able to reduce this turbidity (Figure 2(b)), and on the contrary, it seemed to be more stable in these ternary model systems than in AMy. This behaviour of myricetin 3-O-glucoside in solutions containing mv-3-glc can be of interest when studying the colloidal behaviour of wines with great proportions of My, as can be those made from Tempranillo grapes, where this flavonol can account for 45% of the total flavonol content [7].
In the ternary model systems, the effects of the different MPs on the flavonol-anthocyanin copigmentation interaction were also evaluated from the CIELAB parameters and indexes calculated from them (Table 1). Again, these parameters gave more information about the colour modulation caused by the presence of the different MPs than the simple determinations of λmax and absorbance at λmax. In the ternary model systems with mv-3-glc, isorhamnetin 3-O-glucoside, and MPs (Table 1), CCI values (36–38 in A1I, AFI, ALI, and API and next to 45 in AMI) were statistically lower than that observed in the binary system (AI) (CCI ∼ 67), thus confirming the observation reported above from conventional measurements: the presence of MPs reduced the copigmentation effect observed in AI (binary model system), although they did not totally hinder it. MP-M was the MP that reduced less copigmentation effect. This difference in the CCI values between binary and ternary model systems was maintained over the whole experiment, although it tended to be reduced at the end of the experiment (Figure 4(a) and Table 1), as a consequence of the reduction of CCI in the binary system AI, and a somehow stabilisation in the presence of all the MPs (except MP-P). It seems, therefore, that the addition of the MPs can reduce the copigmentation phenomenon between mv-3-glc and I, but that their presence can stabilise the copigmentation complex over time. However, despite this reduction in the copigmentation magnitude after MP addition, colour differences between these ternary model systems and model system A were still detectable by human eye ( around 5–6) and even at the end of the experiment (except in the presence of MP-P). Concerning individual CIELAB parameters (Table 1), these ternary model systems showed L∗ and values intermediate between the binary model system (AI) and model system A (only mv-3-glc). However, values were quite preserved among all the flavonol-containing samples, above all in the presence of MP-1, MP-F, and MP-L, showing more bluish hues than that only containing the anthocyanin. Over time, CIELAB parameters evolved similarly in binary and ternary model systems of this flavonol, although, as observed for CCI, all the MPs except MP-P seemed to reduce the evolution rate (Figure S2).
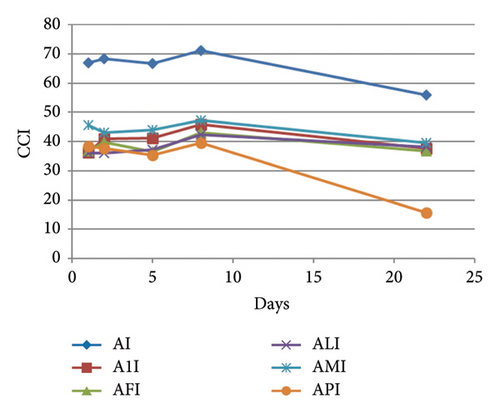
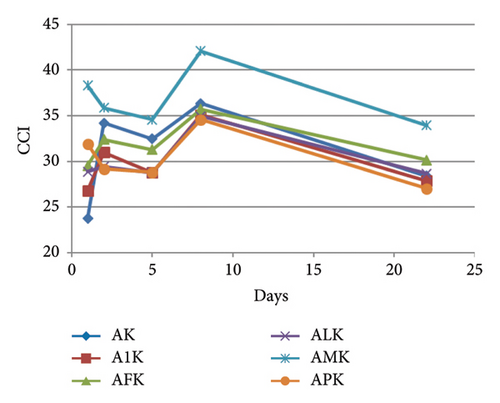
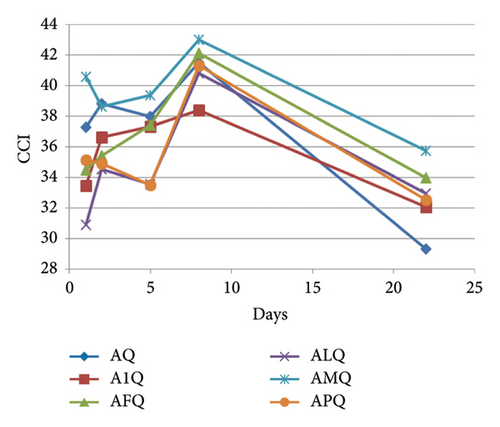
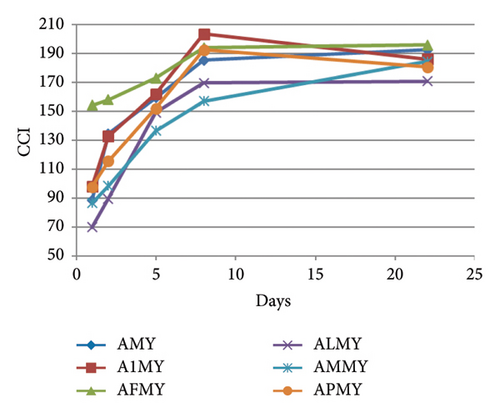
In the ternary model systems prepared with kaempferol 3-O-glucoside, the MPs provoked the opposite effect to that observed in those prepared with I: all the tested MPs increased the CCI of the binary model system AK (Table 1), although it was only statistically significant higher for MP-M and then for MP-P. CCI evolved similarly in all the model systems (Figure 4(b)) and, at day 22, only model system containing MP-M (AMK) still showed greater CCI. The rest of the MPs maintained the copigmentation observed in the binary system AK. between ternary model systems and model system A was >3 in all of the cases and, therefore, observable by the human eye. However, these differences were not statistically significant from that between A and AK, except for model AMK, which indicated again that MP-M was able to cause greater colour changes than the rest of the MPs. This was also observed with individual CIELAB parameters (Table 1): MP-M was the MP that reduced L∗ and increased the most. In contrast, MP-1 was the one that initially maintained the best the bluish hue of the copigmented complex between mv-3-glc and K ( value in A1K was not statistically different from that observed in AK). At day 22, differences in between binary and ternary model systems were not significant, but were still significant in relation to model system A, meaning that all the MPs were able to stabilise the hue of the copigmentation complexes.
Concerning the ternary model systems containing quercetin 3-O-glucoside, all of the MPs except MP-M initially reduced the CCI value observed in the binary model system AQ (Table 1). However, from day 5 onwards, the CCI values of the binary and ternary model systems tended to equalise (Figure 4(c)), and at day 22, AQ showed the lowest CCI pointing to a certain protection of the copigmentation complexes between Q and mv-3-glc by MPs. MP-M, which caused the greatest CCI increases in the ternary model systems containing I or K mostly by decreasing L∗ and increasing , also increased CCI in relation to that of AQ from the beginning of the experiment. This observation pointed to a similar behaviour of MP-M independently of the flavonol present in the ternary model system. However, the magnitude of the effect seemed to depend on the strength of the anthocyanin-flavonol interaction (when CCI in binary system is high, as in AI, MP-M can not increase the magnitude of copigmentation; however, when CCI is low, as in AK or AQ, the presence of MP-M can increase it). Similarly, as occurred in the ternary model systems containing I or K, the smallest modification of the value of the binary model system AQ took place in the presence of MP-1. Thus, it seems that MP-1 allows the stabilisation of the hue expressed by the copigmentation complexes between mv-3-glc and these three flavonols, whereas MP-M affects mainly the lightness and colour purity.
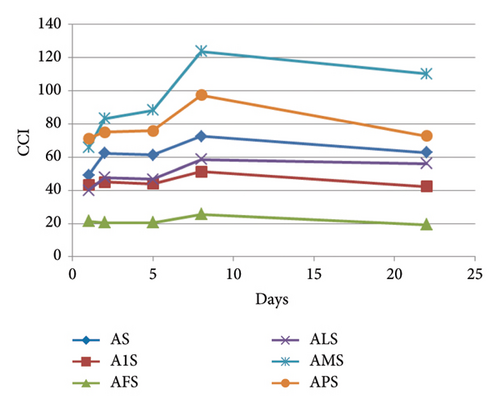
In the ternary model systems containing syringetin 3-O-glucoside, MP-P and MP-M increased CCI in relation to the binary model system (AS) (mainly by lowering L∗), whereas MP-F reduced it and MP-1 and MP-L hardly modified it (day 1, Table 1 and Figure 4(d)). Differences between binary and ternary model systems were not significant for and , and for this reason, colour differences () were mostly affected by lightness. At day 1, the samples added with MP-M or MP-P showed the greatest values, whereas those added with MP-F showed values next to 3. As indicated above, the copigmented aggregates that appeared over time in S-containing model systems made difficult the evaluation of copigmentation in the last sampling points. However, the presence of MP-F or MP-1 allowed a reversion of these aggregates (Figure 2), making acceptable the use of CCI data in these cases. These two model systems showed CCI values lower (AFS) or similar (A1S) to the binary system AS, but it has to be taken into account that the greater CCI values in AS might be due to the formation of copigmented aggregates over time and do not necessarily indicate a greater copigmentation in that binary model system than in ternary ones. The rest of the MPs (MP-L, MP-M, and MP-P) were not able to revert the formation of the copigmented aggregates (Figure 2). However, the greater CCI values at the end of the experiment of APS and AMS in relation to the binary model system (AS) might be indicating that the presence of MP-M or MP-P was causing changes in the size of the coloured aggregates or in their behaviour towards light in the spectrophotometer in relation to the aggregates of the binary model system.
As explained above, none of the MPs were able to revert the turbidity observed in the binary system prepared with mv-3-glc and myricetin 3-O-glucoside (AMy). In terms of CIELAB parameters, this turbidity was detectable from the great CCI values (Table 1) that did not correspond to the visual observation (Figure 2) and seemed to increase over time in all of the samples containing myricetin 3-O-glucoside (Figure 4(e)). This turbidity caused these binary and ternary model systems to show L∗ values much lower than model system A, greater values, and values corresponding to red-orange hues (Figure S2e). Although these results did not provide useful information in what respects copigmentation, they point to a slight ability of MP-M to reduce this turbidity (Figures 4(e) and S2e), highlighting again differences between this MP and the others.
3.3. HPLC-DAD Analysis
Samples were analysed by HPLC-DAD at day 22 to study the evolution of mv-3-glc and the possible formation of derivative pigments in the presence of flavonols and/or MPs. The qualitative and quantitative profile of model system A was taken as a reference. The results of the HPLC-DAD analysis highlighted again the relevance of copigmentation in the colour expression of anthocyanins, since, at day 22, many of the binary and ternary model systems showed lower mv-3-glc contents than model system A (Figure S3a), but, as mentioned above, the colours expressed by some of them were darker, purer, and more bluish than that of model system A (Figures S1 and S2). Furthermore, it was interesting to observe lower decreases in the content of mv-3-glc in the model systems with greater CCI (AI and AMK, for example), pointing to a protective role of some flavonols alone or in combination with certain MPs against mv-3-glc disappearance. Long-term studies would be useful to confirm this preliminary observation.
Since none of the model systems showed additional peaks in the chromatograms at 520 nm that could correspond to derivative pigments, the reduction in mv-3-glc levels should be mainly driven by degradation reactions. Four main peaks corresponding to degradation compounds (already present in model system A at day 1) were monitored at day 22 in the chromatograms at 280 nm. The chromatographic and spectral features allowed the identification of the two main degradation products of mv-3-glc, syringic acid (compound 1), and 2,4,6-trihydroxybenzaldehyde (THB, compound 2) along with the chalcone 3-O-glucoside resulting from the C-ring opening (compound 4) and an intermediary product in the transformation of malvone aglycone to THB (compound 3) that has been reported in previous studies [30–32]. In model system A (Figures S3b–S3e), the greatest changes from day 1 to day 22 were observed in the levels of syringic acid and of compound 3, which increased. In contrast, the levels of THB slightly decreased, probably related to its possible oxidation into 2,4,6-trihydroxybenzoic acid [32, 33]. The levels of chalcone 3-O-glucoside remained quite stable. Taking this behaviour as a reference, these four degradation products were monitored in binary and ternary model systems. In an overview, the results point to a lower formation of these four degradation products in the binary model systems with flavonols and a greater formation of THB and chalcone 3-O-glucoside in the binary model systems with MPs. Flavonols seem, therefore, to reduce the degradation reactions of mv-3-glc, whereas MPs seem to increase the formation of some of the degradation products, probably by reducing their transformation, respectively, into 2,4,6-trihydroxybenzoic acid (from THB) and into the coloured forms in equilibrium with chalcone 3-O-glucoside. These greater levels of chalcone 3-O-glucoside in these binary model systems containing MPs might also partly explain the “anticopigment” effect inferred from the negative CCI values observed for them (Table 1). Interestingly, model system AL, which showed the most negative CCI values, also showed the greatest chalcone 3-O-glucoside content, whereas AP, which showed CCI values next to zero, showed the lowest levels of chalcone 3-O-glucoside (Table 1 and Figure S3e). In ternary model systems, no global conclusions could be drawn from HPLC-DAD results and further studies should be performed for a deeper understanding of the combined effect of flavonols and MPs on the degradation of mv-3-glc.
4. Conclusions
In the present study, both conventional measurements by spectrophotometry and CIELAB parameters have shown that all the tested flavonols (except My) were able to take part in copigmentation interactions with mv-3-glc and that the changes caused in the colour of the solutions were visible to human eye ( > 3). The presence of methoxyl groups in the B-ring of the flavonol increased the magnitude of copigmentation, and CCI values were greater for I and S than for Q and K. Contrarily to flavonols, the tested MPs did not take part in copigmentation interactions with mv-3-glc ( < 1) and they even seemed to exert, in most cases, a slight anticopigment effect (CCI < 0), possibly by disrupting self-association complexes and/or by stabilising chalcone forms of the anthocyanin (as observed in the HPLC-DAD analysis). The effects of MPs in the copigmentation between the anthocyanin and the different flavonols (ternary model systems) seemed to be dependent on the type of MP and on the magnitude of flavonol-anthocyanin copigmentation: when copigmentation in the binary model system was great, the presence of MPs reduced it, but when it was lower, MPs were able to increase or maintain the CCI observed in the binary model system. In any case, the greatest CCI values in all of the ternary model systems were observed for those containing MP-M, pointing to a similar behaviour of this MP independently of the flavonol. Copigmented aggregates appeared and turbidity developed over time in the model systems containing S and My, respectively. In the first case, the formation of the aggregates could be reverted by the presence of MP-F and MP-1. However, none of the MPs were able to reduce the turbidity observed for the My-containing model system. The knowledge of these behaviours will be helpful to understand the expression of colour and the colloidal behaviour of red wines in relation to their flavonol composition and their modification by the presence of MPs.
The results of the present study, along with the determination of the flavonol profile of grapes, will allow winemakers to estimate, to a certain extent, the potentiality of these grapes to produce wines with great levels of copigmentation. The different behaviours observed for the different flavonols, not only in what relates to the interaction with mv-3-glc, but also to their colloidal behaviours, highlight that it is not only important to determine the total flavonol content, but also the proportions of the different compounds. In addition, winemakers could adapt winemaking techniques depending on the flavonol composition of the grapes in order to improve the copigmentation effect by favouring the release of MPs from yeasts or by adding exogenous MPs or by improving flavonol colloidal stability.
Long-term studies are needed to confirm the protective role of flavonols and/or MPs in protecting the anthocyanin against degradation.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This research was financially supported by Grant PID2021-127126OB-C21 funded by MICIU/AEI/10.13039/501100011033 and by “ERDF/EU.”
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.




