Potential Role of APC Mutations in the Prognosis and Targeted Therapy of Gastric Adenocarcinoma
Abstract
Background: Adenomatous polyposis coli (APC) gene, an oncogene, has been implicated in stomach adenocarcinoma (STAD), which is a common type of gastric cancer (GC). Although the relationship between APC gene mutations and gastric adenocarcinoma has been comprehensively studied, the potential role of these mutations in the prognosis and targeted therapy remains known.
Methods: We utilized The Cancer Genome Atlas (TCGA) database to obtain gene expression matrices, clinical information, and mutation data from patients with STAD. The mutation status of the APC gene was analyzed, and its correlation with tumor mutational burden (TMB), microsatellite instability (MSI), and clinical prognosis in STAD was investigated. Gene set enrichment analysis (GSEA) was conducted to explore the pathological role of APC gene mutations in STAD metabolic pathways. Drug sensitivity analysis was conducted to identify potential targeted antitumor drugs for patients with APC gene mutations in gastric adenocarcinoma.
Results: The results revealed that 88% (46/52) of STAD samples had nonsynonymous mutations. The mutation group exhibited a significantly higher TMB than the wild-type group (p < 0.001), and the percentage of high MSI (MSI-H) was significantly higher in the mutation group than in the wild-type group (p < 0.001). Patients with APC mutations had a worse prognosis than those with APC wild-type (p = 0.009). The APC gene mutation group displayed significant enrichment in amino acids, RNA, and several pathways (|NES| > 1 and nominal p value < 0.01). Compared to the wild-type group, the mutation group exhibited a higher infiltration proportion of natural killer (NK) cells resting and eosinophils, whereas a lower infiltration proportion of monocytes and resting mast cells (p value < 0.05). AZD5991 exhibited significant sensitivity in patients with STAD carrying APC mutations (p = 0.028).
Conclusion: APC gene mutations play a crucial role in the prognosis, molecular characteristics, and potential therapeutic strategies for gastric adenocarcinoma.
1. Introduction
Despite a discernible reduction in the global incidence and mortality rates of gastric cancer (GC) in recent decades, it is still considered a persistent burden in China [1]. With 1.089 million new cancer cases and 7,69,000 deaths reported globally, the Global Cancer Statistics 2020 report states that GC not only retains its status as the fifth most prevalent malignant neoplasm but also stands as the fourth principal cause of cancer-related mortality worldwide [2]. Specifically, in 2020, GC accounted for 4,79,000 new cases in China, constituting 44% of the global total, and claimed 3,74,000 lives, representing 48.6% of global fatalities [2–4]. GC exhibits special developmental characteristics, and early symptoms are not obvious [5, 6]. The 5-year survival rate of patients with advanced GC is less than one-fifth, seriously threatening the health of the population [7, 8].
The pathogenesis of GC is complicated and several factors are known to synthetically affect its occurrence and development [9, 10]. These include age, sex, race and genetics, Helicobacter pylori (H. pylori) infection, gastrointestinal microbiota, obesity, unhealthy dietary habits and lifestyle, tobacco and alcohol consumption, and chemical, radiation, or virus exposure [11–15]. Recent research reports the involvement of certain relatively rare risk factors, such as gastroesophageal reflux disease, gastric ulcer, and previous gastric surgery, in the pathogenesis of GC, [16]. Although most cases of GC are sporadic, familial clustering accounts for around 10% of cases. Hereditary GC, related to known cancer susceptibility syndromes and/or genetic causes, accounts for 1%–3% of all GC cases [17]. The three major hereditable syndromes primarily affecting the stomach are hereditary diffuse GC (HDGC), gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS), and familial intestinal GC (FIGC) [18, 19].
The adenomatous polyposis coli (APC) gene is a well-known oncogene [20]. Relevant studies have demonstrated that APC-associated gastric polyposis includes classical familial adenomatous polyposis (FAP), attenuated FAP (AFAP), gastric adenocarcinoma, and GAPPS [21–23]. APC gene mutations inactivate the regulatory function of β-catenin, an oncogenic protein, resulting in the abnormal accumulation of β-catenin in the cytoplasm and its penetration into the nucleus, consequently activating the transcription of the members of the T-cell factor/lymphoid enhancer-binding factor (TCF/LEF) family of transcription factors and leading to tumorigenesis [24, 25]. Although the information pathway of APC mutations in oncogenesis has been initially clarified, the potential function of APC mutations in the prognosis and targeted therapy of gastric adenocarcinoma remains elusive.
In this study, we initially acquired gene expression matrices, clinical data, and mutation profiles from patients diagnosed with stomach adenocarcinoma (STAD). Subsequently, we analyzed the mutation status of the APC gene in these patients and studied the correlation between mutations and tumor mutational burden (TMB) and microsatellite instability (MSI). In addition, we validated the association of APC gene mutations with the clinical prognosis of patients with STAD. Gene set enrichment analysis (GSEA) was performed to investigate the pathological role of APC gene mutations in the metabolic pathways of STAD. Furthermore, we compared the differences in the infiltration of 22 immune cell subtypes between the APC gene wild-type and mutation groups. Finally, a drug sensitivity analysis was conducted to identify potential antitumor drugs for patients with APC gene mutations in gastric adenocarcinoma.
2. Materials and Methods
2.1. Sample Data Sources
Gene expression matrices, clinical data, and mutation profiles of STAD were sourced from The Cancer Genome Atlas (TCGA) database. The path to obtain the gene expression matrix is as follows: https://portal.gdc.cancer.gov/; Repository--Cases--Primary Site: stomach--Program: TCGA--Project: TCGA-STAD--Files--Data Category: transcriptome profiling--Data Type: Gene Expression Quantification. A dataset containing 407 samples was downloaded, including 375 STAD tissues and 32 normal tissues, and was used to extract comprehensive information. This dataset encompassed detailed clinical characteristics, such as age, gender, tumor stage, survival time, and survival status. In addition, the path to obtain the mutation matrix is as follows: https://portal.gdc.cancer.gov/; Repository--Cases--Primary Site: stomach--Program: TCGA--Project: TCGA-STAD--Files--Data Category: simple nucleotide variation--Data Type: Masked Somatic Mutation. A dataset containing 431 samples is downloaded, including sample information, gene name and ID, chromosomal location, mutation type, and base change details. Stringent selection criteria were applied to ensure the inclusion of samples with complete clinical information and mutation profiles, establishing a robust foundation for the methodological approach used in this study.
2.2. Mutation Statistics of APC Gene
Mutation information of the APC gene from the TCGA database was downloaded, and mutations predicted by the VarScan2 variant aggregation and masking software were selected for analysis. A custom Perl script was used to systematically count the number of samples exhibiting mutations in the APC gene. This analysis focused exclusively on mutations causing alterations to the amino acid sequence. For each mutated sample, the specific mutation type (mutant vs. wild-type) was documented. Subsequently, the APC gene mutation landscape was visualized using the R package GenVisR, and the results are presented as a waterfall plot for enhanced graphical representation and interpretation.
2.3. TMB
TMB was calculated by determining the total number of somatic gene encoding errors, base substitutions, insertions, or deletions detected per million bases in the tumor tissue. The samples were categorized into wild-type (wild) and mutation groups based on the mutation status. TMB for each group was computed as the nonsynonymous protein-coding mutation count divided by the total sequencing exonic length (estimated at 38 Mb as the size of exons), excluding gene mutations not altering amino acids. Subsequently, a t-test was used to assess the correlation between APC gene mutations and TMB. The results were visualized using R packages such as grid, cowplot, and patchwork to create a box plot, providing a graphical representation of the findings.
2.4. MSI Analysis
Compared to normal tissues, tumor tissues exhibit the emergence of new microsatellite alleles at specific loci due to the insertion or deletion of repeat units. This phenomenon is indicative of MSI and is attributed to functional deficiencies in DNA mismatch repair in tumor tissues. Such changes can serve as clinically relevant tumor biomarkers. We used the R package plyr to compute the number and percentage of MSI occurrences in patients with STAD for both APC-mutated and wild-type groups. A bar plot was generated to objectively visualize and interpret the results. The means from matched samples were compared using paired Student’s t-test or Wilcoxon matched-pairs signed-rank test.
2.5. Survival Analysis and Independent Prognostic Analysis of APC Gene Mutations
We further evaluated the relationship between APC gene mutations and clinical prognosis. For this, the R packages survival and survminer were utilized to conduct Kaplan–Meier survival analysis. The results were represented as survival curves. Subsequently, the R packages limma and survival were employed for univariate and multivariate independent prognostic analyses, respectively. Forest plots were created to visualize the outcomes. A p value < 0.05 was considered significant.
2.6. GSEA
GSEA_V4.3.3 software (https://www.gsea-msigdb.org/gsea/index.jsp) is extensively utilized to analyze gene expression profile data. It aggregates differences in gene expression profiles to provide statistical significance related to specific pathways or gene sets. Moreover, it considers the overall trend of genes within a gene set rather than individual gene expression. The gene expression matrix of STAD samples and APC mutation data downloaded from the TCGA database were input into the GSEA software. A total of 1000 permutations were conducted to obtain more accurate p values. Pathways with an absolute value of the normalized enrichment score (NES) > 1 and a nominal p value < 0.01 were considered significantly enriched.
2.7. Immune Cell Infiltration
Immune cell infiltration levels in STAD samples were analyzed using the R package limma. Infiltration levels were computed, and a histogram was generated to visualize the results. In addition, pairwise comparisons of infiltration levels among different immune cells were conducted using the R package corrplot. This involved iteratively calculating correlation coefficients for different immune cell comparisons and creating a correlation heatmap to visualize the results. Differences with a significance level of p < 0.05 were considered significant.
2.8. Correlation Analysis of APC Gene Mutations and Immune-Infiltrating Cells
Then, we calculated the differences in immune cell infiltration among different APC gene mutation groups (wild-type and mutation groups) using the R package limma to establish the relationship between APC gene mutations and immune cell infiltration. Subsequently, the R package vioplot was used to visualize the results using violin plots. Differences with a significance level of p < 0.05 were considered significant.
2.9. APC Gene Mutation and Drug Sensitivity Analysis
The Genomics of Drug Sensitivity in Cancer (GDSC), developed by the Wellcome Trust Sanger Institute in the United Kingdom, has been used to compile drug sensitivity and response data for tumor cells, constituting the largest public database in this domain. The term “APC-mut” is input into the search box, and the GDSC dataset is selected along with GC tissues (STAD) to identify drugs exhibiting significant selectivity for APC gene mutations. The results were objectively visualized using volcano plots and scatter plots for clarity.
3. Results
3.1. Mutation Status of the APC Gene
The TCGA-STAD cohort consisted of 17,089 mutated genes and 1,87,748 somatic mutations (Supporting Tables 1 and 2). Figure 1 depicts the mutation landscape of the APC gene. Among the 52 samples with APC gene mutations (Supporting Table 3), 46 samples (88%) manifested nonsynonymous mutations in the APC gene, thereby altering amino acid sequences.
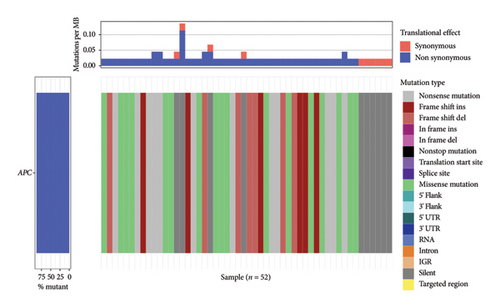
3.2. Correlation Between APC Gene Mutations and TMB and MSI
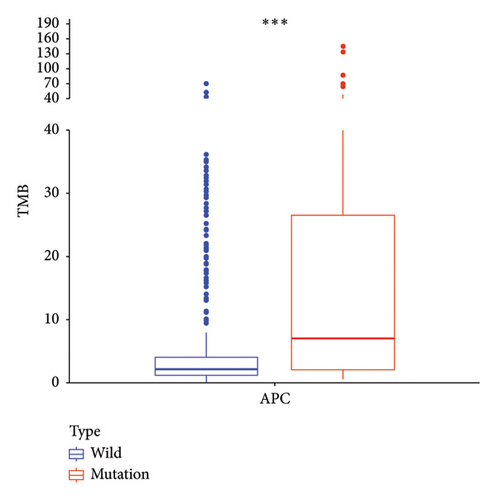
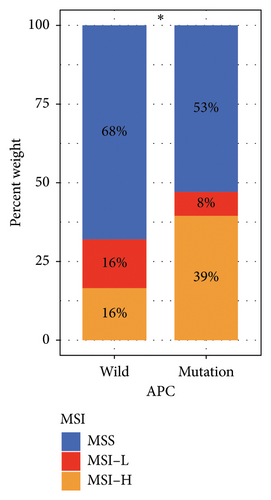
The computed TMB for each STAD sample is detailed in Supporting Table 4. Figure 2(a) depicts that TMB in samples with APC gene mutations is significantly higher than that in wild-type samples (p < 0.001). Subsequently, the MSI status in APC wild-type and mutation groups was analyzed. As depicted in Figure 2(b), the percentage of microsatellite stable (MSS) was higher in the wild-type group (68%), whereas the percentage of MSI-H was higher in the mutation group (39%), and this difference was significant (p < 0.05).
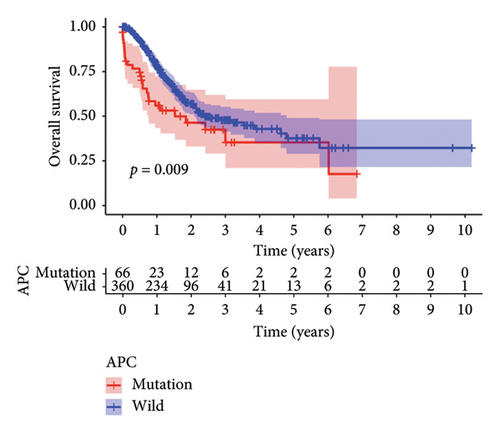
3.3. Verification of Clinical Prognosis of APC Gene Mutations
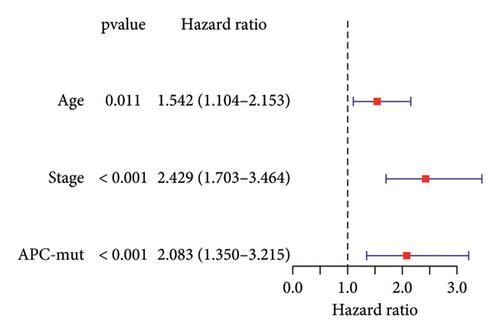
We next validated the correlation between APC gene mutations and the clinical prognosis of STAD patients by conducting survival analysis and independent prognostic analysis. The results demonstrated a correlation between APC mutations and the survival rate of STAD patients, with a poorer prognosis observed in APC-mutated patients compared to APC-wild patients (p = 0.009) (Figure 3(a)). Furthermore, both univariate and multivariate independent analyses revealed hazard ratio values > 1, with p values < 0.05 in APC mutation (Figures 3(b) and 3(c)). This suggests that APC mutation can serve as an independent prognostic factor for predicting the clinical prognosis of patients with STAD.

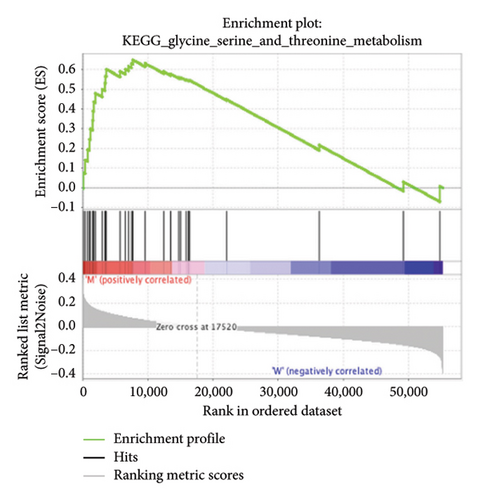
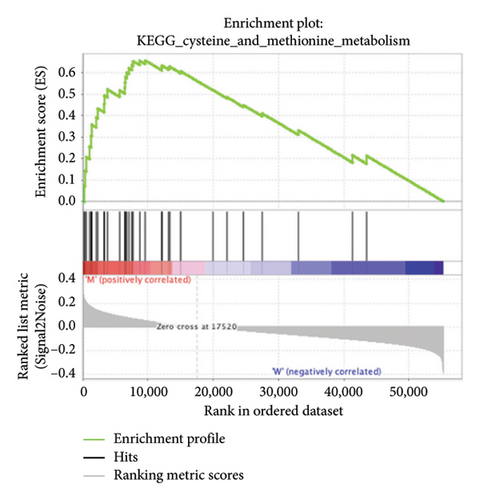
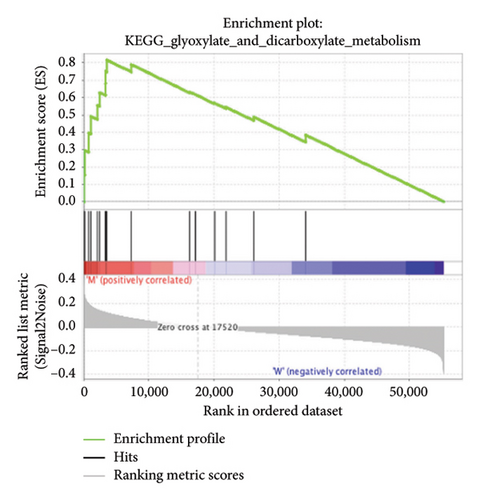
3.4. Enriched Pathways Associated With APC Gene Mutations
The pathological function of APC gene mutations in the metabolic pathways of STAD was studied via the GSEA. As depicted in Figure 4, the APC gene mutation group exhibited significant enrichment in different metabolic pathways, including amino acids, RNA, and gene metabolism (|NES| > 1 and nominal p value < 0.01). These pathways displayed enrichment for GLYCINE_SERINE_AND_THREONONE, CYSTEINE_AND_METHIONINE_METABOLISM, GLYOXYLATE_AND_DICARBOXYLATE_METABOLISM, PROTEASOME, PYRIMIDINE_METABOLISM, SNARE_INTERACTIONS_IN_VESICULAR_TRANSPORT, RNA_DEGRADATION, RNA_POLYMERASE, and ARGININE_AND_PROLINE_METABOLISM.
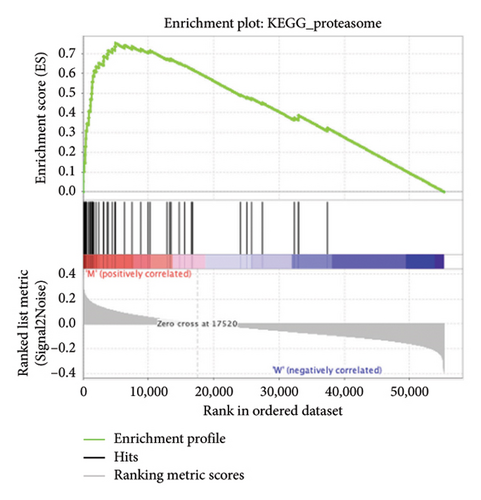
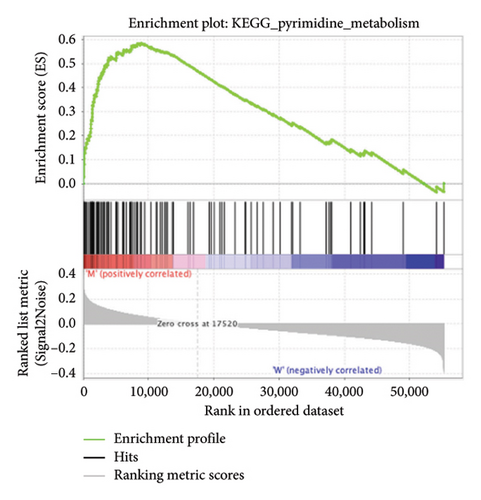
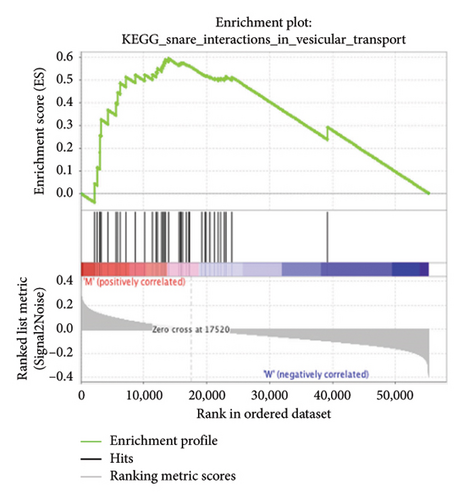
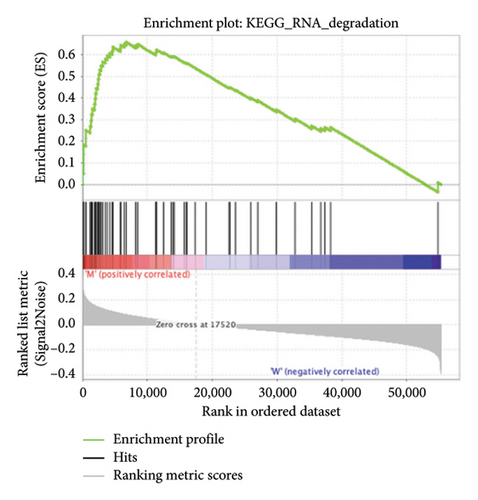
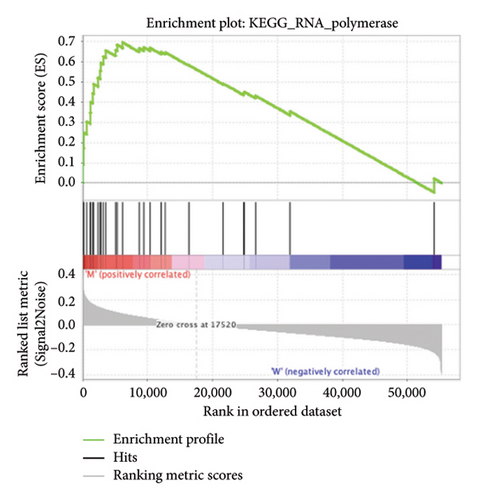
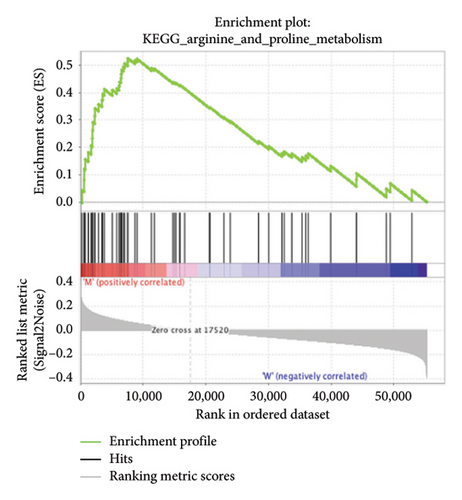
3.5. Immune Cell Infiltration and Their Correlation
The CIBERSORT algorithm was used to conduct an immune cell infiltration analysis for all STAD samples. Figure 5(a) illustrates the immune cell infiltration status among different samples. The detailed proportions of 22 immune cell infiltrations in each sample are depicted in Supporting Table 5. In addition, a correlation matrix for the infiltration levels of 22 immune cells was obtained through a correlation analysis in Figure 5(b).
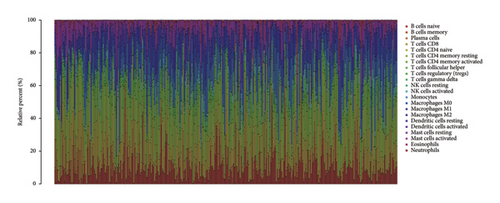
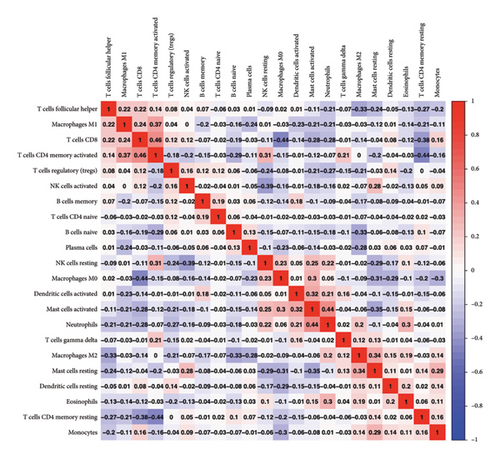
3.6. Difference in Immune Cell Infiltration in Different Groups of Mutations
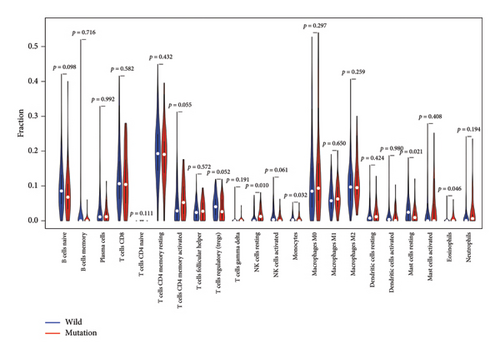
Differences in the proportions of the 22 immune cell subtypes between the APC gene wild-type and mutation groups were identified through correlation analysis. As shown in Figure 6, compared to the wild-type group, the mutation group exhibited a higher infiltration proportion of natural killer (NK) cells resting and eosinophils, whereas a lower infiltration proportion of monocytes and resting mast cells was observed (p value < 0.05).
3.7. STAD Cells Carrying APC Mutations Are Sensitive to the Drug AZD5991
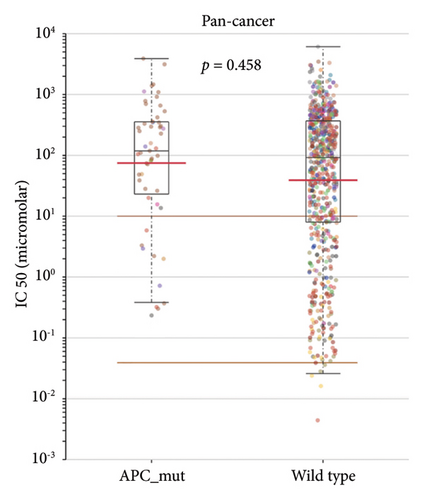
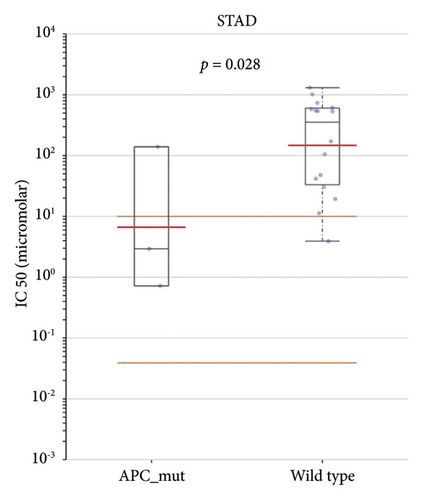
Then, we investigated whether STAD patients carrying APC mutations showed potential for better drug selectivity by searching the GDSC database. Preliminary research reported no significant difference in the IC50 values of AZD5991 between APC mutation and wild-type groups across different cancers (p = 0.458) (Figure 7(a)). However, AZD5991 demonstrates significant sensitivity in STAD patients with APC mutations (p = 0.028) (Figures 7(b) and 7(c)). Current studies suggest that AZD5991 is an effective Mcl-1 inhibitor with high selectivity. It directly binds to Mcl-1, rapidly inducing apoptosis in cancer cells by activating the Bak-dependent mitochondrial apoptotic pathway. Thus, AZD5991 may serve as a potential antitumor drug for STAD patients carrying APC mutations.
4. Discussion
In this study, we found that approximately 80% of STAD samples had nonsynonymous mutations. A significant difference was noted in TMB, MSI, and clinical prognosis between APC mutation and wild-type groups (all p < 0.05). The immune cell infiltration analysis revealed a higher infiltration proportion of NK cells resting and eosinophils, and a lower infiltration proportion of monocytes and mast cells resting (p value < 0.05) in the APC mutation group. AZD5991 exhibited significant sensitivity in STAD patients with APC mutations (p = 0.028).
Current studies suggest that AZD5991 is an effective Mcl-1 inhibitor with high selectivity [26, 27]. Mcl-1, a member of the Bcl-2 protein family, has been known to promote cell survival in several cancers by preventing apoptosis induction [26]. High expression of Mcl-1 causes tumorigenesis and resistance to anticancer therapies, highlighting the potential of Mcl-1 inhibitors as anticancer drugs [28]. AZD5991 directly binds to Mcl-1, rapidly inducing apoptosis in cancer cells by activating the Bak-dependent mitochondrial apoptotic pathway [26, 29, 30]. This action increases the permeability of the outer mitochondrial membrane, releasing apoptotic factors such as cytochrome c into the cytoplasm and activating downstream apoptotic signals [31, 32]. Consequently, AZD5991 rapidly induces apoptosis in cancer cells, exhibiting high selectivity and potency, particularly against cells with specific mutations [33]. In summary, AZD5991 demonstrates great potential as an Mcl-1 inhibitor for cancer treatment, especially for patients with STAD carrying APC mutations. Future studies and clinical trials will further explore its clinical value and offer new options for personalized treatment.
The impact of APC mutations on the prognosis was elucidated, and the differences in immune cell infiltration and TMB between the mutation and wild-type groups were compared. Our findings revealed that patients with APC mutations had a worse prognosis than those with APC wild-type (p = 0.009), and the percentage of MSS was higher in the wild-type group (68%), whereas the percentage of MSI-H was higher in the mutation group (39%), and this difference was statistically significant (p < 0.05). The mutation group exhibited a significantly higher TMB than the wild-type group (p < 0.001), and the percentage of MSI-H was significantly higher in the mutation group than in the wild-type group (p < 0.001). A high TMB indicates an elevated number of mutations in the tumor tissue [34]. Specifically, a high TMB signifies a greater number of mutations per million bases, reflecting genomic instability in tumor cells [35]. Elevated TMB is often associated with increased sensitivity of tumor cells to immunotherapy, as it may generate more tumor-specific antigens, triggering a robust immune response [36–38]. Previous studies have reported that APC mutations could be associated with poor outcomes for immunotherapy in patients with CRC, regardless of their MSI status [39]. Usui et al. reported that germline pathogenic variants in nine genes (APC, ATM, BRCA1, BRCA2, CDH1, MLH1, MSH2, MSH6, and PALB2) were associated with the risk of GC [40].
MSI has emerged as a primary method for molecular subtyping of GC. In recent years, the role of the MSI status in predicting chemotherapy response has been extensively studied. For instance, a retrospective study published in 2020, focusing on stage II/III MSI-H/dMMR patients with GC, predicted the efficacy of adjuvant therapy by establishing a novel immune score system (ISSGC). The study reported that patients with MSI-H/dMMR GC had a longer overall survival after adjuvant chemotherapy (HR: 0.59 (0.38–0.93)] [41]. A meta-analysis of seven studies to explore the prognostic impact of adjuvant chemotherapy reported that patients with MSI-H/dMMR could benefit from adjuvant chemotherapy, with estimated HRs of 0.56 (95% confidence interval (CI): 0.36–0.87; p = 0.010) for DFS and 0.62 (95% CI: 0.45–0.83; p = 0.002) for OS [42].
The importance of immune cell infiltration in GC with APC mutant was studied via the CIBERSORT analysis that compared the differences in APC gene wild and mutant groups. The immune cell infiltration analysis revealed a higher infiltration proportion of NK cells resting and eosinophils and a lower infiltration proportion of monocytes and resting mast cells (p value < 0.05) in the APC mutation group. Eosinophils are a type of white blood cells, specifically granulocytes, that play a role in the immune system [43]. NK cells are a type of immune cells with the capability to kill tumor cells and combat infections. In their resting state, these cells are not actively engaged in immune functions. Under specific immune activation conditions, these cells are activated and perform their immune functions [44, 45]. Monocytes are involved in several immune functions, including phagocytosis (engulfing and digesting pathogens), antigen presentation, and the production of cytokines [46, 47]. They contribute to the defense against infections and the regulation of inflammatory responses in the body [48]. Changes in the infiltration proportion of immune cells can provide insights into the immune landscape and potential immune responses in a specific context of gastric adenocarcinoma.
This study benefitted from comprehensive data derived from the TCGA database, incorporating gene expression, clinical, and mutation information, which resulted in a robust and extensive analysis. The multidimensional approach, exploring gene mutations, TMB, MSI, immune cell infiltration, and clinical prognosis, ensured a thorough investigation. Diverse methodologies such as GSEA and drug sensitivity analysis provide a comprehensive understanding of the biological and clinical implications of APC gene mutations. While this study leverages the richness of TCGA data, offering a detailed exploration of several aspects, it is essential to acknowledge certain limitations. The reliance on a single database may introduce certain biases, and potential homogeneity in the dataset could impact the generalizability of findings. Furthermore, the use of retrospective data and the lack of randomization in patient sample selection could introduce selection bias. The absence of experimental validation using cell lines or animal models is another limitation. Future laboratory experiments and clinical trials are necessary to validate the effects of gene mutations on tumor biology and drug sensitivity and verify the conclusions. In addition, long-term follow-up of patients should be conducted to collect more comprehensive clinical information, including treatment response and survival status, to completely assess the clinical significance of gene mutations. In future studies, we will aim to incorporate diverse datasets and multi-centric cohorts to enhance the robustness and applicability of our results. We will also strive to implement strategies to mitigate the biases associated with a retrospective data analysis. We believe that despite these limitations, our current findings provide a valuable foundation for understanding the sensitivity of AZD5991 in APC mutation-positive STAD patients.
5. Conclusion
This study revealed a high prevalence of APC gene mutations in gastric adenocarcinoma, which was associated with high TMB, MSI-H, and poor clinical prognosis. In addition, the APC mutation group reported significant differences in multiple metabolic pathways and immune cell infiltration, whereas AZD5991 exhibited potential antitumor effects in patients with APC mutations.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Y.H. designed the study. W.Z., Z.H., and J.Y. performed the experiments. All authors read and approved the final manuscript. C.Z. and J.Q. have contributed equally to this work. C.Z. and J.Q. are the co-first authors.
Funding
This work was supported by grants from the key research project of Ningxia Hui Autonomous Region: Application of gene mutation and abnormal expression of circular RNA in risk prediction and early diagnosis of gastric cancer in adenomatous polyposis coli (Fund number: 2021BEG03085), Ningxia Natural Science Foundation: Study on the effect of MiR-153 on apoptosis of GC cells by regulating Wnt signaling pathway combined with the APC gene (Fund number: 2019A0228), Project of National Natural Science Foundation of China: Study on the role and mechanism of sodium ion channel Nax in the pathological process of pulmonary hypertension (Fund number: 82260016), and the key research and development project of Ningxia Hui Autonomous Region: Development of bifidobacterium-activated intestinal epithelial γδT cell technology and its application in the treatment of colorectal tumors (Fund number: 2021BEG03086).
Acknowledgments
This work was supported by grants from the Key Research Project of Ningxia Hui Autonomous Region: Application of Gene Mutation and Abnormal Expression of Circular RNA in Risk Prediction and Early Diagnosis of Gastric Cancer in Adenomatous Polyposis Coli (Fund number: 2021BEG03085), Ningxia Natural Science Foundation: Study on the effect of MiR-153 on apoptosis of GC cells by regulating Wnt signaling pathway combined with APC gene (Fund number: 2019A0228), Project of National Natural Science Foundation of China: Study on the role and mechanism of sodium ion channel Nax in the pathological process of pulmonary hypertension (Fund number: 82260016) and Key Research and Development Project of Ningxia Hui Autonomous Region: Development of bifidobacterium-activated intestinal epithelial γδT cell technology and its application in the treatment of colorectal tumors (Fund number: 2021BEG03086).
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




