Baseline ANA and Anti-Ro60/SSA Antibody as Potential Predictors for Immunogenicity and Poor EULAR Response in Adalimumab-Treated RA Patients
Abstract
What Is Known and Objective. Given that autoantibodies are relatively abundant and easily measured, the detection of antibody biomarkers would be ideal for predicting therapeutic responses. Although anti-Ro60/SSA (anti-TROVE2) antibody has been identified as a useful predictor for the development of immunogenicity and therapeutic failure to adalimumab in RA patients, autoantibodies with similar predictive potentials are not yet sufficiently validated. Methods. We aimed to investigate the baseline autoantibodies, including rheumatoid factor (RF)-IgM, anti-citrullinated peptide antibodies (ACPA), antinuclear antibody (ANA), anti-Ro60/SSA antibody, and anti-La/SSB antibody, for their relationships with drug immunogenicity and therapeutic responses in RA patients assessed at week 48 of adalimumab therapy. We also compared adalimumab drug survival between participants with or without these autoantibodies. Results and Discussion. Our results showed that poor EULAR responders had significantly higher positive rates of baseline ANA, anti-Ro60/SSA antibody, and anti-La/SSB antibody than moderate or good responders. However, no significant differences in circulating levels of RF-IgM or ACPA were observed among groups with different responses. The multivariate logistic regression analysis revealed that ANA and anti-Ro60/SSA antibodies were significant predictors of developing immunogenicity to adalimumab (both p < 0.001). The presence of ANA, anti-Ro60/SSA, and anti-SSB antibodies could significantly predict poor EULAR response in adalimumab-treated RA patients (odds ratio, 4.98, 5.60, and 8.08, respectively, all p < 0.01). In Kaplan–Meier analysis, the adalimumab drug survival rate was significantly lower in RA patients with positivity for ANA, anti-Ro60/SSA, anti-SSB, and anti-drug antibodies than in the seronegative group. What Is New and Conclusion. Our results suggest that baseline ANA and anti-Ro60/SSA antibodies are potential predictive markers of drug immunogenicity and poor EULAR response in adalimumab-treated RA patients.
1. What Is Known and Objective
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by persistent synovitis and bone erosion and often associated with functional disability [1, 2]. The pathogenic role of tumor necrosis factor (TNF)-α in RA is resonated with the effectiveness of biological agents targeting this cytokine [2–4]. It is well known that the development of antidrug antibodies (ADAb) or immunogenicity may be related to low or undetectable drug levels and the resulting poor therapeutic response to TNF-α inhibitors (TNFi) such as adalimumab [5–9]. To pursue a treat-to-target goal [10] and reduce the uncertainty of the therapeutic response to TNFi, there is an unmet need to find baseline biomarkers capable of predicting drug immunogenicity and the effectiveness of biologics.
Using a high-density protein microarray approach [11], we have recently found that baseline anti-Ro60/SSA (anti-TROVE2) antibody might be a useful predictor of the emergence of ADAb and secondary therapeutic failure in RA patients treated with adalimumab [12]. Hagiwara et al. similarly revealed that anti-Ro/SSA antibody-positive RA patients receiving therapy with infliximab, a monoclonal antibody TNFi, had a higher proportion of ADAb development than anti-Ro/SSA antibody-negative patients (50% versus 0%, 0/12), with all patients with positivity for ADAb being poor responders to infliximab [13]. Yukawa et al. also demonstrated that baseline antinuclear antibody (ANA) titers could predict clinical response to infliximab [14]. However, the aforementioned study results have not been further replicated and validated, and whether circulating autoantibodies might predict the immunogenicity or therapeutic responses to adalimumab in patients with RA remained elusive.
This retrospective observational study aimed at investigating the baseline autoantibodies, including rheumatoid factor (RF)-IgM, anti-citrullinated peptide antibodies (ACPA), ANA, anti-Ro60/SSA antibody, and anti-La/SSB antibody, for their association with immunogenicity of and therapeutic response to adalimumab in RA patients. Firstly, we identified baseline autoantibodies that are significantly associated with poor therapeutic responses evaluated at week 48 after adalimumab therapy in patients with RA. Secondly, we compared adalimumab drug survival rates between participants with or without these autoantibodies.
2. Methods
2.1. Patients and Study Design
This retrospective and two-centric study was conducted at China Medical University Hospital and Taichung Veterans General Hospital between April 2014 and March 2021. A total of 221 patients were consecutively enrolled. The inclusion criteria were as the following: (1) aged over 20 years, (2) fulfillment of the 2010 revised criteria of the American College of Rheumatology for RA [15], and (3) initially biologic-naïve, and then received adalimumab therapy for at least 48 weeks. The exclusion criteria in our study if any of the following criteria apply: (1) diagnosis of primary Sjögren’s syndrome [16], or (2) diagnosis of secondary Sjögren’s syndrome for which the underlying disease or predominant diagnosis was not RA. RA disease activity was assessed using the 28-joint disease activity score-erythrocyte sedimentation rate (DAS28-ESR) at baseline and week 48 after therapy with adalimumab, respectively [17]. The therapeutic response was evaluated at week 48 of adalimumab therapy using the EULAR response criteria [18]. Patients were categorized as good, moderate, or poor responders based on the change in the DAS28 and the value of DAS28 measured at week 48 of adalimumab therapy. Patients who have >1.2 decreases of DAS28 from baseline (∆DAS28) and DAS28 ≦ 3.2 at evaluation time were considered good responders. Moderate responders had either ∆DAS28 > 1.2 and a DAS28 > 3.2 or ∆DAS28 of 0.6–1.2 and DAS28 ≦ 5.1; poor responders were those who had either ∆DAS28 < 0.6 or a DAS28 > 5.1. The Institutional Review Board of both medical centers approved this study (TCVGH CF21176A, CMUH110-REC2-106-AR1) and the requirement for written consent was waived due to the retrospective nature of the analysis. As patient data were anonymized before analysis, the written consent was waived.
2.2. Measurement of ADAb and Plasma Adalimumab Trough Levels
Antidrug antibodies’ levels were measured using bridging ELISA (Progenika Biopharma, SA, Derio, Spain) at week 48 of adalimumab therapy according to the described technique [9]. This assay determines circulating levels of free ADAb but lacks sensitivity toward IgG4-ADAb because only the bivalent fraction is detected [19]. Given that the determination of ADAb may be influenced by circulating drug levels, plasma ADAb levels were measured in those samples that had no detectable adalimumab concentration. In this study, the results were transferred to arbitrary units per milliliter (AU/ml) by comparison with dilutions of a reference plasma. The result was regarded as positivity for ADAb if the level was higher than 3.5 AU/ml. To avoid the probability of a false positivity, the result was considered positive if the level was greater than the 3-fold value (10.5 AU/ml) of the detection limit (3.5 AU/ml). Although the rheumatoid factor (RF) may interfere with the determination of ADAb, no interference by the RF was observed in the ADAb assay used in our study. Plasma adalimumab trough levels were measured using the sandwich ELISA (Progenika Biopharma, SA, Derio, Spain) at week 48 according to the manufacturer’s instructions [9]. The minimal detectable level for adalimumab was 0.024 μg/ml.
2.3. Determination of RF-IgM, ACPA, ANA, Anti-Ro60/SSA Antibody, and Anti-La/SSB Antibody
Plasma levels of autoantibodies, including RF-IgM, ACPA, ANA, anti-Ro60/SSA antibody, and anti-La/SSB antibody were determined at baseline and after 48 weeks of adalimumab therapy. Circulating RF-IgM levels was determined by nephelometry (Dade Behring Inc., Newark, DE, USA, positive if ≥14 IU/mL), and ACPA levels by EliA CCP (Phadia, Nieuwegein, The Netherlands, positive if ≥10 U/mL). ANA was detected by indirect immunofluorescence using a Hep-2 cell line as an antigen source (Medical and Biological Laboratories Co., Ltd., Nagoya, Japan). A titer of at least 1 : 160 was considered positive by a senior medical technologist. The anti-Ro60/SSA and anti-La/SSB antibodies were determined using ImmunoCAP fluorescence enzyme immunoassay (Phadia, Uppsala, Sweden); a titer >10EliA U/ml was considered positive.
2.4. Statistical Analyses
The results are illustrated as numbers (percent) or median (interquartile range (IQR)). The chi-square test and Kruskal–Wallis test were used to compare parameters among participants with EULAR good, moderate, and poor responses. Factors associated with poor EULAR responses were calculated using logistic regression analysis in the enter and forward models with the adjustment of statistically significant parameters in the univariate model. We performed the age- and gender-adjusted logistic regression analysis for exploring the variables associated with the presence of anti-adalimumab antibodies. The odds ratio (OR) and 95% confidence interval (CI) for each variable were determined. Ten-year drug survival of adalimumab by the presence of antidrug antibodies, ANA, anti-Ro60/SSA, and anti-SSB antibodies were depicted by the Kaplan–Meier survival analysis. All statistical analyses were performed using the Statistical Package for the Social Science, version 22.0 (SPSS, IBM Corp., Armonk, NY, USA) and MedCalc® Statistical Software version 20.014 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2021). A two-tailed p value <0.05 was considered significant.
3. Results and Discussion
3.1. Clinical Characteristics of RA Patients
Among the enrolled 221 patients with RA, 77 (34.8%) patients were EULAR good responders, 122 (55.2%) moderate responders, and 22 (10.0%) poor responders when evaluated at week 48 of adalimumab therapy. As illustrated in Table 1, patients with low disease activity at baseline were likely to have poor response as compared with others. Significantly higher proportions of positivity for ANA, anti-Ro60/SSA, anti-SSB, and ADAb were observed in poor EULAR responders compared with good or moderate responders. As expected, significantly higher proportions of the biologic switch were found in poor EULAR responders compared with the other two groups. Besides, significantly lower drug levels were found in poor responders compared with good or moderate responders. Compared with moderate responders, a significantly lower proportion of good responders used corticosteroids or csDMARDs because of low disease activity or achieving remission. However, no significant differences in age, the percentage of female, disease duration, or serum levels of RF-IgM or ACPA were observed among the three groups.
| Poor response (n = 22) | Moderate response (n = 122) | Good response (n = 77) | p value | ||||
|---|---|---|---|---|---|---|---|
| Age at onset (years) | 51.5 | (39.8–56.3) | 53.0 | (44.8–60.0) | 53.0 | (46.0–60.0) | 0.362 |
| Gender | |||||||
| Female, n (%) | 21 | (95.5%) | 107 | (87.7%) | 63 | (81.8%) | 0.213 |
| RA duration (yrs) | 11.5 | (10.7–12.9) | 11.5 | (9.5–12.7) | 11.0 | (9.0–13.5) | 0.549 |
| RF-IgM (IU/mL) | 121.8 | (56.0–361.9) | 56.5 | (27.7–152.8) | 69.0 | (29.8–342.5) | 0.064 |
| ACPA (IU/mL) | 242.9 | (29.8–251.0) | 128.9 | (12.4–250.0) | 72.2 | (11.0–234.9) | 0.141 |
| RF-IgM (+), n (%) | 19 | (86.4%) | 85 | (69.7%) | 55 | (71.4%) | 0.274 |
| ACPA (+), n (%) | 18 | (81.8%) | 87 | (71.3%) | 2 | (67.5%) | 0.426 |
| ANA (+), n (%) | 14 | (63.6%) | 29 | (23.8%) | 6 | (7.8%) | <0.001 ∗∗†‡§ |
| Anti-SSA (+), n (%) | 13 | (59.1%) | 19 | (15.6%) | 2 | (2.6%) | <0.001 ∗∗†‡§ |
| Anti-SSB (+), n (%) | 5 | (22.7%) | 11 | (9.0%) | 1 | (1.3%) | 0.003 ∗∗‡ |
| ADAb (+), n (%) | 13 | (59.1%) | 8 | (6.6%) | 0 | (0.0%) | <0.001 ∗∗†‡ |
| Drug levels (µg/mL) | 0.02 | (0.02–0.11) | 1.90 | (1.01–3.34) | 6.30 | (4.50–7.92) | <0.001 ∗∗†‡§ |
| Baseline DAS28 | 5.91 | (5.67–6.28) | 6.42 | (5.95–7.06) | 6.34 | (5.85–6.94) | 0.007 ∗∗† |
| DAS28 at week 48 | 4.93 | (4.72–5.22) | 3.61 | (3.28–4.00) | 2.64 | (2.10–2.86) | <0.001 ∗∗†‡§ |
| Biologic switch, n (%) | 21 | (95.5%) | 35 | (28.7%) | 6 | (7.8%) | <0.001 ∗∗†‡§ |
| Steroids use, n (%) | 20 | (90.9%) | 97 | (79.5%) | 30 | (39.0%) | <0.001 ∗∗‡§ |
| MTX use, n (%) | 19 | (86.4%) | 94 | (77.0%) | 70 | (90.9%) | 0.037 ∗∗§ |
| csDMARD use, n (%) | 10 | (45.5%) | 72 | (59.0%) | 26 | (33.8%) | 0.002 ∗§ |
- aData are illustrated as mean ± standard deviation, number (percentage), or median (interquartile range). RF, rheumatoid factor; ACPA, anti-citrullinated peptide antibody, DAS28, 28-joint disease activity score; MTX, methotrexate; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; EULAR, European League Against Rheumatism; good responder defined as having a decrease in DAS28 from baseline (∆DAS28) >1.2 and a DAS28 ≦ 3.2 at week 48; moderate responder as either ∆DAS28 > 1.2 and a DAS28 > 3.2 or ∆DAS28 of 0.6–1.2 and a DAS28 ≦ 5.1 at week 48; and poor responder as having either ∆DAS28 < 0.6 or a DAS28 > 5.1 at week 48 of adalimumab therapy. The Kruskal–Wallis test was used for among-group comparison of numerical variables. The chi-square test was used to compare binary variables. ∗p < 0.05 and ∗∗p < 0.01; post hoc analysis by the Bonferroni method. †Poor response vs. moderate response; ‡poor response vs. good response; §moderate response vs. good response.
3.2. Logistic Regression Analysis to Identify Predictors of Drug Immunogenicity
As shown in Table 2, age- and gender-adjusted regression analysis revealed the positivity of ANA and anti-SSA antibody as significant predictors of the emergence of ADAb.
| Univariate | Multivariable† | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Age | 0.97 | (0.93–1.00) | 0.065 | |||
| Sex | ||||||
| Female | Reference | |||||
| Male | 0.29 | (0.04–2.28) | 0.242 | |||
| Disease duration | 1.01 | (0.99–1.03) | 0.254 | 1.01 | (0.99–1.03) | 0.187 |
| RF-IgM | 1.00 | (1.00–1.00) | 0.416 | 1.00 | (1.00–1.00) | 0.419 |
| ACPA | 1.00 | (1.00–1.00) | 0.895 | 1.00 | (1.00–1.00) | 0.829 |
| ANA (+) | 7.40 | (2.86–19.17) | <0.001 | 6.44 | (2.45–16.92) | <0.001 |
| Anti-Ro60/SSA (+) | 10.79 | (4.08–28.49) | <0.001 | 9.45 | (3.53–25.33) | <0.001 |
| Anti-SSB (+) | 1.30 | (0.28–6.11) | 0.741 | 0.97 | (0.20–4.70) | 0.968 |
| Baseline DAS28 | 1.02 | (0.57–1.82) | 0.959 | 1.00 | (0.55–1.84) | 0.995 |
- Logistic regression. †Adjust age and sex. OR, odds ratio; 95%CI, 95% confidence interval; RF, rheumatoid factor; ACPA, anti-citrullinated peptide antibodies; ANA, antinuclear antibodies; DAS28, 28-joint disease activity score.
3.3. Logistic Regression Analysis to Identify Predictors of Poor EULAR Response
Using the logistic regression analysis, we identified the potential predictors of poor EULAR response to adalimumab. As shown in Table 3, the multivariate regression analysis revealed the positivity of ANA, anti-Ro60/SSA antibody, anti-SSB antibody, or ADAb as the significant predictors of poor EULAR response. On the contrary, higher baseline DAS28 was related to a better therapeutic response.
| Multivariable-ANA† | Multivariable-SSA† | Multivariable-SSB† | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | OR | 95% CI | p value | |
| Age | 1.00 | (0.95–1.05) | 0.958 | 0.99 | (0.94–1.04) | 0.668 | 1.00 | (0.95–1.05) | 0.934 |
| Gender | |||||||||
| Female | Reference | Reference | Reference | ||||||
| Male | 0.46 | (0.05–4.23) | 0.493 | 0.31 | (0.03–3.79) | 0.359 | 0.31 | (0.03–3.49) | 0.344 |
| ANA (+) | 4.98 | (1.48–16.67) | 0.009 | ||||||
| Anti-SSA (+) | 5.60 | (1.66–18.90) | 0.006 | ||||||
| Anti-SSB (+) | 8.08 | (1.82–35.93) | 0.006 | ||||||
| ADAb (+) | 37.1 | (9.5–144.8) | <0.001 | 29.1 | (7.5–113.2) | <0.001 | 63.4 | (16.0–251.4) | <0.001 |
| Base. DAS28 | 0.29 | (0.13–0.63) | 0.002 | 0.33 | (0.15–0.69) | 0.004 | 0.31 | (0.15–0.65) | 0.002 |
- Logistic regression. †Enter method. EULAR, European League Against Rheumatism; poor responders are those who have either ∆DAS28 (DAS28 decrement) <0.6 or a DAS28 > 5.1 at week 48 of adalimumab therapy; OR, odds ratio; 95% CI, 95% confidence interval; ANA, antinuclear antibodies; ADAb, anti-drug antibody; Base., baseline; DAS28, 28-joint disease activity score.
3.4. Kaplan–Meier Analysis of Ten-Year Adalimumab Survival Rate
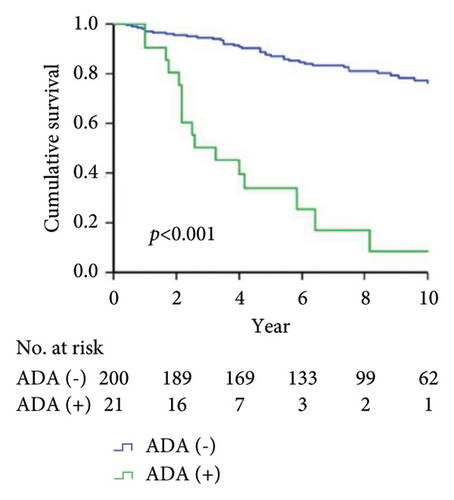
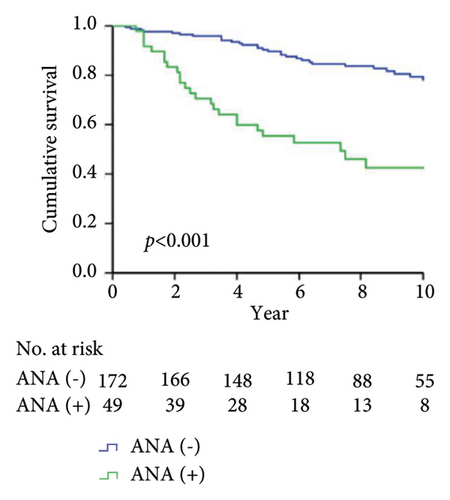
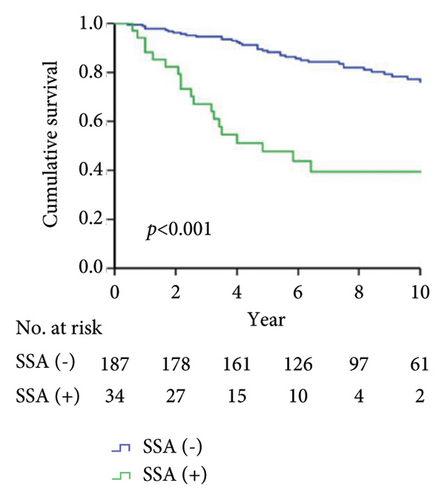
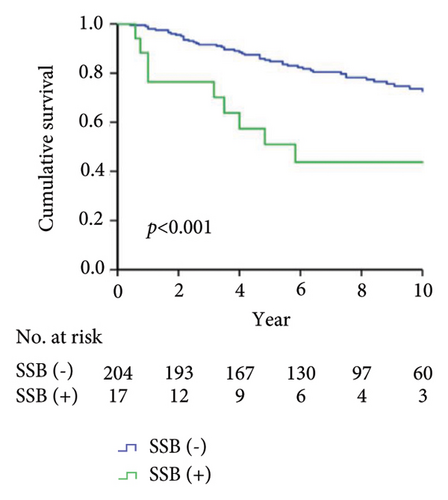
As shown in Figure 1, the Kaplan–Meier analysis depicted the 10-year drug survival of adalimumab therapy in RA patients with the positivity for ADAb (Figure 1(a)), ANA (Figure 1(b)), anti-Ro60/SSA (Figure 1(c)), and anti-SSB (Figure 1(d)) antibodies in RA patients. Compared with participants who did not have baseline positivity of ANA, anti-Ro60/SSA, or SSB antibodies, or did not develop ADAb, the adalimumab drug survival rate is significantly lower in those with these antibodies.
3.5. Changes in Plasma Titers of Autoantibodies after 48 Weeks of Adalimumab Treatment
Among good responders with seropositivity, plasma levels of RF-IgM, anti-Ro60/SSA antibody, and anti-La/SSB antibody significantly declined after 12 months of adalimumab therapy (mean value, 176.6 IU/mL vs. 121.3 IU/mL, p < 0.05; 171.9 IU/mL vs. 80.8 IU/mL, p < 0.005; and 39.7 IU/mL vs. 13.4 IU/mL, p < 0.005; respectively). Among the good responders with baseline ANA negativity (n = 40), 17 (42.5%) had seroconversion to ANA positivity. However, there was no significant change in plasma levels of ACPA after 12 months of adalimumab therapy.
3.6. Discussion
Given that autoantibodies are relatively abundant, highly specific, and easily measured, the detection of antibody biomarkers would be ideal for predicting drug immunogenicity and therapeutic responses [20]. We have recently used the immune-related protein microarray platform to identify the anti-TROVE2 (anti-Ro60/SSA) antibody as a useful predictor of immunogenicity and secondary therapeutic inefficacy in RA patients treated with adalimumab [12] and would like to perform further verification and validation. In this large independent cohort, we evaluated the clinical utility of the potential autoantibody-based biomarkers to predict the immunogenicity of and therapeutic response to adalimumab in RA patients. Our results showed that patients with poor EULAR response had significantly higher positive rates of baseline ANA, anti-Ro60/SSA antibody, or anti-La/SSB antibody than other responders. The multivariate regression analysis revealed that both ANA and anti-Ro/SSA antibodies were significant predictors of the development of immunogenicity to adalimumab. The positivity of ANA, anti-Ro60/SSA, anti-La/SSB, and ADAb could significantly predict poor EULAR response in adalimumab-treated RA patients. In the Kaplan–Meier analysis, the drug survival rate of adalimumab was also significantly lower in patients seropositive for ANA, anti-Ro60/SSA, anti-La/SSB, or anti-drug antibodies than in the seronegative group during a longitudinal follow-up. These observations reveal that baseline ANA and the anti-Ro60/SSA antibody may be candidate markers for predicting the immunogenicity and poor EULAR response to adalimumab therapy in patients with RA.
Although TNFi are effective in RA management, a substantial proportion (20–30%) of patients still show poor therapeutic responses [21, 22]. Several factors may contribute to poor responses to TNFi therapy [23]. TNF-α may not be one of the predominant cytokines involved in RA pathogenesis, which can lead to primary therapeutic failure of TNFi [24, 25]. The variability in the therapeutic response could also be related to genetic heredity, the so-called pharmacogenomics [26]. Clinical parameters, including RA duration, disease activity, and baseline levels of RF and ACPA, may also affect the therapeutic response to TNFi [27]. In this study, we showed that the EULAR poor responders had lower disease activity at baseline compared with other responders. Those with less inflammation at baseline may have less pronounced disease improvement after treatment than those with a higher inflammatory burden at baseline, similar to the findings of a recent study [28]. We also revealed a near-significant trend of higher RF-IgM and ACPA levels in poor responders compared with responders, consistent with the findings of previous studies [29, 30]. However, other clinical characteristics such as demographic data and concomitant csDMARD use may either contribute to only 15–17% of the variance found in the therapeutic response [29] or show conflicting results [31]. Finally, poor therapeutic responses may take the form of secondary inefficacy, which is probably related to ADAb development [5, 32].
Given that biopharmaceuticals could induce immunogenicity [33], long-term use of TNFi may elicit ADAb and result in reduced therapeutic responses [5–9]. Although the positive rates of ADAb reported in patients treated with adalimumab were various, the proportion of positivity for ADAb in our study was in agreement with the data of some other studies [6–8]. Currently, there is no marker that can accurately predict the emergence of ADAb. In our study, the multivariate regression analysis identified the positivity for ANA and anti-SSA antibody as significant predictors of the emergence of ADAb. Mori et al. similarly demonstrated ANA positivity as a risk factor for the appearance of ADAb in patients treated with infliximab or adalimumab [34]. The high link between anti-Ro60/SSA antibody and the emergence of ADAb to adalimumab in this study also resonates with the findings of other studies that anti-Ro60/SSA positivity was significantly associated with therapeutic failure of infliximab [13, 35], a biologic with high immunogenicity [7]. Because the 60 kDa Ro (Ro60)/SSA ribonucleoprotein is a common member of extractable nuclear antigens and a frequent target of humoral immunity, anti-Ro60/SSA antibody is often observed in autoimmune diseases such as primary Sjögren’s syndrome and RA. Cavazzana et al. also revealed that anti-Ro60/SSA-positive RA patients had a broad autoantibody profile, reflected by polyclonal hypergammaglobulinemia and a high proportion of ANA positivity [36]. The significant relationship between anti-Ro60/SSA antibody positivity and the emergence of ADAb in our adalimumab-treated patients suggest that Ro autoantigen probably takes part in the diversification of an autoantibody response through a determinant spreading [37, 38]. These findings indicate that baseline positivity of ANA or anti-Ro60/SSA antibody may predict the emergence of ADAb in RA patients treated with adalimumab.
Given a link between ADAb positivity and reduced therapeutic response, a higher proportion of our ANA-positive patients would have poor therapeutic response compared with seronegative patients. Furthermore, the positivity of baseline ANA strongly predicted poor EULAR response in our adalimumab-treated patients. Ishikawa et al. similarly reported that ANA positivity is related to poor therapeutic response to biological disease-modifying antirheumatic drugs in RA patients [39]. Besides ANA, significantly higher positive rates of anti-Ro60/SSA and anti-SSB were observed in poor EULAR responders compared with responders. Likewise, Hagiwara et al. revealed that anti-Ro60/SSA antibody-positive patients treated with infliximab had a higher proportion of ADAb and poor therapeutic response compared with seronegative patients [13]. It is interesting that anti-La/SSB antibody positivity was also a significant predictor of poor EULAR response to adalimumab in our RA patients, which has not been reported previously. Besides, there was a group of patients with anti-Ro60/SSA and anti-La/SSB copositivity in our cohort. Topfer et al. similarly showed that mice immunized with 60-kDa Ro could produce anti-La/SSB antibodies in addition to high titers of anti-Ro60/SSA antibody, probably through intermolecular spreading [38]. These observations suggest that a subset of autoantibodies such as ANA, anti-Ro60/SSA, and anti-La/SSB antibody may be potential markers to predict poor response to adalimumab. However, these findings require to be further validated in ethnically matched control populations.
Drug survival, also known as drug retention or treatment persistence, is a comprehensive indicator reflecting a combination of effectiveness, safety, and tolerability [40]. Our RA patients with the positivity of ADAb, ANA, anti-Ro60/SSA, or anti-SSB antibodies had a significantly lower survival rate of adalimumab than seronegative patients. Given a close link between autoantibodies’ positivity and poor therapeutic response, it is expected that patients with the positivities of ADAb, ANA, anti-Ro60/SSA, or anti-La/SSB antibodies would have a greater loss of therapeutic effectiveness than seronegative patients. Similarly, biologic switching might be considered when the first use of therapeutic agent exhibits an insufficient efficacy or adverse events [41], and patients with positive ADAb, ANA, anti-Ro60/SSA, or anti-La/SSB antibodies would have a higher proportion of biologic switching. It is clinically important to be aware that RA patients positive for baseline autoantibodies are at increased risk of poor therapeutic response and biologic switching.
The emergence of autoantibodies after anti-TNF-α therapy may suggest that the use of adalimumab acts as the triggering factor for developing autoimmunity in adalimumab-treated patients. Resonated with the findings of previous studies [42, 43], we revealed the seroconversion of ANA in 42.5% of good responders with baseline ANA negativity. Similar to the findings of previous studies [44, 45], we revealed a significant reduction of plasma levels of RF-IgM after 12 months of adalimumab treatment in those with a good therapeutic response. The influence of seroconversion on the effectiveness of adalimumab in the long term is really worth further investigation.
It is worth highlighting that we used autoantibodies as predictive markers, a rare approach in the past, and our results were based on a relatively large cohort with a long-term follow-up. Despite the novel findings herein, some limitations should be addressed. First, the major drawback of our study is its retrospective and observational nature, which may render it liable to confounding bias such as discontinuation or switching of biologics. Given concomitant administration of csDMARDs reduces immunogenicity [46, 47], the utility of baseline antibody markers to predict therapeutic response may be biased since the concomitant csDMARD use was not uniform among all three groups. Lastly, the role of these autoantibodies in the pathogenesis of drug immunogenicity is not elucidated in the present study. Therefore, future studies are needed to examine the utility of antibody biomarkers to predict therapeutic response to TNFi other than adalimumab and elucidate the pathogenic mechanism of autoantibodies in drug immunogenicity.
Our preliminary data have been presented at the annual conference of Taiwan Rheumatology Association [48]. The major differences between the conference article and the submitted manuscript include the following: The conference article was published only as an abstract, not as a full article. The submitted manuscript includes a revised title and a detailed evaluation of the association between baseline disease activity and EULAR therapeutic response, which was not presented in the conference abstract. In addition, the term “anti-Ro/SSA” is specified as “anti-Ro60/SSA” in the manuscript, and the changes in plasma titers of various autoantibodies after 48 weeks of adalimumab treatment are examined, which were not addressed in the conference abstract. Overall, our manuscript presents significant new data and comprehensive analyses that expand upon the preliminary findings shared in the conference abstract.
4. What Is New and Conclusion
Our results indicate that baseline autoantibodies, including ANA and anti-Ro60/SSA antibody, might be useful predictors of the emergence of drug immunogenicity and poor response to adalimumab in patients with RA. The positivity of these autoantibodies was also significantly associated with low drug survival rates and a high proportion of biologic switching. Testing for baseline autoantibodies might help guide the optimal selection of bDMARDs with cost-effective benefits.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors’ Contributions
All authors made substantive intellectual contributions to the present study and approved the final manuscript. Y-MC conceived the study, designed the study, analyzed data, and drafted and revised the manuscript. P-KC conceived the study, analyzed data, and drafted the manuscript. S-HC and H-HC acquired clinical data and analyzed data. J-PC analyzed data with statistical analysis. K-TT and J-LL acquired clinical data. D-YC generated the original hypothesis, designed the study, conceived the study, acquired clinical data, analyzed data, and drafted and revised the manuscript.
Acknowledgments
The authors thank Shiow-Jiuan Wey, MD, of the Chung Shan Medical University Hospital, Taiwan, for manuscript editing. This work was supported by a grant from China Medical University Hospital (DMR-111-200).
Open Research
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.




