Real-World Experience of Secukinumab Treatment in Patients with Moderate-to-Severe Plaque Psoriasis in Greece: 3-Year Interim Results of the SERENA Study
Abstract
SERENA is an ongoing European noninterventional longitudinal study evaluating retention, effectiveness, safety, and quality of life (QoL) in secukinumab-treated patients with active moderate-to-severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis. Herein, 3-year interim results among patients with psoriasis enrolled in Greece are presented. Consented adults receiving secukinumab according to the approved label for ≥16 weeks were included. Of 292 patients enrolled, 290 eligible patients (mean age 48.4 years, 71.7% male) were analyzed. At treatment initiation, 65.9% of patients were biologic-naïve and mean total Psoriasis Area Severity Index (PASI) score was 29.0. At enrolment, mean treatment duration was approximately 1.0 year. The treatment retention rate at 1/2/3 years after enrolment was 94.4/87.3/85.9%; main reasons for discontinuation were lack of effectiveness and adverse events (AEs) (43.6% and 28.2% of discontinuations, respectively). At enrolment, the mean PASI score was 4.0, 61.3% of patients had PASI ≤ 3, 71.7% had Physician’s Global Assessment (PGA) score 0/1, 59.5% had Dermatology Life Quality Index (DLQI) score 0/1, while the mean EuroQoL Visual Analogue Scale (EQ-VAS) score was 82.0. At 1/2/3 years postenrolment, the mean PASI score was 1.9/1.6/1.0, 86.6/89.4/90.0% had PASI ≤ 3, 89.5/94.8/97.5% had PGA 0/1, 71.1/75.9/81.8% had DLQI 0/1, and mean EQ-VAS score was 85.7/90.0/92.0. Of enrolled patients, 7.2% experienced secukinumab-related AEs, while special interest AEs (candida infections, malignancy, and major adverse cardiovascular events) were reported in ≤2 patients, each. These results demonstrate high secukinumab persistence in the Greek population up to three years after study enrolment, accompanied by sustained improvements in both clinical and QoL parameters and a favorable safety profile.
1. Introduction
Psoriasis (PsO) is an inflammatory, chronic skin disease, with a reported lifetime prevalence of 0.5–2.4 in adult populations in Europe [1], and an age-standardized prevalence of approximately 2% in Greece [2]. The multifaceted nature of the disease, alongside an increased risk of comorbid conditions, leads to a substantial psychosocial burden in PsO patients [3–5].
A significant proportion of patients with PsO have a moderate-to-severe condition [6, 7]. Clinical management of moderate-to-severe PsO has been revolutionized with the introduction of biologic agents and small molecule inhibitors [8]. Second-generation biologic agents targeting interleukin IL-17, IL-23, and IL-12/23 have demonstrated improved efficacy and a better safety profile [9–11]; however, biologic treatments are often discontinued or switched over time because of lack or loss of effectiveness or tolerability issues [12–16]. Thus, real-world drug survival reflects a proxy measure of sustainable effectiveness and long-term safety, providing a valuable index of the drug’s clinical utility.
Secukinumab, a fully human IgG1 monoclonal antibody that selectively binds to IL-17A, has shown sustained efficacy in moderate-to-severe PsO for up to 5 years in clinical trial settings [17]. At the time, the present SERENA study was designed, and real-world data to support these findings were scarce and mainly limited to the first year after treatment initiation [13, 18]. Therefore, this ongoing study, which has a 5-year horizon, primarily aims to characterize the longer-term retention, effectiveness, and safety profile of secukinumab as well as its impact on quality of life (QoL) of patients with moderate-to-severe plaque PsO, active psoriatic arthritis (PsA), or active ankylosing spondylitis, in real-life conditions in Europe. This 3-year interim analysis reports data from patients with moderate-to-severe PsO who were enrolled in Greece.
2. Materials and Methods
2.1. Study Design
The design of the SERENA study (CAIN457A3403) has been described in detail previously [19]. In brief, it is an ongoing, longitudinal, observational study conducted at 438 sites across 19 countries in patients with the aforementioned three indications, who had received at least 16 weeks of secukinumab treatment before study enrolment.
The 3-year interim analysis presented herein included both the target analysis set and safety analysis set populations with moderate-to-severe PsO enrolled in Greece. The safety analysis set consisted of patients who received at least one dose of secukinumab treatment after signing the informed consent, while the target analysis set excluded patients who were withdrawn due to protocol deviations.
2.2. Study Population
Detailed inclusion and exclusion criteria have been reported previously [19]. Briefly, patients were included in the study if they were consented adults with a diagnosis of active moderate-to-severe plaque PsO who have been receiving secukinumab for ≥16 weeks before enrolment, for whom the decision to prescribe secukinumab treatment was made by the treating physician regardless of this noninterventional study and according to the European commission approved use. Patients were excluded if they had any condition preventing them from remaining in the study for the initial 2 years post-enrolment. Patients who were currently participating or had previously participated in another secukinumab study or were still in safety follow-up from another such study were also excluded.
2.3. Study Assessments
The primary objective of the SERENA study is to evaluate the long-term retention of secukinumab in daily clinical practice, as well as to identify main reasons affecting this retention [20]. Endpoints for moderate-to-severe PsO patients enrolled in Greece presented herein include the following: retention rates at 1, 2, and 3 years post-enrolment; retention rates at 1, 2, and 3 years after secukinumab treatment initiation; effectiveness measures of the Psoriasis Area and Severity Index (PASI; scored 0 (no psoriasis) to 72 (extremely severe psoriasis)), body surface area (BSA) (assessed by the percentages of areas affected, including head, trunk, upper limbs, and lower limbs), achievement of Physician’s Global Assessment (PGA) score of 0/1 (denoting clear/almost clear skin), the percentage of patients with psoriatic nail involvement; and, lastly, dermatology-specific and general health-related QoL measures based on patient-reported outcomes, including achievement of Dermatology Life Quality Index (DLQI) 0/1 response (no impact on QoL), and change from enrolment of the EuroQoL Visual Analog Scale (EQ-VAS) score (0 (worst imaginable health) to 100 (best imaginable health)), respectively. With regard to safety, the present analysis reports treatment emergent adverse events (AEs), serious AEs (SAEs), and adverse events (AEs) of special interest, including major adverse cardiac events (MACEs), candida infections, and malignancy. Relationship of treatment emergent AEs with secukinumab is also presented. All AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA), version 24.0.
Data on drug retention, effectiveness, impact on QoL, and safety were collected prospectively. Since patients included in the study were pretreated with secukinumab, retention and effectiveness data were also collected retrospectively from the start of the treatment. The retention rate is defined as the percentage of patients who have not discontinued secukinumab, and study enrolment refers to inclusion of patients in the SERENA study (start of observation/baseline visit). All prior biologic treatments administered for PsO were documented without a time limit, whereas all other prior PsO treatments were only documented if taken within 6 months prior to enrolment.
2.4. Statistical Methods
The results of this interim analysis were mainly analyzed based on descriptive statistics, with no data imputation applied. Quantitative data were analyzed based on the statistical parameters of N, mean, and standard deviation (SD), while qualitative variables were presented by absolute and relative frequency distributions. Two-sided 95% Wald confidence intervals (CIs) were provided for secukinumab treatment retention rates. Time to secukinumab treatment discontinuation was estimated using Kaplan–Meier survival analysis. Correlation of DLQI with PASI and PGA was examined using Spearman’s rank correlation coefficient.
3. Results
3.1. Patient Disposition and Profile
Overall, 292 patients with PsO were enrolled in Greece, which comprised the safety analysis set, while the target analysis set excluded two patients who were withdrawn due to protocol deviation. Thus, a total of 290 patients (71.7% males) with a mean age of 48.4 years and a mean body mass index of 28.5 kg/m2 (Table 1) were included in the efficacy and retention analyses. Among patients with available data, 50.6% were either current or former smokers and 27.4% had a family history of PsO, while hypertension was the most frequent comorbidity in 11.4% (Table 1).
| Characteristics | N = 290 |
|---|---|
| Age (n = 290), mean (SD), years | 48.4 (13.9) |
| Male (n = 290), n (%) | 208 (71.7) |
| Caucasian (n = 290), n (%) | 270 (93.1) |
| BMI (n = 189), mean (SD), kg/m2 | 28.5 (5.6) |
| BMI classification (n = 189), n (%) | |
| Normal BMI (BMI < 25 kg/m2) | 44 (23.3) |
| Overweight (25 kg/m2 ≤ BMI < 30 kg/m2) | 88 (46.6) |
| Obese (BMI ≥ 30 kg/m2) | 57 (30.2) |
| Smoking status (n = 249), n (%) | |
| Never smoker | 123 (49.4) |
| Current smoker | 98 (39.4) |
| Former smoker | 28 (11.2) |
| Smoking amount (n = 123), mean (SD), pack/years | 28.0 (22.4) |
| Family history of psoriasis (n = 234), n (%) | |
| First-degree relatives | 43 (18.4) |
| Second-degree relatives | 21 (9.0) |
| Family history of arthritis (n = 218), n (%) | |
| First-degree relatives | 6 (2.8) |
| Second-degree relatives | 2 (0.9) |
| Family history of other inflammatory disease (n = 200), n (%) | |
| First-degree relatives | 1 (0.5) |
| Second-degree relatives | — |
| Relevant medical history/comorbidities in ≥10 patients (n = 290), n (%) | |
| Hypertension | 33 (11.4) |
| Dyslipidaemia (inc. hypercholesterolaemia and hyperlipidaemia) | 19 (6.6) |
| Diabetes mellitus (inc. type 1 and 2) | 18 (6.2) |
| Depression | 10 (3.4) |
- BMI, body mass index; inc., including; N, total number of patients; n, number of patients with available data (i.e., with non-missing data); SD, standard deviation.
At study enrolment, patients had a mean disease duration of 11.8 years, with 10.3% also had a previous diagnosis of PsA (Table 2). Types and manifestations of PsO are presented in Table 2.
| Characteristics | N = 290 |
|---|---|
| Time since diagnosis of psoriasis (n = 288), mean (SD), years | 11.8 (10.7) |
| PsA (n = 290), n (%) | 30 (10.3) |
| Time since diagnosis of PsA (n = 29), mean (SD), years | 9.0 (8.9) |
| Types and manifestations of psoriasis† (n = 290), n (%) | |
| Scalp psoriasis | 92 (31.7) |
| Nail psoriasis | 71 (24.5) |
| Palms psoriasis | 51 (17.6) |
| Soles psoriasis | 32 (11.0) |
| Genital psoriasis | 30 (10.3) |
| Erythrodermic psoriasis | 11 (3.8) |
| Generalized pustular psoriasis | 10 (3.4) |
| Guttate psoriasis | 2 (0.7) |
| Palmoplantar pustular psoriasis | 1 (0.3) |
- N, total number of patients; n, number of patients with available data (i.e., with non-missing data); PsA, psoriatic arthritis; SD, standard deviation. †In this table, psoriasis history as per prior diagnosis, which is ongoing at the baseline, is presented. All patients in this study had plaque PsO, and other types of PsO presented in this table (e.g., erythrodermic PsO) have been reported as additional/secondary involvements.
3.2. Treatment Characteristics
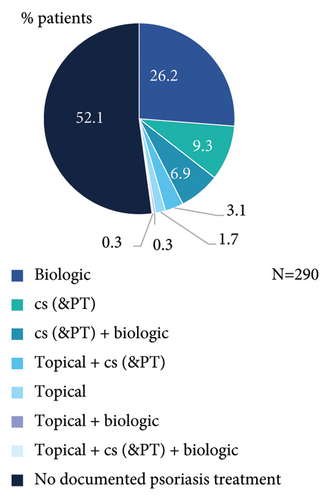
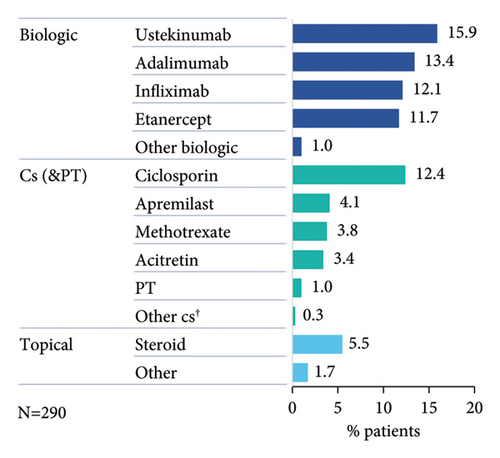
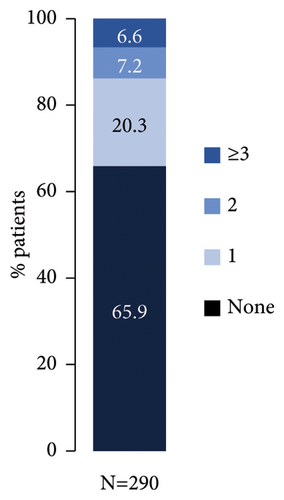
Nearly half of patients had documented PsO treatment taken prior to start of secukinumab (Figure 1(a)), which included at least one biologic (bio-experienced), conventional systemic (cs)/phototherapy, or topical treatment in 33.8% (98/290), 19.7% (57/290), and 5.5% (16/290) of patients, respectively. Most frequent prior biologic agent was ustekinumab (Figure 1(b)), while 13.8% (40/290) had received ≥2 prior biologic treatments (Figure 1(c)).
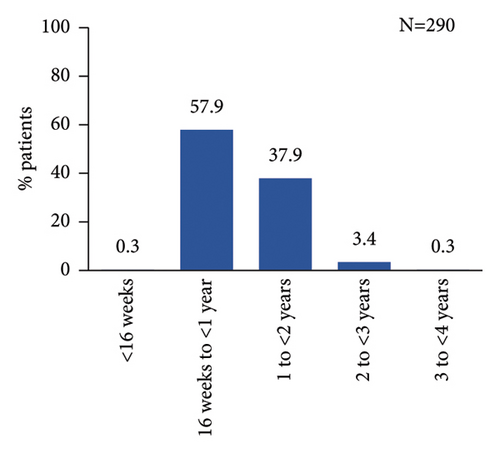
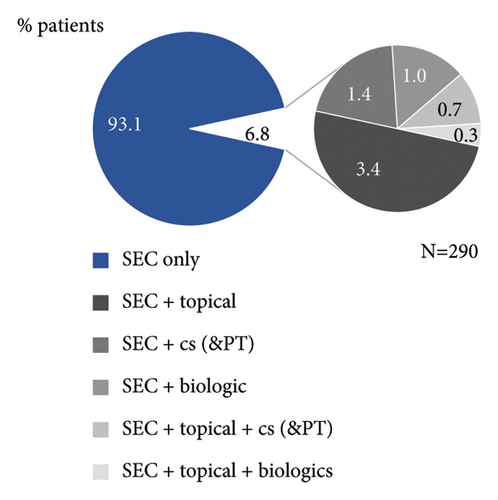
As per study design, all patients had started secukinumab treatment prior to enrolment. At enrolment, patients were treated with secukinumab for a mean (SD) duration of 1.0 (0.5) year, with most patients treated between ≥16 weeks and <2 years (Figure 2(a)). Majority of patients received secukinumab alone (93.1%), while 6.8% of patients were treated with topical and/or other systemic treatments in addition to secukinumab (Figure 2(b)).
3.3. Retention and Effectiveness
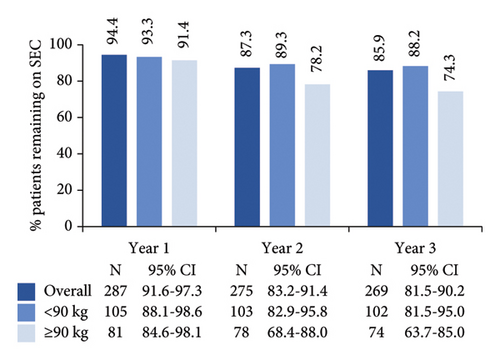
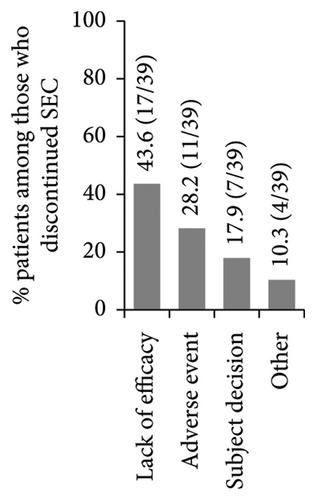
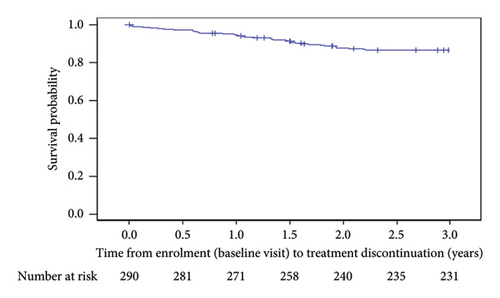
High secukinumab retention rates were observed after 1, 2, and 3 years in the study, with numerically higher rates noted among patients who weighed <90 kg compared to the weight group of ≥90 kg (Figure 3(a)). Secukinumab retention rates at 1, 2, and 3 years of post-treatment initiation were 98.6% (N = 289; 95% CI: 97.1–100.0), 94.7% (N = 284; 95% CI: 91.9–97.5), and 87.7% (N = 276; 95% CI: 83.6–91.7), respectively. A total of 13.1% (38/290) of patients had discontinued secukinumab within 3 years of post-enrolment, mainly due to lack of effectiveness (Figure 3(b)). The Kaplan–Meier-estimated median time from enrolment to secukinumab treatment discontinuation was not reached (Figure 3(c)).
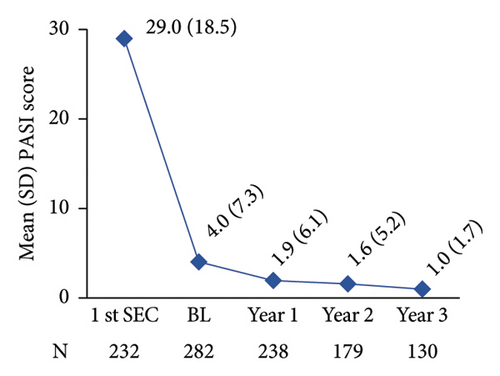
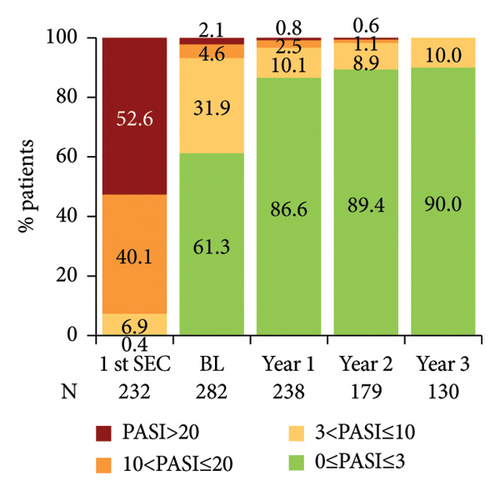
The mean PASI score at first secukinumab dose administration was 29.0, while it was reduced to 4.0 when patients were enrolled in SERENA and was further reduced to 1.0 at 3 years post-enrolment (Figure 4(a)). Notably, at secukinumab initiation, most patients (92.7%; 215/232) had an absolute PASI score >10 (severe disease), whereas from year 1 post-baseline onwards, the vast majority of patients (>86%) had an absolute PASI score ≤3 (Figure 4(b)). The absolute PASI ≤ 2 rate was 53.5% (151/282) at enrolment and 79.0% (188/238), 83.8% (150/179), and 84.6% (110/130) at years 1, 2, and 3 post-enrolment, respectively.
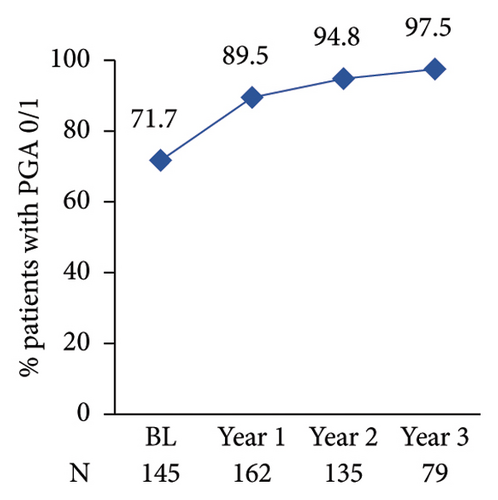
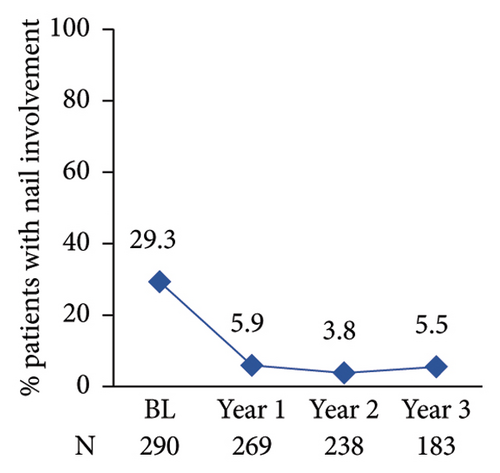
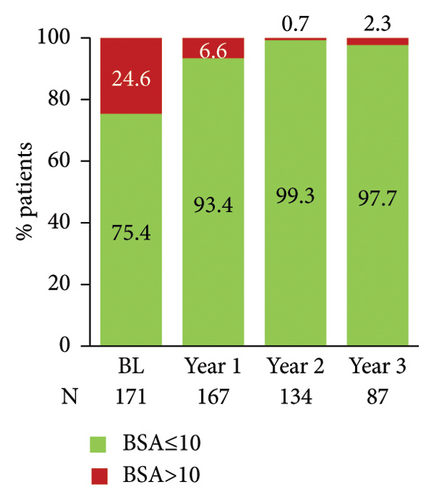
The proportion of patients achieving clear/almost clear skin as assessed by PGA 0/1 increased annually reaching 97.5% at year 3 post-enrolment (Figure 4(c)). The proportion of patients with nail involvement and BSA of >10 decreased from 29.3% to 24.6% at enrolment to 5.9% and 6.6% at year 1 post-enrolment, respectively (Figures 4(d) and 4(e)). A low nail involvement and PsO-affected BSA was sustained at least until 3 years of post-enrolment (Figures 4(d) and 4(e)).
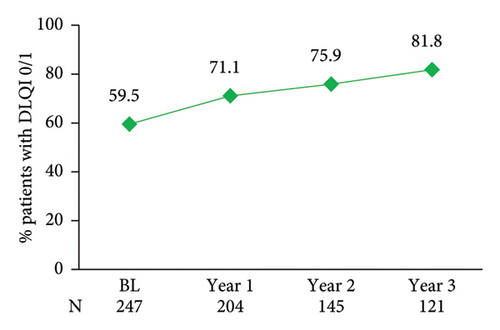
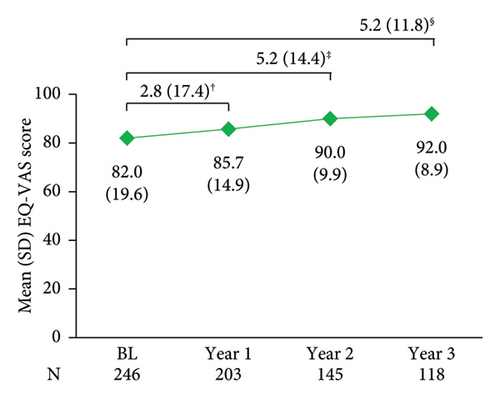
PsO had no impact on patient’s QoL in 59.5% of patients at enrolment based on DLQI (score 0/1), with this proportion increasing annually and reaching 81.8% by year 3 post-enrolment (Figure 5(a)). A similar trend was observed over 3 years for EQ-VAS, a generic QoL measure (Figure 5(b)). Data at treatment initiation for PGA, nail involvement, BSA, DLQI, and EQ-VAS were not available.
A positive correlation of the total DLQI score with the total PASI score was shown at year 1 (r = 0.57), year 2 (r = 0.60), and year 3 (r = 0.68) post-enrolment. The total DLQI score was also positively correlated with a PGA score at year 1 (r = 0.56), year 2 (r = 0.52), and year 3 (r = 0.41) post-enrolment.
3.4. Safety
An overview of the secukinumab safety profile during the 3-year study observation period is presented in Table 3. At year 3 post-enrolment, 17.1% and 2.4% of patients had experienced at least one AE and one SAE, respectively. One patient experienced a fatal event of myocardial infarction which was not considered related to secukinumab treatment. The frequency of secukinumab-related AEs was 7.2% (21/292), mostly comprising drug ineffectiveness (in 18 of 21 cases). The AE-related treatment discontinuation rate was 9.6% (28/292). AEs of special interest (candida infections, malignancies, and major adverse cardiovascular events) were reported in ≤2 patients each. Overall, there were no unexpected safety signals.
| Treatment emergent AEs | Year 1 (N = 292) | Year 2 (N = 292) | Year 3 (N = 292) | |||
|---|---|---|---|---|---|---|
| Number of AEs | 43 | 82 | 86 | |||
| Number of patients with at least one AE, n (%) | 28 (9.6) | 47 (16.1) | 50 (17.1) | |||
| Number of SAEs | 4 | 7 | 7† | |||
| Number of patients with at least one SAE, n (%) | 4 (1.4) | 7 (2.4) | 7 (2.4) | |||
| Number of patients with AEs leading to death, n (%) | — | 1 (0.3) | 1 (0.3) | |||
| Discontinued treatment due to AE, n (%) | 15 (5.1) | 27 (9.2) | 28 (9.6) | |||
| n (%) | IR | n (%) | IR | n (%) | IR | |
| Most common AEs by MedDRA SOC with incidence rate >2 | ||||||
| General disorders and administration site conditions | 14 (4.8) | 5.34 | 25 (8.6) | 4.82 | 25 (8.6) | 3.47 |
| Skin and subcutaneous tissue disorders | 6 (2.1) | 2.49 | 8 (2.7) | 1.86 | 10 (3.4) | 1.54 |
| AEs of special interest by MedDRA SOC and PT | ||||||
| Malignancy | 1 (0.3) | 0.36 | 1 (0.3) | 0.19 | 1 (0.3) | 0.13 |
| Prostate cancer | 1 (0.3) | 0.36 | 1 (0.3) | 0.19 | 1 (0.3) | 0.13 |
| Candida infections | 1 (0.3) | 0.36 | 2 (0.7) | 0.37 | 2 (0.7) | 0.26 |
| Oral candidiasis | 1 (0.3) | 0.36 | 1 (0.3) | 0.19 | 1 (0.3) | 0.13 |
| Vulvovaginal candidiasis | — | — | 1 (0.3) | 0.19 | 1 (0.3) | 0.13 |
| Major adverse cardiovascular events (MACE) | 1 (0.3) | 0.36 | 2 (0.7) | 0.37 | 2 (0.7) | 0.26 |
| Myocardial infarction | 1 (0.3) | 0.36 | 2 (0.7) | 0.37 | 2 (0.7) | 0.26 |
| AEs related to SEC by MedDRA SOC | ||||||
| General disorders and administration site conditions | 8 (2.7) | 3.20‡ | 18 (6.2) | 3.53‡ | 18 (6.2) | 2.57‡ |
| Skin and subcutaneous tissue disorders | 1 (0.3) | 0.36§ | 1 (0.3) | 0.19§ | 2 (0.7) | 0.26§,¶ |
| Ear and labyrinth disorders | — | — | 1 (0.3) | 0.19£ | 1 (0.3) | 0.13£ |
- AE, adverse event; MedDRA, medical dictionary for regulatory activities; IR: incidence rate; N, total number of patients; n, number of patients with available data (i.e., with non-missing data); PT, Preferred Term; SAE, serious adverse event; SOC, system organ class. †PT terms: cerebral ischaemia (n = 1), exposure during pregnancy (n = 1), myocardial infarction (n = 2), pneumonia (n = 1), prostate cancer (n = 1), and sudden hearing loss (n = 1); ‡PT term: drug ineffective; §PT term: nail psoriasis; ¶PT term: psoriasis; £PT term: sudden hearing loss.
4. Discussion
In this 3-year interim analysis of the SERENA study, we provide real-life data on a sizable sample of 290 patients with moderate-to-severe PsO treated with secukinumab in Greece. In this cohort, high retention rates of 94.4%, 87.3%, and 85.9% were reported at 1, 2, and 3 years after enrolment in the study, respectively.
In view of the limited amount of available long-term data on secukinumab retention in real-world settings in Europe [18] at the time of SERENA conduct, the outcomes of this study provide valuable insight into the prolonged clinical value of secukinumab, assessed in a larger pool of patients, compared to prior Greek studies [20–22]. A naïve-indirect comparison with earlier European real-world cohorts indicates that persistence rates for the Greek SERENA cohort appear high. Although not directly comparable, due to differences in study design (e.g., most other studies are retrospective [15, 23–35] or prospective registries/databases [14, 16, 36–38] and in some studies drug survival is Kaplan–Meier estimated [28, 34, 38, 39]), other European real-world studies have previously reported 1-year persistence rates of 72–89% [14–16, 23–28, 34, 36–42]. Furthermore, persistence rates at 24–27 months widely range between 25 and 87% [14–16, 23, 24, 26–28, 33, 38, 39, 41, 42], with 36-month retention rate being 44–50% [16, 26]. It should be noted that the above-described results mostly concern retention from secukinumab initiation [14, 15, 24–30, 35–38, 40] and thus differ from the SERENA study, in which observation began after a mean of 1 year of treatment (for both the overall and the Greek cohort) [43]. Nevertheless, the results of the Greek SERENA cohort are also favourable compared with the overall SERENA cohort in which retention rates were 88%, 76%, and 61% at 1, 2, and 3 years in the study, respectively [43].
The variability in drug survival outcomes may be partly explained by differences in management practices across centres and/or countries, further highlighting the importance of obtaining local real-world data, but may also be attributed to the different patient profiles and the extent of prior exposure to systemic treatment. In this respect, bio-naïve or previously untreated patients tend to show better clinical responses [14, 20, 25, 30, 35] and persistence to therapy [14, 28, 34, 36–38]. Among the Greek SERENA cohort, 66% were bio-naïve, which is higher than that of most other real-life settings of secukinumab (most frequently between 30 and 51% [15, 23, 24, 27, 28, 38, 39, 41] and rarely above 70% [14]).
In line with previous real-world studies, showing that patients with obesity are at an increased risk of discontinuing secukinumab treatment [27, 34] or have worse responses, [25, 40] in the Greek SERENA cohort retention rates among patients with body weight ≥90 kg were numerically lower than their counterparts. Caution should be exercised in interpreting this observation, given the high missing rate in body weight data (>35%). Recent results on dose optimization have now shown that higher secukinumab exposure among patients with higher weight improves efficacy [44]; thus, secukinumab persistence rates in SERENA are expected to be underestimated compared with the current status of increased dosing frequency in psoriasis patients with body weight ≥90 kg.
Supporting the notion that treatment persistence could be a surrogate marker for longer-term effectiveness, a low mean PASI score of 4.0 had been achieved at enrolment, reaching 1.9 at year 1 which was sustained until year 3 (1.0). Similar response rates have been found in previous European observational studies, in which mean PASI was 9.0–17.7 at secukinumab initiation (lower than SERENA) and 1.0–3.1 at 52–60 weeks, [25, 28–30, 32, 35, 36, 40] 2.7 at 2 years [35], and 3.1 at 136 weeks [35]. Although relative PASI has traditionally been utilized in determining treatment success, absolute PASI is increasingly considered a better measure, as it is less error-prone and allows therapeutic decisions to be based on actual disease severity, regardless of baseline PASI [45, 46]. In the Greek SERENA cohort, absolute PASI ≤ 3/≤2 rates at an average of 1 year of treatment (61/54%) and at year 1 post-enrolment (87/79%) were high, supporting the favourable prior European real-world data of 65–73 [25, 30]/56–63% [25, 30, 40] after 1 year of treatment. To provide a wider context for interpreting these data, and since PASI90 has been previously proposed to correspond to PASI ≤ 2, [11] PASI ≤ 2 rates in our study were within the PASI90 range based on the clinical trial (57–76%) [17, 47] and European real-world data (46–86%) [23, 26, 29, 30, 33, 35, 40, 48] after 1 year of treatment. At 2 and 3 years after secukinumab initiation, PASI90 was reported in 67% and 64% of patients in the CLEAR and SCULPTURE trials, respectively, [17] and 71% (at 2 years) and 72% (at 136 weeks) in an Italian retrospective cohort [35]. The high levels of effectiveness based on PASI in our study are reinforced by the findings of sustained high PGA 0/1 response rates as well as low nail involvement and low BSA.
Consistently high effectiveness was also previously observed in other Greek studies. Kaplan–Meier-estimated drug survival at 2 years was 74.5%, [20] while 75.4% remained on secukinumab at 3 years [23]. PASI ≤ 3 rate at year 1/1.5/2 was 73–84 [20, 21]/83 [21]/74%; [20] PGA 0/1 rate at year 1/1.5 was 72/83%; [21] and mean or median PASI at years 1 and 2 was ≤1.8 [20, 21]. Although enrolment data (baseline visit) in SERENA represent results after ≥16 weeks of treatment with secukinumab and thus improvement from treatment initiation cannot be accurately inferred, evidence presented herein strengthen and expand prior local findings showing that response rates are high and sustained over the next 3 years of treatment.
Skin clearance of patients under secukinumab treatment is closely associated with improvements in DLQI-assessed QoL [24, 49]. After 1 year in the study, more than 70% of patients in the Greek cohort of SERENA reported that PsO had no impact on their QoL, based on a DLQI of 0/1, with sustained QoL normalization up to 3 years of post-enrolment (82%). These results are aligned with the previously reported 1-year DLQI 0/1 rate of 72% in the PROSE prospective study of 17 European countries [47]. Furthermore, DLQI 0/1 rates at week 48/96/144/192/240 were 81/90/90/79/86% among patients (required to be on secukinumab ≥192 weeks) in an Italian retrospective cohort [31]. In a Spanish retrospective cohort, the proportions of patients achieving DLQI 0/1 were 74% and 72% at years 1 and 2 after secukinumab initiation [24]. In addition to dermatology-specific QoL findings, favourable outcomes on generic health-related QoL (HRQoL) were also noted in SERENA, based on a mean EQ-VAS score of 82.0 after a mean of 1 year of treatment (i.e., at enrolment/baseline). At 3 years of post-enrolment, mean EQ-VAS scores had reached 92.0, signifying, altogether, a better HRQoL than the general psoriatic population in Greece (mean EQ-VAS score: 74.7) [50].
With regard to safety, no unexpected AEs were observed over 3 years of post-enrolment. Furthermore, the frequency of AEs of special interest, such as those pertaining to infections, malignancies, and major adverse cardiovascular events, was exceptionally low (≤2 patients). No cases of IBD were encountered by our patients, consistent with another Greek cohort [20]. Less than 10% of patients discontinued treatment due to AE, altogether confirming the manageable and well-tolerated safety profile of secukinumab previously reported [17].
This observational study is subject to several limitations which have been previously described in detail [19]. The most pertinent limitation is the selection bias since only patients with initial response and persistence to therapy for ≥16 weeks were included, which precludes study outcomes from being generalizable to a wider population. Further studies investigating parameters influencing secukinumab’s retention and effectiveness, in patients with PsO, are warranted.
5. Conclusions
In conclusion, this interim analysis of real-world data from Greece showed high secukinumab retention rates as well as sustained effectiveness and QoL improvements over 3 years in patients with moderate to severe PsO. Three-year interim results of SERENA also confirmed that secukinumab is well tolerated in the real clinical setting, while the overall incidence of treatment-emergent AEs was low.
Ethical Approval
The study protocol was approved by the institutional review board of each participating site according to local regulations. The study is conducted in accordance with Good Clinical Practice, the Guidelines for Good Pharmacoepidemiology Practices (GPP) of the International Society for Pharmacoepidemiology (ISPE 2008), the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines, the Declaration of Helsinki, and all local regulations. The study was approved by independent ethics committees or institutional review boards of participating centers, i.e., Ippokrateio General Hospital of Thessaloniki (567/12.09.2017), Syggros Hospital (2397/6.12.2016 and 2405/24.04.2017), 401 General Military Hospital of Athens, (03/01.03.2017), University General Hospital of Larissa (1st meeting/22.02.2017), Olympion General Clinic (14.12.2016), Euromedica General Clinic of Thessaloniki (576/26.07.2017), University General Hospital Attikon (17th meeting/28.11.2017), General Hospital of Athens KAT (122/27.04.2017), Papageorgiou General Hospital (277th meeting/23.05.2017), and University General Hospital of Patra (15272/27.06.2017).
Consent
All patients provided written informed consent before any study-related procedures were undertaken.
Conflicts of Interest
D. Ioannides: Clinical trial investigator fees and honoraria for educational events & consultancy from Abbvie, Boehringer-Ingelheim, Genesis, Iasis Pharma, Inovia, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, Pharmathen, Sanofi, UCB & Epsilon Health; D. Rigopoulos: Clinical trial investigator fees and honoraria for educational events & consultancy from Abbvie, Novartis, Genesis, LEO Pharma, UCB & Amgen; M. Papakonstantis: Clinical trial investigator fees and honoraria for educational events & consultancy from Novartis, Abbvie, LEO Pharma, Pfizer, Janssen, Faran, Lilly, Amgen & Genesis Pharma; V. Chasapi: Clinical trial investigator fees from Janssen & UCB, honoraria for lectures from Lilly & Boehringer Ingelheim, congress support from Piere Fabre & Lilly, P. Deligiannis: Clinical trial investigator fees and honoraria for educational events & consultancy from Abbvie, LEO Pharma, Pfizer & Amgen; P. Rigatos: Clinical trial investigator fees from UCB and honoraria for lectures from Abbvie; I. Lefaki: Clinical trial investigator fees from Novartis, LEO Pharma & Genesis Pharma, congress support from Avene, Ducray & Evdermia; E. Papadavid: honoraria for educational events & consultancy from Abbvie, Lilly, Pfizer, Sanofi, Helsin, Takeda, Novartis & UCB, E. Pokas: Clinical trial investigator fees and honoraria for educational events & consultancy from UCB, Amgen & Lilly; S. Tsilifis: Clinical trial investigator fees and honoraria for consultancy from Loreal, LEO Pharma & Frezyderm; A Roussaki-Schulze: none; I Barkis: honoraria for lectures from UCB, Abbvie, LEO Pharma, Lilly, Menarini, Galenica & Novartis; E. Lazaridou: Honoraria for lectures, educational grants and consultancy from Abbvie, Novartis, Janssen, LEO Pharma, UCB, Lilly, Sanofi, Pierre Fabre, L’oreal, Amgen & Pfizer; C. Fotiadou: Honoraria for lectures from AbbVie, UCB Pharma, Lilly, Janssen & L’Oréal; C. Zisimou: Clinical trial investigator fees from Coronis & Novartis; P. Kallidis: Clinical trial investigator fees from LEO Pharma & Viatris; V. Chatzakis: honoraria for lectures from UCB & LEO Pharma; C. Oikonomou: Clinical trial investigator fees from Novartis, UCB & LEO Pharma, honoraria for lectures from UCB; X. Madia: Employee of Novartis Hellas S.A.C.I.
Authors’ Contributions
D Ioannides, D Rigopoulos, M Papakonstantis, V Chasapi, P Deligiannis, P Rigatos, I Lefaki, E Papadavid, E Pokas, S Tsilifis, A Roussaki-Schulze, I Barkis, E Lazaridou, C Fotiadou, C Zisimou, P Kallidis, V Chatzakis, and C Oikonomou were involved in acquisition and interpretation of data, while X Madia was involved in the conception/design of work, analysis/interpretation of data, as well as drafting the manuscript. All authors were responsible for critically revising the work for important intellectual content and for making all content and editorial decisions. All authors had final approval of the manuscript version to be published and are accountable for all aspects of the work in ensuring the accuracy and integrity of this manuscript. Dimitrios Ioannides and Dimitrios Rigopoulos should be considered joint first authors.
Acknowledgments
This work was supported by Novartis Hellas S.A.C.I. The authors wish to thank Qualitis SA, Part of the Optimapharm Group, for medical writing support, which was funded by Novartis Hellas S.A.C.I.
Open Research
Data Availability
Data are available upon reasonable request. The data sets generated and/or analyzed during the current study are not publicly available. Novartis is committed to sharing with qualified external researchers’ access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the study in line with applicable laws and regulations. The data may be requested by writing to the corresponding author.