Insect Pests and Diseases in Stored Sorghum (Sorghum bicolor L.) and Maize (Zea mays L.) in West Hararghe, Ethiopia
Abstract
The presence of major insect pests and microflora in stored sorghum and maize in West Hararghe, Ethiopia, poses a significant threat to food security and agricultural productivity, leading to postharvest losses and economic hardship for local farmers. This study aimed to evaluate and identify the main grain insect pests and diseases of stored maize and sorghum in West Hararghe, Ethiopia. The survey was carried out in five major sorghum and maize-producing districts of the surveyed areas, namely, Mieso, Gemechis, Tullo, Anchar, and Oda Bultum. The results revealed that maize and sorghum grains stored in sacks, ground pits, markets, and hanging (maize cob) storage containers were infested with insect species: maize weevil (S. zeamais), Angoumois grain moth (S. cerealella), rice weevil (S. oryzae), Red flour beetle (T. castaneum), confused flour beetle (T. confusum), and Lesser grain borer (R. dominica). The mean levels of major insect pest infestations and associated percentages of grain damage, weight loss, and germination loss were recorded across all traditional storage systems, including sacking, marketing, hanging or maize cob, and ground pit storage methods. The highest absolute frequency and relative frequency of F. oxysporum (14.53%), followed by A. flavus (13.08%), on maize seeds were recorded in the agar plate test. A total of eight species of fungi, including Fusarium species, A. parasiticus, and A. niger, were identified using the blotter and agar plate method. The incidence of fungal diseases was markedly high (98.33%) in maize grain stored in the Anchar district, followed closely by grain stored in the market in the same district, recording 98.33%. Therefore, it is recommended that further confirmatory identification using molecular tools be conducted to develop novel management strategies for these insect pests and diseases.
1. Introduction
Sorghum (Sorghum bicolor L.) and maize (Zea mays L.) are crucial cereal crops in Ethiopia, covering 2.5 million hectares during the main cropping season and producing nearly 10,500 kg million, ranking second in area coverage and first in total production [1]. However, their production faces hindrances from various factors, including fungal diseases and arthropod pests, both in the field and during storage. Certain fungal species associated with sorghum and maize produce mycotoxins, which pose health risks to animals and humans [2]. Insects penetrate kernels, damaging their surface and endosperm. They exhibit rapid development, a long life, and attack stored grains within kernels or as secondary pests in broken kernels, grain dust, or mold [3]. Their selective feeding promotes pathogen development, accelerating seed deterioration by raising temperature and moisture levels [4, 5].
These losses directly impact food security, human nutrition, and seed viability [6], especially in tropical climates where poor sanitation and inadequate storage exacerbate fungal growth and insect infestations [4, 7]. Insects, responsible for 10%–60% of postharvest losses in grains in developing nations, spread fungal spores and increase the moisture content through digestion [8, 9, 10, 11]. Storage fungi, correlated with insect infestation, significantly contribute to maize grain losses [12, 13], while mycotoxins in grains and feedstuffs pose health risks and diminish seed quality [14].
The occurrence of insect pests and diseases in stored sorghum and maize poses a significant challenge to agricultural productivity in the West Hararghe region of Ethiopia. This study aimed to identify the specific insect pests and diseases affecting stored sorghum and maize in this region. By understanding these challenges, we could develop effective strategies for pest and disease management to improve agricultural productivity and food security. The scope of the study includes identifying common insect pests and diseases and exploring potential methods for their control and management. The information gathered would contribute to the development of targeted interventions and policies to mitigate the impact of insect pests and diseases on stored sorghum and maize in the West Hararghe region of Ethiopia. Therefore, this study aimed to assess and identify the main grain insect pests and diseases in stored maize and sorghum in West Hararghe, Ethiopia, providing vital data to mitigate these issues.
2. Materials and Methods
2.1. Description of Study Areas
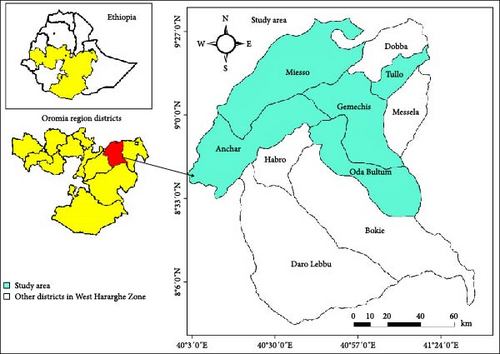
The survey was carried out in five major sorghum- and maize-producing districts of West Hararghe, namely, Mieso, Gemechis, Tullo, Anchar, and Oda Bultum. The laboratory study was carried out at Oda Bultum University College of Agriculture, Plant Science Laboratory in 2020/2021. West Hararghe is situated at 7° 55′–9° 33′N latitude and 40° 01′–41° 39′E longitude. The selected districts vary in their geographical location and mean weather variables (Figure 1). The Mieso district is located 300 km east of Addis Ababa, between 40° 9′30.1′ E and 40° 56″44′ E longitude and 9° 19′52′N and 8° 48″12 N latitude. Its altitude ranges from 1,107 to 3,106 m above sea level, with a mean annual temperature of around 21°C and average annual rainfall between 635 and 945 mm [15]. The Anchar district is located 390 km east of Addis Ababa, with an altitude ranging from 1,200 to 4,500 m above sea level. The mean annual rainfall is 1,950 mm, and the minimum and maximum average temperatures range from 12.4 to 24°C, respectively [16]. The Gemechis district, located 343 km east of Addis Ababa, has longitude and latitude coordinates between 8° 40′0′ and 9° 04′0′ N and 41° 12′0′ E. It experiences minimum and maximum temperatures of 20 to 30°C, with rainfall ranging from 850 to 1,000 mm [15]. The Tullo district, positioned 370 km southeast of Addis Ababa, has an altitude of 1,750 m above sea level. It experiences a mean annual rainfall of 1,850 mm and a mean annual temperature of 23°C. The Oda Bultum district has an altitudinal range of 1,600–2,400 m above sea level, with annual rainfall between 900 and 1,100 mm. Located at a longitude and latitude of 8.0 30′ 0′ to 9.0 0′0′N and 40.020′0′– 40.0 40′00′E, it is 362 km east of Addis Ababa. The district experiences a mean maximum temperature of 28°C and a mean minimum temperature of 25°C [17].
2.2. Sample Collection and Treatment
From each district, four areas were selected based on the availability of storage types for both crops. Grains were collected from specific storage areas in each district: Gemechis district (Sororo, Walargi, Daro, and Harabafano), Oda Bultum district (Hara Dimtu, Saphalo, Ido Bariso, and Burka Misoma), Tulo District (Kira Kufis, Lubu Dakab, Terkanfata, and Ifa Bas), Mieso district (Oda Bal’aa, Dalacha, Hameressa, and Ittisa), and Anchar district (Seradiya, Daro Gura, Lafto Goba, and Metekoma) (Figure 1). Composite grains of maize and sorghum were collected from underground sorghum pits and from markets and sacks for both sorghum and maize, while hanging maize cob samples were collected for maize. A total of 60 grain samples were collected for each crop, making a total of 120 samples assessed during the study. Each location was systematically selected based on pest availability and crop production potential.
From each selected container, 500 g of maize and sorghum grain samples were collected. Each sample was placed in a paper bag and labeled with the necessary information. Samples of the same agroecology were mixed and placed in a cloth bag for further inspection in the laboratory [18]. The inspection of the samples began within 2 weeks of collection at Oda Bultum University, College of Agriculture, Plant Science Laboratory.
2.3. Insect Pest Assessment
2.3.1. Major Insect Pest Distribution, Composition, and Identification
Each grain sample was collected from various agroecologies in different areas in western Hararghe in the eastern Ethiopia, and it was collected into separate containers. For the study, the samples were sieved using a 2 mm mesh sieve following the method described by Tadesse [19]. Living and dead insects were counted, identified, and the distribution and composition of pests were analyzed using the insect identification procedures outlined by Mandali [20]. The samples were kept at room temperature (25°C). Internal infestation was assessed after approximately 1 month, and newly emerged insects were counted and recorded. The number of living and dead insects per sample was documented for each identified insect [21].
2.3.2. Evaluation of Grain Damage and Weight Loss
U = weight of undamaged grains, D = weight of insect-damaged grains,
Nu = number of undamaged grains, and Nd = number of insect-damaged grains.
2.3.3. Seed Germination Test
2.3.4. Relative Abundance and Relative Species of Storage Insect Pests
2.4. Identification and Assessment of Fungal Grain Mold
The fungal species identified from the maize and sorghum grains stored in various containers (sacks, ground pits, market, and hanging or maize cob) were evaluated in different agroecologies: Mieso and Anchar in lowland areas, Tulo as an intermediate agroecology, and Gemechis and Oda Bultum in highland agroecology. The incidence of fungal pathogens was calculated for both types of grains. The incidence of diseases was determined by the number of infected seeds out of the total seeds examined. A total of 120 samples from the stored maize and sorghum grains, with 60 samples for each type of storage (maize and sorghum), were inspected for the incidence of fungal diseases.
2.4.1. Blotter Method
Seeds from each source were tested using the standard blotter method. Three pieces of 90-mm blotting paper were moistened with distilled water and placed in sterilized 90 mm Petri plates after excess water was drained. Untreated seeds were then placed in the Petri plates, with 10 seeds for maize and 15 seeds for sorghum, evenly spaced. The plates were then incubated at room temperature (25°C). After 8 days of incubation, the seeds were examined under a stereoscopic binocular microscope to identify associated fungi according to their “habit and colony characters” [27]. The experiment followed a completely randomized design with three replications.
2.4.2. Agar Plate Method
Potato dextrose agar (PDA) was utilized for fungal isolation, prepared according to the manufacturer’s instructions. Specifically, 39 g of potato dextrose agar was measured and mixed with distilled water to achieve a total volume of 1,000 ml. The medium was then autoclaved at 121° C for 15 min and allowed to cool to approximately 50°C before adding 0.1 g/L streptomycin. After gentle swirling, the mixture was poured into 90 mm diameter sterile Petri dishes and allowed to solidify before sample inoculation. Before plating, the sorghum and maize seeds were surface disinfection with sodium hypochlorite for 1 min, followed by rinsing three times with sterile distilled water and drying on sterile blotter paper for 2 min [28]. Subsequently, a maximum of 10 seeds for maize and 15 seeds for sorghum were plated on sterile PDA poured into each Petri dish. These seed plated, arranged in three replicates, were then incubated at 25°C for 7 days to observe colony growth stages. Subculture was conducted using PDA to obtain pure cultures, with all procedures performed in sterile working environments. After a week of incubation, the total number of fungal colonies, frequency of fungal isolation (%), the relative density of isolated fungi (%), and fungal incidence (%) were recorded and calculated according to Marasas et al. [29]. Fresh PDA plates were used for subculturing, where different fungal colonies from primary cultures were excised using a sterile scalpel and transferred to fresh PDA plates to obtain pure cultures. The inoculated plates were sealed with parafilm wax and incubated at room temperature (25°C) in the dark for 7 days as Warham et al. [30]. The experiment followed a completely randomized design with three replications.
2.4.3. Identification of Storage Fungi
The fungi were identified according to the morphology of the spore and colony characteristics [31]. Slide preparations of fruiting structures and spores of specific fungi were created and examined under a compound microscope to confirm their identities, referencing literature on seed health analysis [32, 33]. Cultures were further purified using a single spore isolation technique [34]. The percentage occurrence of fungi was calculated [35].
2.4.4. Incidence, Isolation Frequency, and Relative Density of Storage Fungi
2.5. Data Analyses
Data on grain damage, weight loss, grain germination percentage, abundance and relative abundance of insect pest species, incidence of fungi, isolation frequency, and relative density of fungi for each district and different storage types were summarized and organized using Microsoft Office Excel. Statistical analysis was performed using the SAS 9.4 software package. Means were compared using the least significant difference (LSD) test, with significance levels set at 5% and 1%.
3. Results and Discussion
3.1. Assessment of Maize and Sorghum Grain Losses
3.1.1. Occurrence of Maize Storage Insect Pests
There were differences in insect pest distribution and infestations, suggesting potential factors such as storage types and forms of maize grain stored by farmers, which may affect insect pest population distribution differently at the local habitat level. In the districts surveyed, the hanging (maize cob) exhibited a significant difference (p < 0.01) compared to the sack and grain purchased from the market. The results of the survey revealed that storage insect pests were present in the maize growing districts of the level of surveyed zone, but the distribution varied based on storage type (Figures 2). It was observed that stored maize grains in the West Hararghe were affected by various insect pests. Maize weevil (S. zeamais), Angoumois grain moth (S. cerealella), rice weevil (S. oryzae), confused flour beetle (T. confusum), sawtooth grain beetle (O. surinamenis), lesser grain borer (R. dominica), red flour beetle (T. castenum), and rusty grain beetle (C. ferugineus) were recorded as major pest in maize grains stored in sack, market, and hanging (maize cob) storage containers (Table 1).




| Districts | Types of grain storage | Sitophilus zeamais | Sitotroga cerealella | Sitophilus oryzae | Tribolium castaneum | Tribolium confusum | Oryzaephilus surinamenis | Cryptolestes ferrugineus | Rhyzopertha dominica |
|---|---|---|---|---|---|---|---|---|---|
| Coleoptera | Lepidoptera | Coleoptera | Coleoptera | Coleoptera | Coleoptera | Coleoptera | Coleoptera | ||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| Mieso | Sack | 117.00 ± 3.00a | 105.60 ± 5.10a | 71.30 ± 5.50b | 35.60 ± 8.30ab | 61.00 ± 3.60b | 46.30 ± 7.50a | 35.30 ± 5.60a | 28.00 ± 9.60a |
| Market | 85.33 ± 6.50b | 76.30 ± 5.50b | 91.60 ± 7.20a | 54.30 ± 17.90a | 80.30 ± 8.30a | 25.30 ± 5.50b | 45.60 ± 11.50a | 17.30 ± 5.60ab | |
| Hanging | 63.30 ± 12.40c | 55.30 ± 18.80b | 44.30 ± 13.60c | 20.60 ± 11.50b | 34.60 ± 13.30c | 6.00 ± 7.90c | 13.30 ± 5.70b | 5.30 ± 4.10b | |
| p Value | 0.0054 | 0.0055 | 0.0026 | 0.0552 | 0.0029 | 0.0013 | 0.0066 | 0.0197 | |
| CV (%) | 13.90 | 14.70 | 13.70 | 35.80 | 15.80 | 27.02 | 25.60 | 40.80 | |
| LSD | 24.70 | 23.30 | 18.90 | 26.43 | 18.60 | 14.10 | 16.20 | 13.70 | |
| Anchar | Sack | 101.00 ± 2.60a | 90.00 ± 12.00a | 81.00 ± 15.60a | 37.60 ± 25.30a | 57.30 ± 14.60a | 11.00 ± 4.30b | 25.00 ± 11.30ab | 9.30 ± 0.50b |
| Market | 80.33 ± 8.30ab | 64.60 ± 7.20b | 58.00 ± 7.00ab | 25.60 ± 8.30a | 31.30 ± 1.50b | 34.60 ± 13.60a | 37.30 ± 19.60a | 23.30 ± 4.50a | |
| Hanging | 51.67 ± 25.30b | 46.00 ± 14.70b | 38.67 ± 13.40b | 14.30 ± 5.10a | 17.00 ± 12.10b | 4.60 ± 5.50b | 9.60 ± 5.00b | 6.00 ± 7.80b | |
| p Value | 0.0221 | 0.0107 | 0.0176 | 0.2664 | 0.0114 | 0.0135 | 0.1131 | 0.0109 | |
| CV (%) | 19.91 | 17.50 | 21.20 | 60.50 | 31.20 | 52.70 | 55.80 | 39.40 | |
| LSD | 30.90 | 23.40 | 25.00 | 31.30 | 21.90 | 17.60 | 26.70 | 10.40 | |
| Tulo | Sack | 91.00 ± 13.70a | 81.30 ± 16.60a | 48.00 ± 7.00ab | 51.30 ± 8.90a | 44.60 ± 5.60b | 13.30 ± 5.70a | 61.00 ± 7.20a | 30.00 ± 5.50a |
| Market | 68.33 ± 7.60b | 56.00 ± 6.20ab | 71.00 ± 15.60a | 71.60 ± 12.40a | 67.30 ± 14.60a | 3.60 ± 0.50b | 29.60 ± 10.50b | 14.00 ± 6.20b | |
| Hanging | 32.66 ± 10.40c | 35.00 ± 14.70b | 27.60 ± 13.40b | 25.00 ± 14.70b | 26.00 ± 12.10b | 1.30 ± 0.50b | 14.00 ± 9.60b | 6.60 ± 7.20b | |
| p Value | 0.0016 | 0.0168 | 0.0176 | 0.0111 | 0.0143 | 0.0105 | 0.0019 | 0.0110 | |
| CV (%) | 16.90 | 23.07 | 25.50 | 24.70 | 24.70 | 55.00 | 26.10 | 37.80 | |
| LSD | 21.70 | 26.60 | 25.00 | 24.50 | 22.80 | 6.70 | 18.40 | 12.70 | |
| Oda Bultum | Sack | 74.33 ± 14.00a | 68.60 ± 17.90a | 60.00 ± 13.80a | 24.60 ± 16.00a | 36.00 ± 17.60a | 2.60 ± 1.50b | 21.00 ± 6.50ab | 8.60 ± 3.70ab |
| Market | 56.33 ± 14.50a | 51.00 ± 7.20ab | 35.30 ± 9.80ab | 14.00 ± 7.20a | 16.30 ± 2.80ab | 7.30 ± 3.00a | 37.60 ± 14.50a | 16.60 ± 6.00a | |
| Hanging | 26.33 ± 10.01b | 30.00 ± 13.20b | 20.30 ± 12.30b | 6.00 ± 5.20a | 7.00 ± 4.50b | 1.00 ± 1.00b | 6.60 ± 3.50b | 4.00 ± 5.10b | |
| p Value | 0.0124 | 0.0351 | 0.0197 | 0.1795 | 0.0413 | 0.0223 | 0.0195 | 0.0426 | |
| CV (%) | 24.70 | 26.90 | 31.20 | 69.70 | 53.10 | 56.00 | 43.20 | 50.30 | |
| LSD | 25.90 | 27.00 | 24.20 | 21.20 | 21.30 | 4.10 | 18.80 | 10.10 | |
| Gemechis | Sack | 56.30 ± 16.2a | 47.66 ± 5.80a | 46.60 ± 15.80a | 21.30 ± 8.00a | 48.00 ± 15.70a | 6.30 ± 2.30a | 14.30 ± 5.10a | 9.00 ± 5.10a |
| Market | 43.00 ± 11.0ab | 33.00 ± 9.80a | 30.00 ± 8.70ab | 31.60 ± 24.00a | 24.60 ± 0.50b | 5.00 ± 2.00a | 10.60 ± 3.70a | 2.00 ± 0.01b | |
| Hanging | 21.00 ± 9.8b | 15.66 ± 6.60b | 15.60 ± 8.60b | 11.30 ± 6.10a | 11.60 ± 5.50b | 1.30 ± 0.50b | 2.60 ± 1.10b | 1.60 ± 2.00b | |
| p Value | 0.0426 | 0.0064 | 0.0518 | 0.2943 | 0.0101 | 0.0177 | 0.0225 | 0.0542 | |
| CV (%) | 31.30 | 23.80 | 37.20 | 69.00 | 33.80 | 39.40 | 40.50 | 76.50 | |
| LSD | 25.30 | 15.20 | 23.10 | 30.00 | 19.20 | 3.50 | 7.40 | 6.40 | |
- Means followed by different letters within columns are significantly different, p < 0.05 using least significant difference (LSD).
In this study, it was observed that the weevil (S. zeamais) emerged as the major insect pest that caused significant damage to maize grains, followed by the Angoumois grain moth (S. cerealella). S. zeamais exhibited a notably high mean number compared to other insect species in the major districts. The occurrence of insect species varied from one district to another, influenced by agroecology and storage types. The Mieso district had the highest number of insect species, followed by Anchar, relatively fewer in Gemechis and Oda Bultum, and intermediate in Tulo (Table 1). Farmers predominantly utilized traditional containers for maize storage (Figure 2), and there was a highly significant difference (p < 0.01) in the type of storage structure used across major districts.
Similar findings were reported in Ethiopia by Abamecha et al. [37] and Taddese et al. [38], highlighting widespread insect pest attacks on maize among farmers. According to Midega et al. [39], storing maize in unshelled form appeared to result in fewer pest attacks, while the majority of farmers stored their maize in shelled form. Regarding the mean number of insect species across major districts in maize grains, the order was as follows: S. zeamais > S. cerealella > S. oryzae > T. confusum > T. castaneum > C. ferrugineus > O. surinamenis > R. dominica.
3.1.2. Abundance and Relative Abundance of Maize Grain Insect Pests
S. zeamais had the highest relative abundance (24.87%) in the Oda Bultum district and the highest abundance of (22.14%) in the Mieso district. Its relative abundance was the lowest in the Tulo district (19.79%) and the lowest abundance in the Gemechis district (10.03%) (Table 2). S. cerealella had the highest relative abundance (23.70%) in the Oda Bultum district and the highest abundance of (19.77%) in the Mieso district. Its relative abundance was the lowest in the Tulo district (17.76%) and the lowest abundance in the Gemechis district (8.03%). S. oryzae had the highest relative abundance (18.60%) in the Anchar district and the highest abundance of (17.27%) in the Mieso district. Its relative abundance was the lowest in the Tulo district (15.11%) and the lowest abundance in the Gemechis district (7.68%). T. castaneum had the highest relative abundance (15.25%) in the Tulo district and the highest abundance of 12.33% in the same district. Its relative abundance was the lowest in the Oda Bultum district (7.07%) and the lowest abundance in the same district (3.72%). T. confusum had the highest relative abundance (16.51%) in the Gemechis district and the highest abundance of 14.66% in the Mieso district. Its relative abundance was the lowest in Oda Bultum district (9.39%) and the lowest abundance in the same district (4.94%). O. surinamensis had the highest relative abundance (6.37%) in the Mieso district and the highest abundance of (6.47%) in the same district. Its relative abundance was the lowest in the Oda Bultum district (1.73%) and the lowest abundance in the same district (0.91%). C. ferrugineus had the highest relative abundance (10.78%) in the Tulo district and the highest abundance of 8.72% in the same district. Its relative abundance was the lowest in the Gemechis district (5.39%) and the lowest abundance in the same district (2.29%). R. dominica had the highest relative abundance (5.22%) in the Tulo district and the highest equal abundance of (4.22%) in both the Mieso and Tulo districts. Its relative abundance was the lowest in the Gemechis district (2.47%) and the lowest abundance in the same district (1.05%) (Table 2).
| Storage insect pests | Districts | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oda Bultum | Anchar | Mieso | Tulo | Gemechis | |||||||||||
| Mean | Relative abundance | Abundance | Mean | Relative abundance | Abundance | Mean | Relative abundance | Abundance | Mean | Relative abundance | Abundance | Mean | Relative abundance | Abundance | |
| Sitophilus zeamais | 156.99 | 24.87 | 13.08 | 233.00 | 24.40 | 19.42 | 265.63 | 21.79 | 22.14 | 191.99 | 19.79 | 16.00 | 120.3 | 23.59 | 10.03 |
| S. cerealella | 149.6 | 23.70 | 12.47 | 200.60 | 21.00 | 16.72 | 237.20 | 19.46 | 19.77 | 172.30 | 17.76 | 14.36 | 96.32 | 18.89 | 8.03 |
| S. oryzae | 115.6 | 18.31 | 9.63 | 177.67 | 18.60 | 14.81 | 207.20 | 17.00 | 17.27 | 146.60 | 15.11 | 12.22 | 92.20 | 18.08 | 7.68 |
| T. castaneum | 44.6 | 7.07 | 3.72 | 77.50 | 8.11 | 6.46 | 110.50 | 9.07 | 9.21 | 147.90 | 15.25 | 12.33 | 64.2 | 12.59 | 5.35 |
| T. confusum | 59.3 | 9.39 | 4.94 | 105.6 | 11.06 | 8.80 | 175.90 | 14.43 | 14.66 | 137.90 | 14.22 | 11.49 | 84.20 | 16.51 | 7.02 |
| Oryzaephilus surinamenis | 10.9 | 1.73 | 0.91 | 50.20 | 5.26 | 4.18 | 77.60 | 6.37 | 6.47 | 18.20 | 1.88 | 1.52 | 12.60 | 2.47 | 1.05 |
| C. ferrugineus | 65.20 | 10.33 | 5.43 | 71.90 | 7.53 | 5.99 | 94.20 | 7.73 | 7.85 | 104.60 | 10.78 | 8.72 | 27.50 | 5.39 | 2.29 |
| R. dominica | 29 | 4.59 | 2.42 | 38.6 | 4.04 | 3.22 | 50.60 | 4.15 | 4.22 | 50.6 | 5.22 | 4.22 | 12.60 | 2.47 | 1.05 |
| Total | 631.19 | — | — | 955.07 | — | — | 1,218.83 | — | — | 970.09 | — | — | 509.92 | — | — |
3.1.3. Occurrence of Sorghum Storage Insect Pests
The survey results revealed that storage insect pests were present in sorghum-growing districts within the surveyed zone, but their distribution varied depending on the storage type (Table 3). In this experiment, sorghum sourced from sacks, ground pits, and grains purchased from local markets were infested with five insect species: S. zeamais, S. cerealella, T. castaneum, T. confusum, and R. dominica. Significant differences (p < 0.05) were observed among storage types ground pit, sack, and grain purchased from the market. This finding is consistent with Mendesil et al. [40], suggesting that regardless of the storage structure type, stored sorghum is susceptible to insect pest infestations in all traditional farm storages. This susceptibility may be attributed to poor storage conditions and inadequate storage hygiene in most traditional farm stores. Farmers in the study areas predominantly utilized ground pit storage, which facilitates the development of temperature and moisture conducive to grain respiration and insect pest activity. These factors contribute to significant postharvest losses in both the quantity and quality of sorghum.
| Districts | Types of grain storage | Sitophilus zeamais | S. cerealella | T. castaneum | T. confusum | R. dominica |
|---|---|---|---|---|---|---|
| Coleoptera | Lepidoptera | Coleoptera | Coleoptera | Coleoptera | ||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| Mieso | Sack | 113.30 ± 8.50a | 99.33 ± 10.00ab | 71.00 ± 5.50a | 75.60 ± 5.10ab | 55.60 ± 14.00b |
| Market | 93.00 ± 6.00b | 79.67 ± 17.60b | 99.60 ± 20.00a | 53.60 ± 10.90b | 33.60 ± 7.00c | |
| Ground pit | 72.00 ± 10.80c | 110.33 ± 14.50a | 92.00 ± 16.00a | 98.00 ± 19.00a | 78.00 ± 1.70a | |
| p Value | 0.0034 | 0.0542 | 0.1334 | 0.0170 | 0.0030 | |
| CV (%) | 9.30 | 14.90 | 17.30 | 17.20 | 16.30 | |
| LSD | 17.30 | 28.80 | 30.30 | 26.00 | 18.20 | |
| Anchar | Sack | 106.60 ± 16.00a | 86.67 ± 22.70a | 63.30 ± 5.70a | 63.30 ± 5.70a | 21.60 ± 12.50a |
| Market | 81.30 ± 11.00a | 61.33 ± 18.70a | 87.60 ± 21.50a | 71.30 ± 23.20a | 39.60 ± 17.40a | |
| Ground pit | 91.30 ± 11.50a | 95.33 ± 6.40a | 83.60 ± 14.30a | 76.00 ± 22.60a | 52.60 ± 17.50a | |
| p Value | 0.1348 | 0.1199 | 0.1946 | 0.7247 | 0.1361 | |
| CV (%) | 14.00 | 21.40 | 19.50 | 27.10 | 42.10 | |
| LSD | 26.10 | 34.80 | 30.60 | 38.00 | 32.00 | |
| Tulo | Sack | 73.60 ± 15.90a | 76.30 ± 25.10ab | 39.30 ± 12.60b | 39.30 ± 12.60a | 26.30 ± 14.00a |
| Market | 81.00 ± 17.40a | 51.60 ± 17.60b | 62.60 ± 17.60ab | 65.00 ± 26.10a | 42.60 ± 15.30a | |
| Ground pit | 103.30 ± 14.30a | 101.00 ± 4.30a | 85.30 ± 25.10a | 81.00 ± 37.90a | 33.30 ± 11.90a | |
| p Value | 0.1239 | 0.0447 | 0.0541 | 0.2538 | 0.4061 | |
| CV (%) | 18.40 | 23.30 | 30.50 | 44.60 | 40.50 | |
| LSD | 31.80 | 35.80 | 38.30 | 55.10 | 27.60 | |
| Gemechis | Sack | 57.60 ± 6.00b | 45.00 ± 14.10a | 61.30 ± 34.20a | 40.00 ± 16.50a | 19.30 ± 9.20a |
| Market | 70.60 ± 4.90a | 39.60 ± 21.70a | 33.00 ± 9.00a | 42.30 ± 17.80a | 26.30 ± 12.60a | |
| Ground pit | 62.60 ± 5.00ab | 65.00 ± 12.70a | 52.00 ± 19.10a | 50.60 ± 20.50a | 32.00 ± 15.30a | |
| p Value | 0.0516 | 0.2053 | 0.3760 | 0.7769 | 0.4662 | |
| CV (%) | 8.30 | 33.20 | 47.60 | 41.20 | 48.40 | |
| LSD | 10.60 | 33.30 | 46.40 | 36.70 | 25.30 | |
| Oda Bultum | Sack | 72.30 ± 7.50a | 54.30 ± 10.00a | 61.60 ± 16.00a | 61.60 ± 16.00ab | 36.60 ± 8.30a |
| Market | 67.30 ± 7.60a | 25.30 ± 11.70b | 61.30 ± 35.00a | 75.00 ± 17.30a | 28.30 ± 5.50ab | |
| Ground pit | 60.00 ± 5.00a | 48.30 ± 18.30ab | 42.60 ± 3.20a | 38.60 ± 5.50b | 19.30 ± 5.10b | |
| p Value | 0.1578 | 0.0538 | 0.5614 | 0.0546 | 0.0454 | |
| CV (%) | 10.20 | 32.10 | 40.20 | 23.80 | 22.70 | |
| LSD | 13.60 | 33.30 | 44.60 | 28.00 | 12.90 | |
- Means followed by different letters within columns are significantly different, p < 0.05 using least significant difference (LSD).
3.1.4. Abundance and Relative Abundance of Insect Pest Species in Sorghum Grain
S. zeamais exhibited the highest relative abundance (27.37%) in the Gemechis district and the highest abundance (23.27) in the Anchar district. Its lowest relative abundance was observed in the Mieso district (22.73%), with the lowest abundance also recorded in the Gemechis district (15.90) (Table 4). S. cerealella had the highest relative abundance (23.81%) in the Tulo district and the highest abundance (24.11) in the Mieso district. Conversely, its lowest relative abundance was noted in the Oda Bultum district (17.00%), with the lowest abundance occurring in the same Oda Bultum district (10.66). T. castaneum displayed the highest relative abundance (22.00%) in the Oda Bultum district and the highest abundance (21.88) in the Mieso district. Conversely, its lowest relative abundance was observed in the Tulo district (19.47%), with the lowest abundance recorded in the Gemechis district (12.19). T. confusum exhibited the highest relative abundance (23.29%) in the Oda Bultum district and the highest abundance (18.93) in the Mieso district. Its lowest relative abundance was noted in the same Mieso district (18.55%), with the lowest abundance occurring in the Gemechis district (11.08). R. dominica displayed the highest relative abundance (13.65%) in the Mieso district and the highest abundance (13.93) in the same Mieso district. Conversely, its lowest relative abundance was observed in the Anchar district (10.52%), with the lowest abundance recorded in the Gemechis district (6.47) (Table 4). Most of the species identified and recorded in both maize and sorghum grains in this study belonged to the order Coleoptera (beetles). This finding corroborates with Upadhyay and Ahmad [41], which highlights that stored cereal pests, particularly insects of the Coleopteran order, are highly diversified and destructive, with beetles posing a more significant threat than moths among postharvest pests.
| Storage insect pests | Districts | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oda Bultum | Anchar | Mieso | Tulo | Gemechis | |||||||||||
| Mean | Relative abundance | Abundance | Mean | Relative abundance | Abundance | Mean | Relative abundance | Abundance | Mean | Relative abundance | Abundance | Mean | Relative abundance | Abundance | |
| Sitophilus zeamais | 199.6 | 26.53 | 16.63 | 279.20 | 25.82 | 23.27 | 278.30 | 22.73 | 23.19 | 257.90 | 26.82 | 21.49 | 190.80 | 27.37 | 15.90 |
| S. cerealella | 127.9 | 17.00 | 10.66 | 243.33 | 22.50 | 20.28 | 289.33 | 23.63 | 24.11 | 228.90 | 23.81 | 19.08 | 149.60 | 21.46 | 12.47 |
| T. castaneum | 165.5 | 22.00 | 13.79 | 234.50 | 21.68 | 19.54 | 262.60 | 21.44 | 21.88 | 187.20 | 19.47 | 15.60 | 146.30 | 20.98 | 12.19 |
| T. confusum | 175.20 | 23.29 | 14.60 | 210.60 | 19.47 | 17.55 | 227.20 | 18.55 | 18.93 | 185.30 | 19.27 | 15.44 | 132.90 | 19.06 | 11.08 |
| R. dominica | 84.20 | 11.19 | 7.02 | 113.80 | 10.52 | 9.48 | 167.20 | 13.65 | 13.93 | 102.20 | 10.63 | 8.52 | 77.60 | 11.13 | 6.47 |
| Total | 752.40 | — | — | 1,081.43 | — | — | 1,224.63 | — | — | 961.50 | — | — | 697.20 | — | — |
3.2. Comparative Extent Damage, Weight Loss, and Germination Percentage of Maize and Sorghum
The mean levels of major insect pest infestations and associated percentages of grain damage, weight loss, and germination loss were recorded across all traditional storage types for both maize and sorghum. However, germination percentage, weight loss, and grain damage varied depending on the storage type (sack, market, hanging (maize cob), and ground pit). During seed germination testing, sorghum seeds initiated germination within 2 days, whereas maize seeds began germinating within 3 days. Notably, maize stored on hanging (maize cob) exhibited a high germination percentage. The Gemechis district recorded the highest germination from maize cobs (77.08%), while the lowest germination percentage was observed from sacks in the Mieso district (23.92%). The mean germination percentage of maize cob seeds was consistently high across all assessed districts compared to sack and market grain (Table 5).
| Districts | Types of grain storage | Grain damage (%) | Weight loss (%) | Germination percentage | |||
|---|---|---|---|---|---|---|---|
| Maize | Sorghum | Maize | Sorghum | Maize | Sorghum | ||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| Tulo | Sack | 47.00 ± 20.00a | 44.50 ± 23.00a | 15.60 ± 2.90a | 10.20 ± 3.40b | 37.08 ± 17.20b | 48.67 ± 16.60ab |
| Market | 40.00 ± 28.70ab | 27.25 ± 17.80a | 6.09 ± 2.40b | 5.10 ± 2.60c | 55.92 ± 7.40a | 69.33 ± 8.70a | |
| Hanging | 30.75 ± 22.40b | — | 4.22 ± 2.50b | — | 68.75 ± 7.90a | — | |
| Ground pit | — | 58.50 ± 13.10a | — | 20.97 ± 19.20a | 26.99 ± 11.42b | ||
| p Value | 0.0325 | 0.1745 | 0.0537 | 0.0029 | 0.0003 | 0.0360 | |
| CV (%) | 44.92 | 47.60 | 96.20 | 29.40 | 18.02 | 28.40 | |
| LSD | 15.75 | 32.72 | 8.10 | 4.80 | 14.57 | 23.50 | |
| Gemechis | Sack | 23.50 ± 13.20a | 52.50 ± 4.40a | 6.28 ± 1.20a | 11.0 ± 5.10ab | 54.16 ± 15.9b | 34.40 ± 12.80b |
| Market | 17.00 ± 9.30a | 55.75 ± 9.00a | 4.46 ± 1.10a | 17.03 ± 4.80a | 64.58 ± 7.90ab | 22.90 ± 7.20b | |
| Hanging | 12.75 ± 8.00a | — | 1.62 ± 1.30b | — | 77.08 ± 7.90a | — | |
| Ground pit | — | 37.00 ± 6.60b | — | 8.03 ± 5.80b | — | 52.2 ± 11.20a | |
| p Value | 0.3807 | 0.0009 | 0.0015 | 0.1022 | 0.0532 | 0.0112 | |
| CV (%) | 58.78 | 13.44 | 29.90 | 44.10 | 17.28 | 29.40 | |
| LSD | 16.69 | 11.13 | 1.97 | 8.40 | 18.04 | 17.15 | |
| Oda Bultum | Sack | 23.75 ± 8.09a | 28.50 ± 4.7c | 6.96 ± 1.20a | 5.085 ± 2.30b | 31.25 ± 17.4b | 51.389 ± 10.30a |
| Market | 14.75 ± 8.22ab | 42.750 ± 7.6b | 4.21 ± 2.53ab | 12.964 ± 5.70a | 52.78 ± 14.8ab | 36.472 ± 7.60ab | |
| Hanging | 7.75 ± 6.02b | — | 2.78 ± 2.21b | — | 68.13 ± 9.30a | — | |
| Ground pit | — | 54.500 ± 6.8a | — | 17.682 ± 4.70a | — | 22.778 ± 9.70b | |
| p Value | 0.0430 | 0.0011 | 0.0506 | 0.0100 | 0.0165 | 0.0063 | |
| CV (%) | 48.74 | 15.51 | 44.34 | 37.70 | 28.20 | 25.30 | |
| LSD | 12.02 | 10.40 | 3.30 | 7.10 | 22.80 | 14.90 | |
| Anchar | Sack | 43.25 ± 27.20a | 65.75 ± 16.50a | 11.80 ± 11.50a | 24.98 ± 3.10a | 43.75 ± 10.40b | 31.25 ± 9.20b |
| Market | 41.25 ± 26.90a | 45.50 ± 15.70a | 10.70 ± 9.30a | 6.00 ± 1.70b | 41.60 ± 11.70b | 50.75 ± 5.80a | |
| Hanging | 14.75 ± 10.70a | — | 2.50 ± 2.70a | — | 63.03 ± 19.40a | — | |
| Ground pit | — | 55.50 ± 16.80a | — | 12.93 ± 3.50a | — | 35.83 ± 9.90b | |
| p Value | 0.2023 | 0.2688 | 0.1494 | 0.0260 | 0.0481 | 0.0249 | |
| CV (%) | 69.40 | 29.49 | 85.70 | 30.50 | 22.30 | 21.70 | |
| LSD | 36.70 | 26.20 | 13.90 | 4.60 | 17.60 | 13.60 | |
| Mieso | Sack | 58.00 ± 18.80a | 60.25 ± 11.80a | 16.50 ± 2.90a | 23.80 ± 6.30a | 23.92 ± 8.60b | 22.90 ± 10.90a |
| Market | 28.00 ± 4.80b | 40.5 ± 9.10b | 9.70 ± 5.30b | 9.80 ± 3.10b | 43.75 ± 17.10ab | 30.6 ± 13.50a | |
| Hanging | 19.50 ± 8.20b | — | 4.60 ± 3.90b | — | 62.50 ± 10.70a | — | |
| Ground pit | — | 28.5 ± 12.30b | — | 5.62 ± 3.80b | — | 35.8 ± 9.80a | |
| p Value | 0.0038 | 0.0095 | 0.0096 | 0.0009 | 0.0210 | 0.3279 | |
| CV (%) | 34.68 | 26.06 | 40.70 | 35.57 | 36.30 | 38.78 | |
| LSD | 19.50 | 17.90 | 6.70 | 7.47 | 25.20 | 18.49 | |
- Means followed by the same letters within columns are not significantly different at the p > 0.05 level of probability by LSD (0.05) comparison. NB “—” revealed that the storage type of ground pit is not available for maize and hanging was not available for sorghum.
Significant differences were observed in the germination percentage of maize seeds among different storage types in the study areas. Maize damage and weight loss were also documented for these storage types, with less damage and weight loss noted for maize stored on hanging (maize cob) across districts. Conversely, relatively high maize damage and weight loss were recorded for sack storage types. The Mieso district exhibited the highest damage and weight loss rates for maize grain (58.0% and 16.5%, respectively) (Table 5).
In terms of sorghum, the maximum grain damage and weight loss were recorded from Anchar sack storage types (65.75% and 24.98%, respectively) (Table 5). The germination percentages of sorghum seeds varied across districts, with the lowest recorded from the Oda Bultum district ground pit (22.77%). Sorghum grains collected from different districts exhibited varying levels of grain damage, with the highest damage observed in the Mieso district (60.25%) for sacks.
Studies by Suleiman and Rugumamu [42] revealed that weight losses of up to 13.12% in threshed sorghum and 8.34% in unthreshed sorghum are due to insect pests. Similarly, Waktole and Amsalu [43] reported that weight losses of 41%–80% in maize and sorghum stored grains are caused by significant S. zeamais infestation under traditional storage.
These findings underscore the critical importance of appropriate storage methods in preserving grain viability and germination capacity. Factors such as storage type, insect damage, and fungal diseases significantly contribute to grain damage, weight loss, and reduced germination capacity. Higher grain damage in grains stored in traditional storage has been consistently reported in various studies [44]. The current study’s findings indicate that sorghum germination percentage or capacity was generally lower than that of maize, likely due to higher infestation and grain damage observed in sorghum. This observation aligns with findings by Lemessa et al. [22], which suggested that insect grain damage reduces grain germination percentage in seeds stored using traditional methods.
3.3. Distribution and Incidence of Fungal Species in Stored Maize and Sorghum Grains
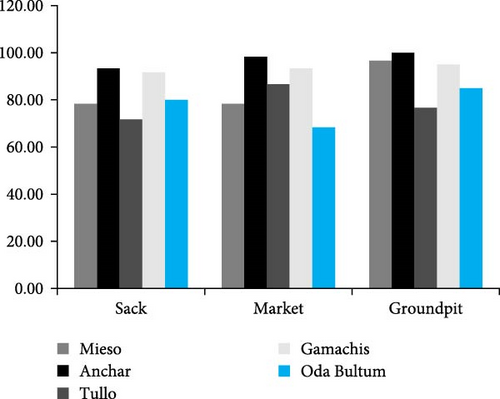
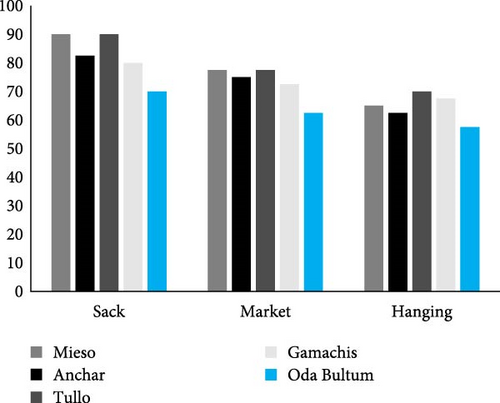
The incidence of fungal diseases was notably high, reaching 100%, in sorghum grains stored in ground pits collected from the Anchar district, followed closely by sorghum grains obtained from the market in the same district, recording 98.33% (Figure 3). Conversely, for maize grains, the highest occurrence of fungal diseases was observed in the sack storage type of the Tulo and Mieso districts, both at 90%, with the sack storage type of the Anchar district following suit (Figure 4). These findings suggest the need for continued monitoring of sorghum stored in pits to mitigate postharvest diseases.
3.3.1. Total Colony-Forming Units/Plate of Fungus Species on Blotter and Agar Plates
(1) Fungal Colony on Maize Grains. In this laboratory study, a significant difference (p < 0.05) in total fungal colonies was noted among samples collected from different storage areas using both the blotter and agar plate methods. On the agar plate method, the highest fungal colony count (37.125) was observed in samples collected from the Tullo district stored in sacks, followed by 35.87 fungal colonies in maize grains purchased from the market in the same district. Conversely, the lowest fungal colony count (26.12) was recorded in samples collected from Anchar, where the maize cobs were hung (Table 6). In the blotter test, the highest fungal colony count (18.87) per plate was found in maize sacks from Oda Bultum, while the lowest (4.5) was observed in maize cobs from Anchar (Table 6). Overall, significant differences were observed among samples collected from different storage types across districts using both agar plate and blotter methods, with a few exceptions. Notably, there were more fungal colonies detected on the agar plate method compared to the blotter method, possibly due to the presence of nutrients (PDA) facilitating the germination and growth of pathogens on maize seeds. Maize cobs consistently exhibited the lowest fungal colony counts across the districts compared to sacks and purchased grains.
| District | Types of grain storage | Blotter test method | Agar plate method |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Anchar | Market | 7.00 ± 3.18cde | 32.37 ± 1.94abc |
| Hanging | 4.50 ± 1.06e | 26.12 ± 1.23c | |
| Sack | 8.12 ± 1.59bcde | 33.00 ± 0.70abc | |
| Gemechis | Market | 5.50 ± 0.70e | 28.83 ± 5.42bc |
| Hanging | 5.87 ± 1.59e | 31.25 ± 6.71abc | |
| Sack | 6.62 ± 1.59de | 28.12 ± 1.23bc | |
| Miesso | Market | 11.87 ± 4.06bcd | 34.62 ± 4.77ab |
| Hanging | 8.50 ± 3.88bcde | 32.75 ± 5.65abc | |
| Sack | 8.62 ± 2.65bcde | 2.62 ± 2.29abc | |
| Tullo | Market | 9.00 ± 3.18bcde | 35.87 ± 5.48ab |
| Hanging | 6.00 ± 1.76e | 28.12 ± 0.53bc | |
| Sack | 7.50 ± 3.53cde | 37.12 ± 3.00a | |
| Oda Bultum | Market | 12.62 ± 1.94bc | 30.12 ± 1.59abc |
| Hanging | 13.75 ± 0.35ab | 32.37 ± 2.29abc | |
| Sack | 18.87 ± 4.41a | 34.37 ± 3.35ab | |
| p Value | 0.0051 | 0.0903 | |
|
|
|
|
- Means followed by different letters within columns are significantly different, p < 0.05 using LSD.
(2) Fungal Colony on Sorghum Grains. In this study, total fungal colonies among the samples collected from different storage areas of sorghum grains were assessed using both the blotter and agar plate methods. On the agar plate method, the highest fungal colony count (44.37) was observed in samples collected from the Mieso district stored in ground pits, followed by 42.25 fungal colonies recorded from the same district in sorghum grain sacks. Conversely, the lowest fungal colony count (32.12) was recorded in samples collected from Tulo, where the storage type was sacks. In the blotter test, the highest fungal colony count (19.25) per plate was found in sorghum ground pits from Mieso, while the lowest (10.37) was observed in sorghum grains purchased from the market in Anchar. Consistently, fungal colonies were more abundant on the agar plate method compared to the blotter method, likely due to the presence of nutrients (PDA) facilitating the germination and growth of pathogens on sorghum seeds (Table 7).
| District | Types of grain storage | Blotter test method | Agar plate method |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Anchar | Ground pit | 13.25 ± 1.06a | 37.75 ± 4.94abc |
| Market | 10.37 ± 7.60a | 38.37 ± 4.41abc | |
| Sack | 12.75 ± 4.24a | 37.87 ± 5.12abc | |
| Gemechis | Ground pit | 14.62 ± 5.48a | 34.87 ± 4.41bc |
| Market | 12.00 ± 1.06a | 35.91 ± 2.23abc | |
| Sack | 12.12 ± 0.88a | 34.00 ± 1.41bc | |
| Mieso | Ground pit | 19.25 ± 7.42a | 44.37 ± 3.35a |
| Market | 16.75 ± 8.13a | 40.75 ± 8.83abc | |
| Sack | 15.62 ± 9.72a | 42.25 ± 6.71ab | |
| Tulo | Ground pit | 13.12 ± 2.65a | 36.00 ± 0.00abc |
| Market | 13.00 ± 7.42a | 33.37 ± 0.88c | |
| Sack | 12.25 ± 8.48a | 32.12 ± 0.88c | |
| Oda Bultum | Ground pit | 15.25 ± 7.07a | 38.12 ± 3.71abc |
| Market | 13.50 ± 2.47a | 32.87 ± 2.29c | |
| Sack | 16.37 ± 1.59a | 34.62 ± 0.17bc | |
| p Value | 0.98 | 0.22 | |
|
|
|
|
- Means followed by different letters within columns are significantly different, p < 0.05 using LSD, where Ns = nonsignificant.
Fungal species were distributed across the surveyed areas. F. oxysporum was found in all samples collected from the Oda Bultum, Tulo, and Anchar districts but less so in the Mieso district and moderately in the Gemechis district (Table 8). A. flavus and A. parasiticus were present in all samples collected from Oda Bultum but were less prevalent in the Gemechis district. A. niger was found in all samples collected from Tulo but less so in the Mieso district. A. alternata was more common in samples from Oda Bultum but less prevalent in the Mieso district. Rhizopus species were more prevalent in samples from the Oda Bultum district but less so in those from the Anchar district. F. verticillioides was more widespread in samples from the Anchar district but less so in those from the Mieso district. D. maydis was more prevalent in samples from the Tullo district, while it was absent in grain samples from the Gemechis and Anchar districts (Table 8). These variations may be attributed to differences in storage conditions and chemical treatments applied to the grain.
| Districts | F. oxysporum | A. flavus | A. parasaticus | A. niger | A. alternata | Rhizopus spp. | F. verticilliodes | D. maydis | Total |
|---|---|---|---|---|---|---|---|---|---|
| Oda Bultum | 12 | 12 | 12 | 11 | 11 | 10 | 8 | 1 | 77 |
| Tullo | 12 | 11 | 6 | 12 | 7 | 8 | 6 | 8 | 70 |
| Gemmechis | 9 | 5 | 7 | 4 | 4 | 8 | 9 | 0 | 40 |
| Miesso | 5 | 6 | 9 | 3 | 3 | 7 | 4 | 8 | 45 |
| Ancher | 12 | 11 | 10 | 5 | 8 | 3 | 9 | 0 | 58 |
| Total | 50 | 45 | 44 | 35 | 33 | 36 | 36 | 17 | 296 |
The highest absolute frequency and relative frequency of F. oxysporum (14.53%), followed by A. flavus (13.08%), on maize seeds were recorded in the agar plate test. The lowest absolute frequency and relative frequency of D. maydis (4.94%) were also recorded (Table 9). This finding is similar to that of Krnjaja et al. [45], who stated that various fungal genera such as Alternaria, Aspergillus, Fusarium, Penicillium, Rhizopus, and others were identified in stored maize grains with varying levels of occurrence.
| Fungal species | No. of samples | Absolute frequency | Relative frequency |
|---|---|---|---|
| F. oxysporum | 50 | 14.53 | 14.53 |
| A. flavus | 45 | 13.08 | 13.08 |
| A. parasaticus | 44 | 12.79 | 12.79 |
| A. niger | 35 | 10.17 | 10.17 |
| A. alternata | 33 | 9.59 | 9.59 |
| Rhizopus spp. | 36 | 10.47 | 10.47 |
| F. verticilliodes | 36 | 10.47 | 10.47 |
| D. maydis | 17 | 4.94 | 4.94 |
| Total | 296 | 86.04 | 86.04 |
Fungal species were distributed in the surveyed areas of the collected sorghum grains. Fusarium spp. were distributed in more samples collected from Gemechis and Mieso districts and medium in other districts (Table 10). A. flavus and A. parasiticus were distributed in all samples collected from Anchar but were more distributed in other districts. A. niger, A. alternata, and Rhizopus spp. were distributed in more sample collection districts. D. maydis was less distributed across districts and not found in the samples from the Anchar and Oda Bultum districts, while Bipolaris spp. was distributed to a medium extent across districts.
| District | Fusarium spp. | A. flavus | A. parasaticus | A. niger | A. alternata | Rhizopus spp. | D. maydis | Bipolaris spp. | Total |
|---|---|---|---|---|---|---|---|---|---|
| Oda Bultum | 9 | 10 | 11 | 8 | 9 | 8 | 0 | 9 | 64 |
| Tullo | 8 | 9 | 12 | 7 | 11 | 9 | 6 | 4 | 66 |
| Gemmechis | 11 | 8 | 9 | 7 | 12 | 6 | 1 | 7 | 61 |
| Miesso | 11 | 7 | 11 | 10 | 7 | 3 | 2 | 5 | 56 |
| Anchar | 9 | 12 | 12 | 5 | 8 | 9 | 0 | 6 | 61 |
| Total | 48 | 46 | 55 | 37 | 47 | 35 | 9 | 31 | 308 |
The highest absolute frequency and relative frequency of A. parasiticus (17.85%) and 16.22%, respectively, followed by F. oxysporum (15.58%) and 14.15%, respectively, on sorghum seeds were recorded on the agar plate test (Table 11). The lowest absolute frequency and relative frequency of D. maydis (2.92% and 2.65%, respectively) were also recorded. This finding is similar to that of Sreenivasa et al. [46], who stated that the most dominant fungal genera were Fusarium and Aspergillus spp., with a high frequency, while other fungal genera such as Alternaria and Penicillium spp. were also isolated with different levels of frequency and relative percentage from sorghum grains.
| Fungal species | No. of samples | Absolute frequency | Relative frequency |
|---|---|---|---|
| F. oxysporum | 48 | 15.58 | 14.15 |
| A. flavus | 46 | 14.93 | 13.56 |
| A. parasaticus | 55 | 17.85 | 16.22 |
| A. niger | 37 | 12.01 | 10.91 |
| A. alternata | 47 | 15.25 | 13.86 |
| Rhizopus spp. | 35 | 11.36 | 10.32 |
| D. maydis | 9 | 2.92 | 2.65 |
| Bipolaris spp. | 31 | 10.064 | 9.14 |
| Total | 308 | 99.96 | 90.81 |
A total of eight species of fungi, including Fusarium species, A. flavus, A. parasiticus, A. niger, A. alternata, Rhizopus species, Bipolaris species, and D. maydis, were identified in the laboratory using the agar plate method on both maize and sorghum grains. However, their incidence levels differed based on storage types and the conditions and agroecologies of the collected sample districts. Among these isolated fungal species, Fusarium species, A. flavus, and A. parasiticus were commonly distributed in maize and sorghum grain samples collected from various districts (Table 12), indicating their presence in most or all samples collected from different storage types in the districts. This observation is supported by Ayalew [47] and Abe et al. [48], who confirmed that the major genera of fungi commonly encountered on maize grain in tropical regions are Fusarium, Aspergillus, and Penicillium spp. The results indicated that the genus Fusarium was the most frequently found, followed by the genus Aspergillus. This finding aligns with Camila et al. [48], who reported Fusarium as the most frequently found genus in maize grains. However, Bipolaris species were not detected in collected maize grain samples but was present in collected sorghum grain samples. Meanwhile, D. maydis showed a lower distribution across the districts of collected samples (Table 12). This finding is consistent with Weledesemayat et al. [49], who found contamination rates of approximately 56.7%, 16.7%, and 23.3% with A. flavus, A. niger, and A. parasiticus, respectively, in sorghum samples. Additionally, the results of this study are in agreement with those of Tegegne et al. [50], who detected various grain mold fungi in Ethiopia, including Fusarium, Penicillium, Aspergillus, and Nigropora species, in maize samples collected from various locations. This finding is also supported by Tsedaley [51], who identified Aspergillus, Fusarium, Penicillium, and Cladosporium as the predominant fungal genera associated with stored grain.
| District | Total number of samples | Fungal species | No of sample containing fungal spp. in maize grains | No of sample contain the spp. | % of each spp. occurred | No. of sample contain fungal spp. in sorghum grains | No of sample contain the spp. | % of each spp. occurred | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hanging | Sacks | Market | ||||||||||
| Ground pit | Sack | Market | ||||||||||
| Oda Bultum | 12 | Fusarium spp. | 4 | 4 | 4 | 12 | 100 | 4 | 3 | 2 | 9 | 75.00 |
| A. flavus | 4 | 4 | 4 | 12 | 100 | 4 | 4 | 2 | 10 | 83.33 | ||
| A. parasaticus | 4 | 4 | 4 | 12 | 100 | 4 | 4 | 3 | 11 | 91.67 | ||
| A. niger | 4 | 4 | 3 | 11 | 91.67 | 3 | 3 | 2 | 8 | 66.67 | ||
| A. alternata | 3 | 4 | 4 | 11 | 91.67 | 3 | 4 | 4 | 9 | 75.00 | ||
| Rhizopus spp. | 3 | 4 | 3 | 10 | 83.33 | 3 | 4 | 3 | 8 | 66.67 | ||
| Cladosporium | 2 | 4 | 2 | 8 | 66.67 | 0 | 0 | 0 | 0 | 0.00 | ||
| D. maydis | 0 | 1 | 0 | 1 | 8.33 | 0 | 0 | 0 | 0 | 0.00 | ||
| Bipolaris spp. | 0 | 0 | 0 | 0 | 0 | 4 | 3 | 2 | 9 | 75.00 | ||
| Tullo | 12 | Fusarium spp. | 4 | 4 | 4 | 12 | 100 | 3 | 3 | 2 | 8 | 66.67 |
| A. flavus | 3 | 4 | 4 | 11 | 91.67 | 3 | 3 | 3 | 9 | 75.00 | ||
| A. parasaticus | 1 | 4 | 1 | 6 | 50 | 4 | 4 | 4 | 12 | 100.00 | ||
| A. niger | 4 | 4 | 4 | 12 | 100 | 4 | 2 | 1 | 7 | 58.33 | ||
| A. alternata | 1 | 4 | 2 | 7 | 58.33 | 4 | 4 | 4 | 11 | 91.67 | ||
| Rhizopus spp. | 3 | 3 | 2 | 8 | 66.67 | 3 | 3 | 3 | 9 | 75.00 | ||
| Cladosporium | 2 | 3 | 1 | 6 | 50 | 0 | 0 | 0 | 0 | 0.00 | ||
| D. maydis | 2 | 3 | 3 | 8 | 66.67 | 2 | 3 | 1 | 6 | 50.00 | ||
| Bipolaris spp. | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 4 | 33.33 | ||
| Gemmechis | 12 | Fusarium spp. | 3 | 4 | 1 | 9 | 75 | 4 | 4 | 3 | 11 | 91.67 |
| A. flavus | 1 | 3 | 1 | 5 | 41.67 | 3 | 3 | 2 | 8 | 66.67 | ||
| A. parasaticus | 2 | 4 | 1 | 7 | 58.33 | 4 | 3 | 1 | 9 | 75.00 | ||
| A. niger | 1 | 2 | 1 | 4 | 33.33 | 3 | 2 | 2 | 7 | 58.33 | ||
| A. alternata | 1 | 1 | 2 | 4 | 33.33 | 4 | 4 | 4 | 12 | 100.00 | ||
| Rhizopus spp. | 2 | 3 | 3 | 8 | 66.67 | 2 | 3 | 1 | 6 | 50.00 | ||
| Cladosporium | 2 | 3 | 4 | 9 | 75 | 0 | 0 | 0 | 0 | 0.00 | ||
| D. maydis | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 8.33 | ||
| Bipolaris_spp. | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 3 | 7 | 58.33 | ||
| Mieso | 12 | Fusarium spp. | 2 | 2 | 0 | 5 | 41.67 | 4 | 4 | 3 | 11 | 91.67 |
| A. flavus | 1 | 3 | 0 | 6 | 50 | 1 | 3 | 3 | 7 | 58.33 | ||
| A. parasaticus | 2 | 4 | 3 | 9 | 75 | 4 | 3 | 4 | 11 | 91.67 | ||
| A. niger | 1 | 2 | 0 | 3 | 25 | 4 | 4 | 2 | 10 | 83.33 | ||
| A. alternata | 1 | 2 | 0 | 3 | 25 | 2 | 2 | 3 | 7 | 58.33 | ||
| Rhizopus spp. | 3 | 3 | 1 | 7 | 58.33 | 2 | 1 | 0 | 3 | 25.00 | ||
| Cladosporium | 0 | 2 | 2 | 4 | 33.33 | 0 | 0 | 0 | 0 | 0.00 | ||
| D. maydis | 2 | 3 | 3 | 8 | 66.67 | 0 | 2 | 0 | 2 | 16.67 | ||
| Bipolaris spp. | 0 | 0 | 0 | 0 | 0 | 4 | 2 | 0 | 5 | 41.67 | ||
| Anchar | 12 | Fusarium spp. | 4 | 4 | 4 | 12 | 100 | 3 | 3 | 3 | 9 | 75.00 |
| A. flavus | 4 | 4 | 3 | 11 | 91.67 | 4 | 4 | 4 | 12 | 100.00 | ||
| A. parasaticus | 2 | 4 | 4 | 10 | 83.33 | 4 | 4 | 4 | 12 | 100.00 | ||
| A. niger | 0 | 3 | 2 | 5 | 41.67 | 0 | 3 | 2 | 5 | 41.67 | ||
| A. alternata | 2 | 3 | 3 | 8 | 66.67 | 2 | 3 | 3 | 8 | 66.67 | ||
| Rhizopus spp. | 1 | 2 | 0 | 3 | 25 | 3 | 2 | 4 | 9 | 75.00 | ||
| Cladosporium | 3 | 3 | 3 | 9 | 75 | 0 | 0 | 0 | 0 | 0.00 | ||
| D. maydis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 16.67 | ||
| Bipolaris_spp. | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 2 | 6 | 50.00 | ||
- NB “0” revealed that fungal species are not observed on the collected storage type.
3.4. Relationship between Fungal Diseases and Insect Pests in Maize and Sorghum Grain Storages
The presence of insect pests in grain elevates the temperature due to their feeding activity, leading to the formation of “hot spots.” These spots, in turn, concentrate humidity within the grains, promoting seed deterioration and facilitating fungal activity. The elevated levels of temperature, relative humidity, and moisture commonly found in soil pits typically favor insect infestation and enable the invasion of storage fungi, hastening grain spoilage. Upon visual inspection of collected grain samples, it was observed that maize and sorghum grains devoid of insects showed no signs of fungal species. Conversely, grains with few insects exhibited a lower presence of fungal species, while grains with a high insect population demonstrated a corresponding increase in fungal species. This correlation underscores the direct relationship between insect pests of stored grains and fungal diseases, as insects serve as vectors for disease transmission. This observation aligns with the findings of Befikadu [52], who noted that insect pest infestation introduces moisture, leading to an increase in grain moisture content sufficient to trigger mold growth, thereby perpetuating a cycle of deterioration at an escalating rate.
4. Conclusions and Recommendations
The current findings revealed that all 120 total sample grain stores of maize and sorghum were significantly infested with insect pests and fungal diseases. It is evident from the study that these diseases and insect pests were the primary factors responsible for damage, weight loss, reduced germination percentage, and diminished quantity and quality of stored maize and sorghum. Among the recorded insect pests, maize weevil (S. zeamais) and angoumois grain moth (S. cerealella) were particularly abundant and widely distributed across major stored items. Grain damage and reduced seed germination capacity were observed in grain samples collected from various traditional storage systems, including sacking, marketing, hanging (maize cob), and ground pit storage methods.
Fungal diseases such as Fusarium spp., A. flavus, A. parasiticus, A. niger, A. alternata, Rhizopus spp., Bipolaris spp., and D. maydis were identified in both maize and sorghum grain storage types using both blotter and agar test methods. The survey indicated that farmers experience significant losses in both the quantity and quality of stored maize and sorghum due to insect pests and diseases. Therefore, it is recommended that further confirmatory identification using molecular tools be conducted to develop novel management strategies for these pests and diseases. Additionally, sharing adequate information in the form of training for farmers about the effects of mycotoxins on human and animal health, as well as the storage fungi associated with different storage types of sorghum and maize grains in the study areas, is essential. This educational effort can help farmers better understand the risks associated with these pests and diseases and adopt appropriate management practices to mitigate their impact.
Conflicts of Interest
The authors declare that they have no competing interests.
Acknowledgments
The authors extend their heartfelt appreciation to Oda Bultum University for generously providing laboratory instruments, vehicles, encouragement, and financial support for this study. This project was funded with the Oda Bultum University research grant funding code of RVP 135/2019/20.
Open Research
Data Availability
The authors declare that the materials and data presented in this manuscript are available upon reasonable request.




