Combined Biologic and Surgical Interventions for Hidradenitis Suppurativa: A Systematic Review
Abstract
Introduction. Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease characterized by recurrent painful or suppurative lesions due to follicular occlusion. Biologics and other treatment modalities such as surgical excision are commonly used in the treatment of severe HS. However, despite the frequent use of biologics and surgical interventions in the treatment of patients with HS, an assessment of their combined effects is lacking. This systematic review aims to qualitatively analyze the efficacy of combined biologic and surgical treatment for HS. Methods. The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines were followed. The databases PubMed (MEDLINE), Embase, Cochrane (CENTRAL), ClinicalTrials.gov, MedRxiv.org, and the International Clinical Trial Registry were searched from inception until May 1, 2023. Results. A total of 1,145 studies were screened, with eight studies included for data extraction. Patients receiving combined biologic and surgical treatment showed greater improvement in the severity measurements of HS, including HS Impact Assessment, HS Physician Global Assessment, HS Sartorius Score, International Hidradenitis Suppurative Severity Score, HS recurrence rate, and Dermatology Life Quality Index. However, three studies reported a prolongation of wound healing with combined biologic and surgical treatment. Conclusion. Our systematic review highlights the additive effects of using biologics and surgery together to treat HS compared to either treatment alone. However, when both treatment modalities are used simultaneously, the potential risk of prolonged wound healing must be considered. Due to the limited number and heterogeneity of the included studies, more clinical trials are needed to establish diagnostic conclusions.
1. Introduction
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease affecting 0.1% of the US population and is characterized by recurrent painful or suppurative lesions distributed in the areas of the apocrine sweat glands, such as the axillae, groin, inframammary folds, or anogenital regions [1–3]. Occlusion of the folliculopilosebaceous unit is a central component in the development of HS and is caused by multiple factors, including genetics, environmental triggers (e.g., obesity and tobacco use), and immune dysregulation [2, 4]. Due to the role of inflammation in the pathogenesis of HS, anti-inflammatory and targeted immunotherapy treatments are commonly prescribed for disease management [2, 4]. Other common treatment modalities include lifestyle changes and surgical excision of HS-affected areas [2, 5].
Many studies have shown the success of biologic and surgical interventions for the treatment of severe or recalcitrant lesions in HS, although an assessment of their collective effects is lacking [5]. Given the frequency of patients receiving treatment in advanced stages of the disease, a combined biologic and surgical treatment evaluation is needed. This systematic review aims to qualitatively investigate and summarize studies that analyze the efficacy of combined biologic and surgical treatment for HS.
2. Methods
The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines were followed, and the protocol was registered in PROSPERO (CRD42023390118) [6].
The databases PubMed (MEDLINE), Embase, Cochrane (CENTRAL), ClinicalTrials.gov, MedRxiv.org, and the International Clinical Trial Registry were searched from inception until May 1, 2023.
Randomized controlled trials, completed clinical trials with result data, cross-sectional surveys, observational (retrospective and prospective) studies, and pilot studies were included for data collection. Case reports or series, reviews (systematic, scoping, narrative, or literature), protocols, abstracts, non-English texts, manuscripts with incomplete data or texts, manuscripts that did not evaluate the efficacy of combined biologic and surgical therapy, and manuscripts that did not compare combined biologic and surgical therapy with biologic or surgical-only treatment were excluded. Patients of all ages and sexes, and studies reporting the evaluation of combined therapy along with biologics or surgery-only were included in our analysis.
The studies were independently screened by two authors (CJI and ACH) using Rayyan.ai (Boston, MA), and the data were independently extracted by two authors (CJI and ACH) using Microsoft Excel (Redmond, WA). Any discrepancy was resolved by the senior author (PAL).
Items extracted included the following: first author’s name; year of study; country of study; study type; Hurley stage; number of patients; number of men and women in each study and respective cohort; evaluation of the progression of HS symptoms; biologic used; dosage of biologic; time between the initiation of biologic therapy and surgery; average duration of biologic therapy; type of surgery employed; smoking history; surgical site complications; treatment groups; study outcomes (extracted only if data specifically compare combined treatment with biologic or surgery alone); and patient demographics. Missing or incomplete values were excluded from the qualitative analysis. Level of evidence for each included study was assigned per the Oxford Center for Evidence-Based Medicine, and the GRADE approach (Grading of Recommendations, Assessment, Development, and Evaluation) to evaluate quality of evidence regarding HS symptom improvement was applied to the study outcomes [7, 8].
3. Results
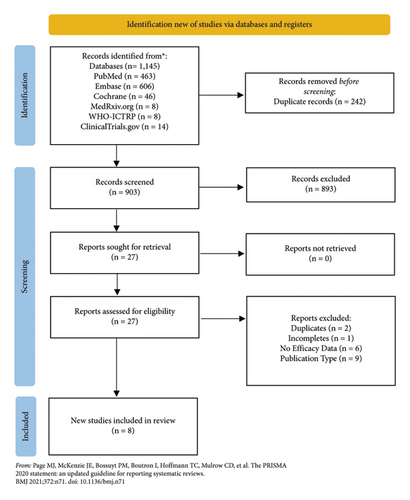
A total of 1,145 studies were screened, with eight studies included for data extraction (Figure 1 and Table 1) [9–16]. The included studies involved a total of 733 patients, with an average age ranging from 31 to 45 years old (Table 2) [9–16]. Studies involving adalimumab, infliximab, and ustekinumab amounted to six, six, and three, respectively [9–16]. Study-specific treatments are specified in Table 1 [9–16].
| Authors | Biologic used (no. of patients) | Biologic regimen | Type of surgery | Study type | Country of study/year | Level of evidencem | GRADE qualityn |
|---|---|---|---|---|---|---|---|
| Aarts et al. [9] | Adalimumab (n = 62) | 12-month treatment course | Wide excision, limited excision, and deroofing with secondary intention healing | Pragmatic randomized control trialj | Netherlands/2023 | Ib | High |
| Bechara et al. [10] | Adalimumab (n = 103) | 24-week treatment course | Wide excisionc with secondary intention healing | Randomized controlled trialk | Canada, Czechia, Colombia, Spain, Germany, France, Italy, United Kingdom, United States, Turkey, Greece, Belgium, Netherlands, Romania, Mexico, Russia, Denmark, Portugal, Poland, and Norway/2021 | Ib | High |
| DeFazio et al. [11] | Infliximab (n=8) | Average initiation on postoperative Day 16b | Radical resectiond with delayed primary closuree,f | Retrospective cohort | United States/2016 | 2b | Moderate |
| Ustekinumab (n = 3) | |||||||
| Prens et al. [12] | Adalimumab (n = 4) | Postoperatively held for 1 week | Major surgery (not specified) | Prospective cohort | Netherlands/2019 | 2b | Moderate |
| Infliximab (n = 1) | Postoperatively held for 2 weeks | ||||||
| Salvador-Rodriguez et al. [13] | Adalimumab (n = 17) | Completed ≥16 weeks preoperatively | Excision with secondary intention healing | Prospective cohort | Spain/2020 | 2b | Moderate |
| Infliximab (n=2) | |||||||
| Ustekinumab (n=2) | |||||||
| Shanmugam et al. [14] | Adalimumab (unspecified) | Not specified | Not specified | Prospective cohortl | United States/2018 | 2b | Moderate |
| Infliximab (unspecified) | |||||||
| Ustekinumaba (unspecified) | |||||||
| Van Rappard and Mekkes [15] | Infliximab (n=30) | Not specified | Deroofing procedures and small to large excisions with secondary intention healing, primary closure, or grafting procedures | Retrospective cohort | Netherlands/2012 | 2b | Moderate |
| Worden et al. [16] | Adalimumab (unspecified) | Preoperatively held for >2 weeks | Incisiong, excisionh, and excision with repairi | Retrospective cohort | United States/2020 | 2b | Moderate |
- aUstekinumab was chosen due to the presence of one or more autoantibodies, a history of drug reaction from tumor necrosis factor-alpha (TNF-a) inhibitor, or coexistent psoriasis. bFour patients remained on biologic treatment at the time of follow-up. cComplete excision of lesions with more than 50% but leaving parts of the anatomic area. dExcision of all hair-bearing skin within an affected region, including a clear margin of 1 centimeter. eAll patients underwent serial debridement before definitive closure. fDelayed primary closure with nondissolvable vertical mattress sutures. gIncision and drainage with fulguration of cavities and sinus tracts with electrocautery. hExcision of an involved area with the wound left open to drain. iRepair included excision of involved areas followed by direct defect closure, skin graft, or skin flap. jNCT03221621. kNCT02808975. lEligible patients determined from Wound Etiology and Healing (WE-HEAL) cohort (IRB 041408, NCT01352078). m2009 Oxford Center for Evidence-Based Medicine: Levels of Evidence. nGRADE method (Grading of Recommendations, Assessment, Development, and Evaluation) used to assess quality of evidence regarding overall hidradenitis suppurativa symptom improvement.
| Authors | Cohort | No. of patients | Average age (years ± SD) | Female (no. of patients, %) | Average BMI (kg/m2) | Surgical site Hurley stage (no. of patients, %) | |
|---|---|---|---|---|---|---|---|
| Adalimumab | |||||||
| Aarts et al. [9] | CTC | 31 | 40.2 ± 11.7 | 17 (55%) | 29.5 ± 6.6 | I | 1 (3%) |
| II | 22 (71%) | ||||||
| III | 8 (26%) | ||||||
| BOC | 31 | 37.5 ± 12.7 | 17 (55%) | 30.2 ± 6.1 | I | 1 (3%) | |
| II | 22 (71%) | ||||||
| III | 8 (26%) | ||||||
| Bechara et al. [10] | CTC | 103 | 38.5 ± 11.7 | 51 (49.5%) | 32.6 | II | 53 (51%) |
| III | 50 (48%) | ||||||
| PSC | 103 | 36.8 ± 10.8 | 55 (53%) | 31.7 | II | 54 (52%) | |
| III | 49 (47%) | ||||||
| Adalimumab, infliximab | |||||||
| Prens et al. [12] | CTC | 5 | 41.0 ± 10.8 | 4 (80%) | 31.3 | IIBa | 2 (40%) |
| IIIa | 3 (60%) | ||||||
| SOC | 34 | 39.3 | 30 (88%) | 28.7 | IA/Ba | 2 (6%)/2 (6%) | |
| IIA/B/Ca | 6 (18%)/19 (56%)/4 (12%) | ||||||
| IIIa | 1 (3%) | ||||||
| Worden et al. [16] | CTC | 248 | 45.0 ± 17 | 186 (75%) | 26 | Hurley stage sites affected (no. of sites, %) | |
| I | 131 (17%) | ||||||
| SOC | II | 442 (56%) | |||||
| III | 210 (27%) | ||||||
| Adalimumab, infliximab, ustekinumab | |||||||
| Salvador-Rodriguez et al. [13] | CTC | 21 | 40.6 ± 15.2 | 9 (43%) | 31.5 | II | 12 (57%) |
| III | 9 (43%) | ||||||
| SOC | 38 | 35.6 ± 9.9 | 27 (71%) | 30.8 | II | 33 (87%) | |
| III | 5 (13%) | ||||||
| Shanmugam et al. [14] |
|
68 | 40.4 ± 14 | 45 (66%) | 34 | 0 | 2 (3%) |
| I | 6 (9%) | ||||||
| II | 13 (19%) | ||||||
| III | 43 (63%) | ||||||
| Unspecified | 4 (6%) | ||||||
| Infliximab, ustekinumab | |||||||
| DeFazio et al. [11] | CTC | 11 | 31 | 7 (64%) | 36 | III | 11 (100%) |
| SOC | 10 | 33 | 7 (70%) | 35.5 | 10 (100%) | ||
| Infliximab | |||||||
| Van Rappard and Mekkes [15] | CTC | 24 | 44b | 13 (43%) | Not reported | II | 4 (13%) |
| BOC | 6 | III | 26 (87%) | ||||
- aRefined Dutch Hurley Stage Classification 2017. bRange given was age 19 to 63. %, percent; ±, plus or minus; BMI, body mass index (calculated as weight in kilograms divided by height in meters); BOC, biologic-only cohort (patients treated with only biologic therapy); CTC, combined treatment cohort (patients receiving biologic and surgical therapy); kg/m2, kilogram per meter squared; No., number; PSC, placebo and surgical cohort; SD, standard deviation; SOC, surgery-only cohort.
3.1. HS Symptom Outcomes
3.1.1. Symptom Severity
The results of the included studies demonstrate significantly improved outcomes when combining adalimumab, infliximab, or ustekinumab with surgery (Table 3).
| Authors | Measure | Combined cohort | Surgical cohort | Biologic cohort | p value |
|---|---|---|---|---|---|
| (i) Adalimumab | |||||
| (a) HS symptom improvement | |||||
| Aarts et al. [9] | DLQI mean change | −8.2 ± 6.2 | — | −4 ± 7.7 | 0.02 |
| ∆HS-PGA ≥2 points | 18/31 (58%) | — | 4/31 (18%) | <0.001 | |
| HiSCR % achieved | 59% | — | 38% | 0.22 | |
| ∆IHS4, mean ± SD | −19.1 ± 11.3 | — | −7.8 ± 11.8 | <0.001 | |
| Bechara et al. [10] | hs-CRP | −2.32 | −0.56 | — | 0.464 |
| DLQI | −5.4 | −3.1 | — | 0.01 | |
| HS-PGA-SP | −1.9 | −0.9 | — | 0.011 | |
| HSIA overall | −1.87 | −1.05 | — | 0.014 | |
| HSSA | −2.35 | −1.62 | — | 0.057 | |
| Authors | Comparison | Site infections | Wound dehiscence | Bleeding emergencies | Other complications |
| (b) Adverse effects following surgery | |||||
| Aarts et al. [9] | CTC vs BOC | No increased risk (12% vs 12%) | — | — | Total adverse events: 68% vs. 94% (p = 0.001) Acute HS flare: 22% vs. 34% |
| Bechara et al. [10] | CTC vs SOC | No increased risk | — | No increased risk | No increased risk |
| Authors | Measure | Combined cohort | Surgical cohort | Biologic cohort | p value |
| (ii) Adalimumab, infliximab | |||||
| (a) HS recurrence | |||||
| Prens et al. [12] | Ratea | 0% | 23.70% | — | — |
| Authors | Measure | Combined cohort | Surgical cohort | Biologic cohort | p value |
| (b) Time to complete wound healing | |||||
| Prens et al. [12] | Days to complete healingb | 185 days | 67.9 days | — | <0.0001 |
| Worden et al. [16] | CTC vs SOC: biologic used within 2 weeks before surgery (OR (95% CI))c | 0.23 (0.08–0.64) | — | — | 0.005 |
| CTC vs SOC: biologic held >2 weeks before surgery (OR (95% CI))c | 1.99 (0.79–5.01) | — | — | 0.146 | |
| Authors | Comparison | Site infections | Wound dehiscence | Bleeding emergencies | Other complications |
| (c) Adverse effects following surgery | |||||
| Prens et al. [12] | CTC vs SOC | No increased risk | — | — | — |
| Worden et al. [16] | CTC vs SOC | — | — | — | — |
| (iii) Adalimumab, infliximab, ustekinumab | |||||
| (a) HS symptom improvement | |||||
| Shanmugam et al. [14] | HSS (decrease in points) | −27 | −2 | — | 0.013 |
| Reduction in AN units | 0.9 | 0.1 | — | 0.033 | |
| Authors | Measure | Combined cohort | Surgical cohort | Biologic cohort | p value |
| (b) HS recurrence | |||||
| Salvador-Rodriguez et al. [13] | Rated | 9.52% | 26.31% | — | 0.1 |
| Authors | Measure | Combined cohort | Surgical cohort | Biologic cohort | p value |
| (c) Time to complete wound healing | |||||
| Salvador-Rodriguez et al. [13] | Days to complete healinge | 70.28 days | 57.68 days | — | <0.01 |
| CTC vs SOC (β(SD))f | 6.33 (1.73) | — | — | <0.01 | |
| Authors | Comparison | Site infections | Wound dehiscence | Bleeding emergencies | Other complications |
| (d) Adverse effects following surgery | |||||
| Salvador-Rodriguez et al. [13] | CTC vs SOCe | 4.76% vs 0%, p = 0.35 | — | 19.05% vs 2.63% (p = 0.04) | — |
| CTC vs SOC (β(SD))f | — | — | 0.78 [0.67] (p = 0.24) | — | |
| Shanmugam et al. [14] | CTC vs SOC | — | — | — | — |
| CTC vs BOC | — | — | — | — | |
| Authors | Measure | Combined cohort | Surgical cohort | Biologic cohort | p value |
| (iv) Infliximab, ustekinumab | |||||
| (a) HS recurrence | |||||
| DeFazio et al. [11] | Sites affected | 19% | 38.50% | — | <0.01 |
| Mean time | 18.5 months | 6 months | — | <0.001 | |
| Authors | Measure | Combined cohort | Surgical cohort | Biologic cohort | p value |
| (b) New HS lesions | |||||
| DeFazio et al. [11] | Patients affected | 18% | 50% | — | <0.001 |
| Mean time | 18 months | 8.5 months | — | <0.05 | |
| Authors | Comparison | Site infections | Wound dehiscence | Bleeding emergencies | Other complications |
| (c) Adverse effects following surgery | |||||
| DeFazio et al. [11] | CTC vs SOC | No significant difference | No significant difference | — | — |
| Authors | Measure | Combined cohort | Surgical cohort | Biologic cohort | p value |
| (v) Infliximab | |||||
| (a) HS symptom improvement | |||||
| Van Rappard and Mekkes [15] | PGA | 3.3 | — | 2.8 | <0.001 |
| Authors | Comparison | Site infections | Wound dehiscence | Bleeding emergencies | Other complications |
| (b) Adverse effects following surgery | |||||
| Van Rappard and Mekkes [15] | CTC vs BOC | — | — | — | Not observed |
- aIn 12-month follow-up duration. bReported data available from 4 out of 5 patients. cMultivariate logistic regression was used to predict poor healing of involved sites. dRate at 24 weeks. eUnivariate analysis. fMultivariate logistic regression analysis was used to identify confounding variables associated with bleeding emergencies and time to complete wound healing. AN, active nodule; AN75, 75% reduction in active nodule count; BOC, biologic-only cohort; CI, confidence interval; CTC, combined treatment cohort; DLQI, Dermatology Life Quality Index; HiSCR, Hidradenitis Suppurativa Clinical Response; hs-CRP, high-sensitivity C-reactive protein; HS-PGA-SP, HS patient’s global assessment of skin pain; HS, hidradenitis suppurativa; HSIA, HS Impact Assessment; HSS, Hidradenitis Suppurativa Sartorius Score; HSSA, HS symptom assessment; IHS4, International Hidradenitis Suppurativa Severity Score System; OR, odds ratio; p, p value; PGA, Physician Global Assessment; SD, standard deviation; SOC, surgery-only cohort; %, percent; β, beta coefficient; ∆, difference.
Specifically, combined adalimumab and surgical treatment showed an 11.3-point greater decrease in the International Hidradenitis Suppurative Severity Score (IHS4) compared to adalimumab monotherapy (p < 0.001) [9, 17]. Moreover, combined treatment led to a 40% higher increase in the number of subjects who achieved at least a 2-point change in the Global Hidradenitis Suppurativa Physician’s Assessment (HS-PGA) compared to adalimumab monotherapy (p < 0.001) [9]. While the proportion of patients who achieved Hidradenitis Suppurativa Clinical Response (HiSCR) was 21% higher with combined treatment versus adalimumab monotherapy, this difference did not reach statistical significance (p = 0.22) [9, 18]. However, when comparing combined treatment with surgery alone, a notable 0.82 and 0.73 greater decrease in HS Impact Assessment (HSIA) and HS Symptom Assessment (HSSA) was achieved with combined treatment, respectively (p = 0.014 and p = 0.057, respectively) [10]. Additionally, two studies investigating combined therapy indicated a significant reduction in the Dermatology Life Quality Index (DLQI) compared to adalimumab monotherapy or surgery-only (p = 0.02 and p = 0.01, respectively) [9, 10, 19].
Moreover, patients who received adalimumab, infliximab, or ustekinumab in conjunction with surgery experienced a 25-point decrease in the Hidradenitis Suppurativa Sartorius (HSS) score compared to those who underwent surgery alone (p = 0.013) [14].
In a study investigating infliximab therapy, the combination of infliximab and surgical therapy demonstrated a 0.5-point greater increase on a 4-point Physician Global Assessment (PGA) scale compared to infliximab monotherapy (p < 0.001) [15].
3.1.2. HS Recurrence
A total of three studies analyzed HS recurrence as an outcome measure (Table 3) [11–13].
When infliximab or ustekinumab was combined with surgery, a significant extension of time without HS recurrence was observed compared to surgical monotherapy. The mean difference in the period without recurrence was 12.5 months (p < 0.001) [11].
A study examining combined surgical treatment with adalimumab, infliximab, or ustekinumab reported a 16.79% reduction in the rate of HS recurrence (e.g., abscesses, inflammatory nodules, and draining tunnels) compared to surgery alone, although this was not statistically significant (p = 0.10) [13].
Furthermore, a study investigating adalimumab and infliximab revealed a 23.70% HS recurrence rate with surgical monotherapy, while the combined biologic and surgical treatment cohort exhibited a 0% recurrence rate [12]. However, no statistical significance value was provided for this comparison [12].
3.1.3. Wound Healing
Patients who underwent combined biologic and surgical treatment with adalimumab, infliximab, or ustekinumab experienced a significant prolongation in wound healing compared to the surgery-only cohort (Table 3) [12, 13].
Specifically, a study reported that patients treated with adalimumab, infliximab, or ustekinumab in conjunction with surgery had a significant prolongation of wound healing of 12.6 days compared to the surgery-only cohort (p < 0.01) [13].
Similarly, in another study, patients treated with adalimumab or infliximab in combination with surgery exhibited a significant prolongation of wound healing by 117.1 days compared to the surgery-only group (p < 0.0001) [12].
Notably, one study reported a significant decrease in wound healing when adalimumab or infliximab was used in conjunction with surgery (p = 0.005); however, when withholding biologic treatment for more than two weeks prior to surgery, the prolonging effect on wound healing was found to be nonsignificant (p = 0.146) [16].
3.1.4. Adverse Effects
When examining studies involving adalimumab, infliximab, or ustekinumab treatment in conjunction with surgery, no notable differences were observed for surgical site infections compared to biologic or surgical monotherapy (Table 3) [9–13].
In the context of adjuvant surgical treatment with ustekinumab or infliximab therapy, no significant differences were found in the occurrence of wound dehiscence compared to surgical monotherapy [11]. Furthermore, a study comparing combined surgical and biologic treatment (adalimumab, infliximab, or ustekinumab) with surgery-only reported a 16.42% higher increase in bleeding emergencies (p = 0.04) with combined treatment [13]. Notably, however, when stratifying confounding variables, such as age, sex, and Hurley stage, no significant differences in bleeding emergencies were found (p = 0.24) [13]. Similarly, adalimumab combined with surgery did not show a difference in bleeding emergencies compared to surgery-only [10].
Moreover, a randomized clinical trial reported a 26% greater decrease in the proportion of patients who experienced an adverse effect with combined adalimumab and surgical treatment compared to adalimumab monotherapy (p = 0.01) [9]. The most reported adverse events included HS flares, viral infections, bacterial infections such as urinary tract infections or tonsillitis, hematoma at the injection site, mild bleeding at the surgical site, and postoperative pain [9].
Importantly, all reported severe adverse events observed in studies involving adalimumab, infliximab, or ustekinumab with surgery were determined to be unrelated to the interventions being studied [9–11, 15].
4. Discussion
Aberrant immunity plays a crucial role in the development of inflammatory follicular occlusion commonly seen in HS [4]. This is supported by studies demonstrating a significant increase in inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) in the lesional and perilesional skin of HS patients [20]. As a result of this immune dysregulation, anti-inflammatory agents are typically used in disease management, with clinical studies showing substantial success in reducing the severity of HS through the use of TNF-α and IL-12 and IL-23 inhibitors [21]. Nevertheless, surgical intervention remains the primary curative therapy for severe lesions [22].
Despite the advanced stages of HS often requiring biologic or surgical treatment, there remains a scarcity of clinical studies that have explored their combined therapeutic effect. Our systematic review sheds light on the additive benefits attained by integrating surgical and biologic therapy, leading to a substantial reduction in the symptoms of HS compared to either treatment in isolation. This can be attributed to the anti-inflammatory effects of biologics on active HS lesions during the perioperative period, reducing inflammation, and allowing less extensive surgery or decreased active flares postoperatively. Similarly, since surgery for patients with HS is recommended when inflammation is at a minimum, the use of biologics can facilitate remission of the disease prior to operative treatment, preventing delays and further disease progression [23]. Additionally, combined biologic and surgical treatment offers the distinct advantage of targeting persistent draining tunnels, whereas biologic monotherapy often exhibits limited therapeutic efficacy [9]. Draining tunnels not only potentiate flares but can also facilitate new lesions by perpetuating inflammatory stimulation [9]. Notably, Aarts et al. reported a significant decrease in draining tunnels with combined treatment compared to biologic monotherapy [9].
Despite the anti-inflammatory actions of biologics, the studies included in our review reported no significant increase in postoperative infections or bleeding complications when combined with surgery. Conversely, three cohort studies demonstrated the potentially negative impact of biologics on postoperative wound healing, possibly due to biologic’s interference with the physiological role of inflammation in wound closure [24]. However, it should be noted that patients may have concurrent autoimmune conditions requiring biologic therapy. In Prens et al., one of the four subjects who received adalimumab also had Crohn’s disease, a condition associated with impaired wound healing [12, 25]. Furthermore, differences in HS severity can be a confounding factor when comparing the rate of wound healing. Salvador-Rodriguez et al. demonstrated that the cohorts within the observational study were not entirely equivocal, with 42.86% of the surgical sites in the biologic cohort being Hurley Stage III compared to only 13.16% in the nonbiologic cohort [13]. Thus, variations in disease severity at surgical sites could affect the apparent impact of biologics on wound healing. A systematic review that analyzed the impact of biologics on surgical site infections and wound healing in patients with rheumatoid arthritis found there was no increase in the risk of these complications, further supporting the safety profile of biologics [26]. However, given the inherent limitations of controlling for confounding variables in observational studies, randomized clinical trials are needed to draw definitive conclusions.
While our study emphasizes the advantages observed with combined biologic and surgical therapy, it is essential to recognize the limitations resulting from the heterogeneity among the included studies and the paucity of available studies and clinical trials investigating this integrated approach. Additionally, the presence of only two double-blind randomized clinical trials in our review restricts the overall quality of the evidence, given the inherent limitations of observational studies. Despite these limitations, our qualitative analysis provides valuable information on the potential risks and benefits associated with combined biologic and surgical therapy in a clinical setting.
5. Conclusion
In conclusion, our review highlights the potential added benefits that can be achieved through combined biologic and surgical interventions for the treatment of HS. However, it is essential to consider the potential risks of prolonged wound healing, as daily wound care can impose a significant burden on patients and lead to significant psychosocial distress. Given the limited number of high-quality studies, further exploration through randomized clinical trials is imperative to further elucidate all confounders and draw diagnostic conclusions. As a result, this can enhance the optimization of patient management for individuals with HS, leading to more effective and tailored treatment approaches.
Ethical Approval
The study protocol was registered on PROSPERO (CRD42023390118).
Conflicts of Interest
Dr. Lio reports being on the speaker’s bureau for AbbVie, Arcutis, Eli Lilly, Galderma, Hyphens Pharma, Incyte, La Roche-Posay/L’Oreal, Pfizer, Pierre-Fabre Dermatologie, Regeneron/Sanofi Genzyme, Verrica; reports consulting/advisory boards for Alphyn Biologics (stock options), AbbVie, Almirall, Amyris, Arcutis, ASLAN, Bristol-Myers Squibb, Burt’s Bees, Castle Biosciences, Codex Labs (stock options), Concerto Biosci (stock options), Dermavant, Eli Lilly, Galderma, Janssen, LEO Pharma, Lipidor, L’Oreal, Merck, Micreos, MyOR Diagnostics, Regeneron/Sanofi Genzyme, Sibel Health, Skinfix, Suneco Technologies (stock options), Theraplex, UCB, Unilever, Verdant Scientific (stock options), Verrica, Yobee Care (stock options). In addition, Dr. Lio has a patent pending for a Theraplex product with royalties paid and is a Board member and Scientific Advisory Committee Member emeritus of the National Eczema Association. Christopher J. Issa and Aubrey C. Hong have no conflicts of interest to declare.
Authors’ Contributions
Christopher J. Issa conceptualized the study, extracted and analyzed the data, and drafted and edited the manuscript. Aubrey C. Hong extracted and analyzed the data, drafted, and edited the manuscript. Peter A. Lio reviewed and edited the manuscript.
Open Research
Data Availability
The study data used to support the findings of this study are included within the article.