RuCo@P Core-Shell Nanoparticles Filled with Carbon Nanotubes for Highly Effective Catalytic Hydrolysis of Ammonia Borane
Abstract
The doping of nonmetals in multiphase metal catalysts can effectively improve the catalytic performance and reduce the catalyst cost. In this study, we synthesized transition metal phosphide (RuCo@P/CNT) catalysts loaded on carbon nanotubes (CNT) using sodium borohydride (NaBH4) as the reducing agent. The microstructure and phase composition of RuCo@P/CNT nanoparticles were investigated using X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), transmission electron microscopy (TEM), and BET. RuCo@P/CNT nanoparticles show superior catalytic activity and cycle stability in the catalytic ammonia borane hydrolysis process compared to Ru/CNT and RuCo/CNT nanoparticles, retaining 65.74% of their initial catalytic activity after 5 reaction cycles. The test yielded values for turnover frequency and activation energy of 327.33 min-1 and 36.77 kJ·mol-1, respectively. Additionally, a kinetic isotope effect value of 2.61 for H2O/D2O showed that O-H bond breaking in proportional acceptor water is the decisive step in the dehydrogenation of ammonia borane, and based on this discovery, a specific mechanism for the catalytic hydrolysis of AB by RuCo@P NPs is postulated.
1. Introduction
Transition metal phosphides (TMPs) are highly regarded for their excellent activity and long-term durability. Its particular structure gives it properties similar to those of precious metals and is known as a “quasi-platinum catalyst” with a wide range of catalytic applications, having been extensively applied in hydrodesulfurization and hydrogen evolution reactions [16–18]. Recently, numerous studies have investigated the effective TMPs in the catalytic dehydrogenation of AB. Yang et al. [19] synthesized a Ni-Fe-P/Ni ternary catalyst using a one-step chemical plating method, which exhibited better catalytic activity compared to the Ni-Fe/Ni catalyst. Wang et al. [20] reported that the noble metal-free Co@Co2P/N with CNT as a carrier has better catalytic performance than the Co@Co2/N catalyst formed by undoped nonmetallic P. Qu et al. [21] obtained good outcomes getting NiCoP/OPC-300 loaded into oxygen-doped porous carbon transformed with ZIF-67. Although transition metal phosphides are easy to obtain and have good catalytic efficiency, precious metals still have unparalleled advantages in the field of catalytic AB hydrogen evolution. The incorporation of precious metals as cocatalysts stands out as a potent strategy for significantly enhancing the activity of heterogeneous catalysts. Asim et al. [22] reported catalysts Au/Ni2P and Au/CoP with high synergistic effects between gold nanoparticles and metal phosphides. Compared with the original Ni2P and CoP, the activity increased by 4.8 and 1.7 times, respectively. Qu et al. [11] synthesized transition metal phosphide nanoparticles (Pd@Co@P) supported by reduced graphene oxide using a one-step in situ synthesis method. It was found that the synergistic electronic interaction between metal phosphides can effectively improve catalytic performance. Wan et al. [23] achieved a visual-driven strategy of generating H2 through AB hydrolysis using NiPt nanoparticles supported by phosphorus-doped titanium dioxide (NiPt/P-TiO2) as photocatalysts. Ru is recognized as one of the best metal catalysts for the hydrolysis of AB [24]; however, there are few researches on Ru-based phosphides for this reaction. This scarcity serves as inspiration for our investigation into Ru-based transition metal phosphide catalysts.
Dispersion of metal nanoparticles (NPs) is a crucial factor in the activity of catalysts. Therefore, surface modification of catalyst systems using porous matrices can control the stability of dispersions and improve catalytic activity by increasing the number of active sites [25]. Carbon nanotubes (CNT) are widely used in catalysis because of their excellent electron conduction properties, large specific surface area, and good stability, making them ideal catalyst carriers. The application of CNT in loaded Ni, Co-based alloy catalysts has been reported extensively recently [26]. Wang et al. [20] embedded uniformly dispersed Co@Co2P nanoparticles into N-doped carbon nanotube polyhedral, which exhibited excellent catalytic performance. Liu et al. [27] synthesized a nitrogen-fixing carbon nanotube that encapsulates Fe and Co nanoparticles under a nitrogen atmosphere. Chen et al. [28] loaded the Pt-Ru nanoparticles on carbon nanotubes and explored the synergistic effect of bimetallic catalysts.
In this research, we used a one-pot coreduction technique to synthesize RuCo@P/CNT. Synthetic product Ru1Co10@P5/CNT catalyzes AB hydrolysis to produce hydrogen at 25°C with a turnover frequency value (TOF) value of 327.33 min-1, which is twice of the Ru1Co10/CNT catalyst (159.61 min-1), and its reaction activation energy (Ea) is 36.77 kJ·mol-1. Thus, Ru1Co10@P5/CNT has high catalytic activity and good kinetic performance.
2. Experimentation
2.1. Chemical Substances
There was no purification of any chemicals; they were all commercially available. The reaction solvent is deionized water. The following are the chemical substances used: ruthenium trichloride (RuCl3, Merck Reagent, 98%), cobalt chloride hexahydrate (CoCl2∙6H2O, Merck Reagent, 98%), ammonia borane (NH3BH3, Merck Reagent, 98%), sodium hypophosphite (NaH2PO2, Merck Reagent, 99%), anhydrous ethanol (C2H5OH, Sinopharm Reagent, 99.8%), sodium borohydride (NaBH4, Sinopharm Reagent, 96%), and carbon nanotubes (CNT, Merck Reagent, 98%).
2.2. Synthesis of RuCo@P/CNT Catalysts
Initially, a 25 mL double-necked flask was utilized to disperse 10 mg of carbon nanotubes (CNT) into 5 mL of deionized water through a five-minute sonication process, ensuring uniform dispersion. Subsequently, a mixture was prepared by adding 1.25 mL of a cobalt chloride solution (CoCl2, 0.04 mol·L-1), 1 mL of a sodium hypophosphite solution (NaH2PO2, 0.05 mol·L-1), and 1 mL of a ruthenium chloride solution (RuCl3, 0.005 mol·L-1) to the flask. The mixture was then vigorously stirred at a rate of 500 r/min.
To introduce the reducing agent NaBH4 (1 mol·L-1), a continuous pressure-falling funnel was connected to one neck of the flask. The other neck was attached to a gas tube, which was employed to collect the gas generated during the reaction. Throughout the reduction process, the magnetic stirrer was consistently operated at 500 rpm, while the temperature of the water bath was maintained at 25°C.
Subsequently, the RuCo@P/CNT catalyst was synthesized through a series of steps involving centrifugation and multiple washes using anhydrous ethanol and deionized water. The resulting particles underwent an additional drying phase within a vacuum furnace, held at a temperature of 25°C, for a duration of eight hours.
Finding the RuCo@P/CNT nanocatalyst with the highest catalytic effect requires choosing the appropriate Ru, Co, and P ratios. We synthesized RuCo@P/CNT nanoparticles with different material ratios by the same method as above under the condition of maintaining n(Ru): n(AB) = 0.05. The molar ratios of Co/Ru were altered across a range of values, including 0, 2.5, 5, 7.5, 10, 12.5, and 15. Similarly, the molar ratios of P/Ru were modified within the values of 2.5, 5, 7.5, 10, and 15.
2.3. Material Characterization
A JEM-1400Plus 120 kV transmission electron microscope was employed to capture the TEM pictures. An Empyrean X-ray diffractometer scanning at a two angle between 5° and 80° was used to acquire XRD patterns. XPS spectra were acquired with a Thermo Scientific ESCALAB Xi+X-ray photoelectron spectroscopy. The element content was verified by ICP-OES (Prodigy 7). BET was scanned with the ASAP 2020 to test specific surface area and pore volume.
2.4. Catalytic Activity Tests
Ru1Co10@P5/CNT catalyst was introduced to the reaction vessel, keeping its molar proportion of catalyst and AB constant, and four different temperatures, spanning from 298 K to 313 K, were carefully chosen to conduct the hydrolytic dehydrogenation reaction. Subsequently, the obtained experimental data were employed to calculate the reaction rate (k) as well as activation energy (Ea) for the catalyst through appropriate calculations and analysis. Similarly, we tested the number of reaction steps of ammonia borane-catalyzed hydrolytic dehydrogenation by changing the catalyst concentration value. The reaction temperature was set at 25°C and 1 mmol of AB was fixed.
In order to figure out the decisive step within the hydrolytic dehydrogenation of AB, the dehydrogenation experiments of AB were carried out with D2O instead of H2O, and the experimental conditions remained unchanged to obtain the graphs of the dehydrogenation of AB catalyzed by both, respectively. The linear part of the reactions was taken to calculate the reaction rate k, and the kinetic isotope effect values (kH2O/kD2O) were obtained by comparison.
3. Results and Discussions
3.1. Characterization
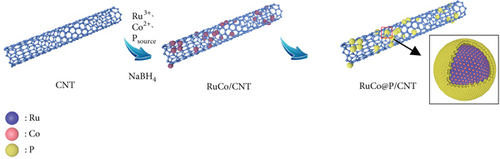
By adding NaBH4 to the solution of RuCl3, CoCl2, NaH2PO2, and CNT at room temperature, we obtained CNT-loaded RuCo@P NPs. NaH2PO2 is a kind of weak Bronsted acid, and when the content is high, it will easily hydrolyze to produce NaOH [29]. The reducibility of NaBH4 sodium borohydride will be reduced in the presence of NaOH [30]. Therefore, the reduction sequence of metal ions is changed, providing conditions for forming a core-shell structure with higher catalytic activities with stronger stabilities [25, 31, 32]. Ru3+ has the highest reduction potential of the three ions (E0 (Ru3+/Ru) = +0.40 eV vs. SHE), followed by Co2+ (E0 (Co2+/Co) = −0.28 eV vs. SHE), and H2PO2- has the lowest reduction potential ( eV vs. SHE). Due to the reduction of the reducibility of NaBH4, Ru3+ and Co2+ with higher reduction potential were reduced firstly as the core. The formed M-H (M = Ru, Co) with strong reducibility reduced the H2PO2- with the lowest reduction potential subsequently to form the shell. Figure 1(a) shows the formation process of the RuCo@P/CNT NPs.

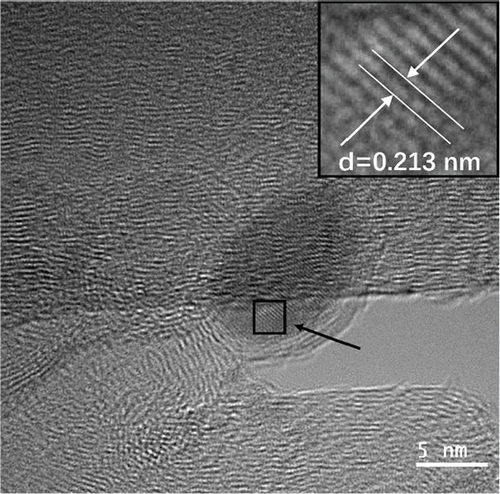
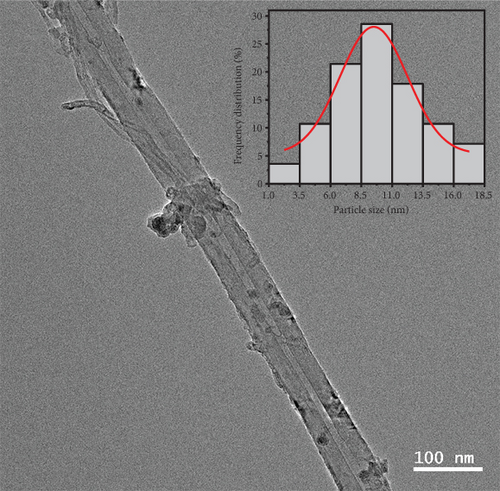
Transmission electron microscopy (TEM) was used to examine the catalyst’s morphological properties and the microstructure pictures of Ru1Co10@P5/CNT shown in Figures 1(b) and 1(c). As shown in Figure 1(b), Ru1Co10@P5/CNT nanoparticles are uniformly distributed on the carbon nanotubes, which indicates that the carbon nanotubes can well prevent the aggregation of nanoparticles. The wall stripe of the carbon nanotube is visible in Figure 1(c). The Ru1Co10@P5 nanoparticle on CNT has a core-shell structure, and the core part is measured to have a lattice stripe of 0.213 nm, which is identified as the crystal plane of Ru (002), indicating that Ru3+ was reduced firstly due to its high electric potential. The absence of lattice stripes in Co is probably related to the fact that it often exists in an amorphous form. Figure 1(d) shows the particle size distribution of Ru1Co10@P5/CNT nanoparticles, which was measured to have an average particle size of 8.9 nm.
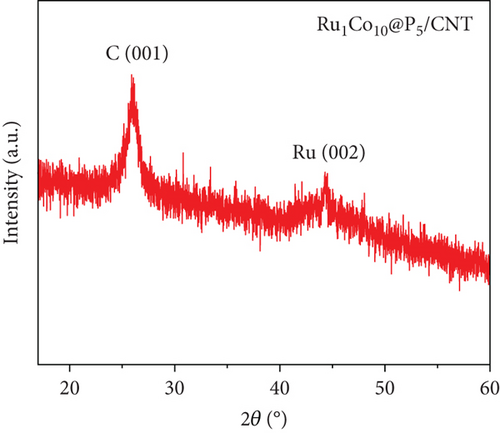
Figure 2 shows the XRD pattern of Ru1Co10@P5/CNT after calcination. The peaks at 26° and 44.3° correspond to the C (001) and Ru (002) (PDF#06-0663) crystalline plane of Ru1Co10@P5/CNT products, respectively. The XRD results do not show peaks of Co and P, indicating that Co and P exist in the amorphous form [11], which is also consistent with the TEM results.
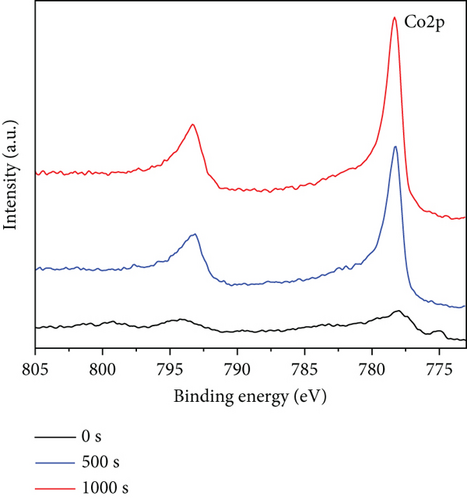
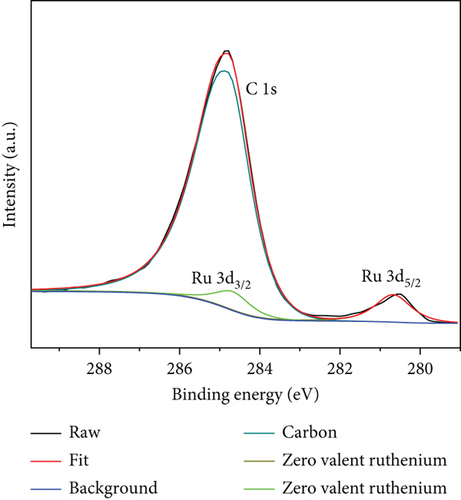
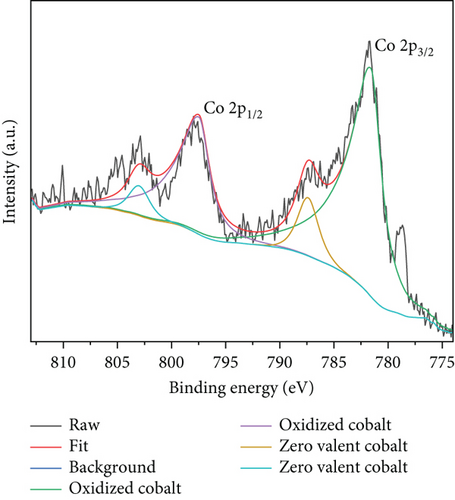
The precise chemical makeup and electronic states of the Ru1Co10@P5/CNT catalysts and the synthesis mechanism were further clarified using X-ray photoelectron spectroscopy (XPS). Figures 3(a)–3(c) show the XPS spectra of Ru, Co, and P elements after etching for 0 s, 500 s, and 1000 s, respectively. Due to the interference of the C 1 s peak, the peak around 285 eV cannot be used as a reference basis for judging the elemental content, and it can be seen that there is no Ru 3d5/2 peak on the surface of nanoparticles in Figure 3(a). After 500 s and 1000 s of surface etching, the material on the nanoparticles’ surface is removed, while the peak at around 280.2 eV appears. It can be judged that the metal Ru does not exist in the outermost layer of the nanoparticles but is basically distributed in the core part, and it is also consistent with the lattice spacing measured in the TEM imaging. Similarly, according to Figure 3(b), the XPS spectra of Co 2p can be seen that after 500 s and 1000 s of surface etching, the peaks of Co 2p1/2 and Co 2p3/2 appear significantly enhanced in height and area. It can be judged that only a small amount of Co exists on the surface of the nanoparticles, and most of it is distributed in the core part, like Ru. In addition, according to the elemental content analysis of the XPS outcomes, the atomic content of Ru and Co within the sample increased by 20% and 30%, respectively, after 1000 s of etching compared with that before etching, which indicates that the Ru and Co elements increased significantly during the etching process. This further confirms that Ru and Co are preferentially reduced and attached to the CNT in the form of a core, guiding P with lower reduction potential to be coated on the surface in the form of a shell. Moreover, since the TEM images show the lattice spacing of the inner layer of nanoparticles is Ru, it can be concluded that Co primarily exists in an amorphous form.
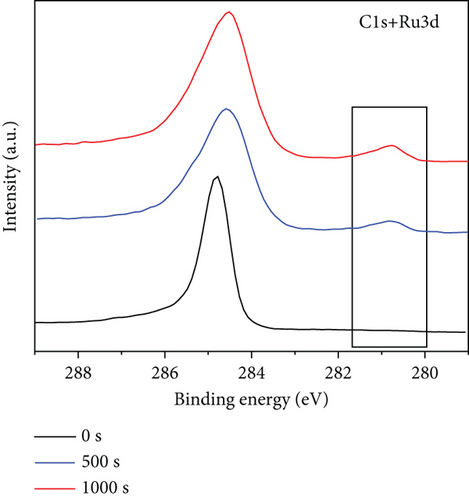
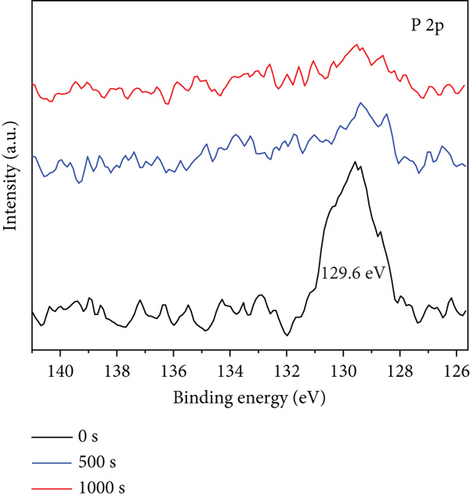
In Figure 3(c) (XPS pattern of P 2p), it can be seen that the characteristic peak of P 2p at 129.6 eV appears at 0 s of etching, indicating the oxidation of P to form P3+ during the sample preparation. After 500 s of surface etching, the characteristic peak at 129.6 eV has been less prominent, and after 1000 s of surface etching, the characteristic peak at 129.6 eV has almost disappeared, which further indicates that the P element is distributed in the outermost layer of this nanoparticle, which coincides with TEM outcomes.
The outcomes of TEM, XRD, and XPS reveal that the reduction sequence changes due to the reduced NaBH4, Ru3+ (E0(Ru3+/Ru) = +0.40 eV vs. SHE), and Co2+ (E0(Co2+/Co) = −0.28 eV vs. SHE) are first reduced to RuCo alloy nanoparticles due to the relatively high reduction potential and then used as in situ seeds, inducing the formation of RuCo-H, a highly reducing intermediate. The generated RuCo-H then reduces H2PO2- (E0 eV vs. SHE) exhibiting the potential of low reduction to P0, so that P, as the shell in the core shell, continuously grows on the surface with RuCo alloy as the core.
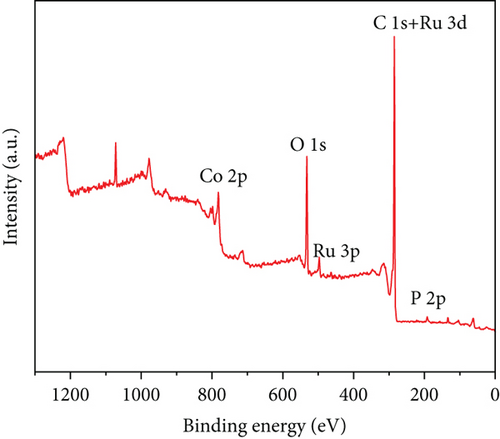
The entire spectrum of Ru1Co10@P5/CNT (Figure 3(d)) clearly shows the presence of Ru, Co, P, C, and O, which indicates that Ru, Co, and P are successfully loaded on the carbon nanotubes. The prominent Ru peak in the zero-valence state can be seen in Figure 3(e), indicating that the metal Ru is stable as a core. However, due to the close closeness of the C 1s and Ru 3d peaks at 284.5 eV, it took work to corroborate the existence of Ru. Contrarily, the XPS survey scan revealed a clear Ru 3p peak at around 463 eV, indicating the presence of Ru. Two peaks at 781.7 eV and 797.6 eV in the spectrum, which represent zero-valent Co, and two peaks at 787.3 eV and 803.1 eV, which represent the oxidized state of Co, are shown as the peaks of Co 2p in Figure 3(f). The outcomes suggest that partial oxidation of Co may have occurred during the synthesis and catalysis of the sample leading to the production of the oxidized state of Co [33, 34].
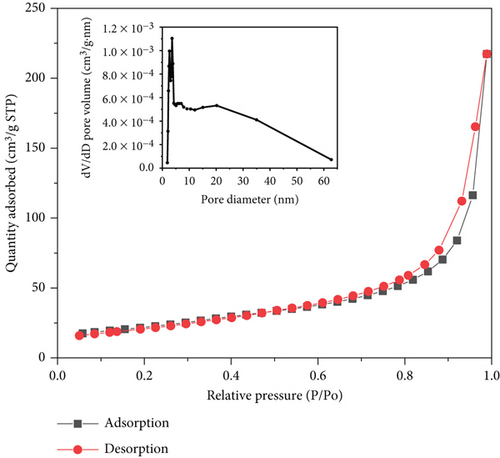
The textural properties of the nanoparticles were further tested by BET. The introduction of the CNT can appreciably increase BET surface area and mesopore volume of the nanoparticles [35]. Figure 4 shows the nitrogen adsorption curve of Ru1Co10@P5/CNT sample calculated using the BET model, and the material displays a typical type IV behavior, indicating that the Ru1Co10@P5/CNT NPs occupy the orifice of the CNT. The surface area of Ru1Co10@P5/CNT was calculated to be 72.528 m2·g-1 using the Brunauer-Emmett-Teller (BET) model [36]. The average pore size is 17.13 nm, concentrated at 2-20 nm, and determined as a mesoporous material.
3.2. Catalytic Activity of RuCo@P/CNT NPs
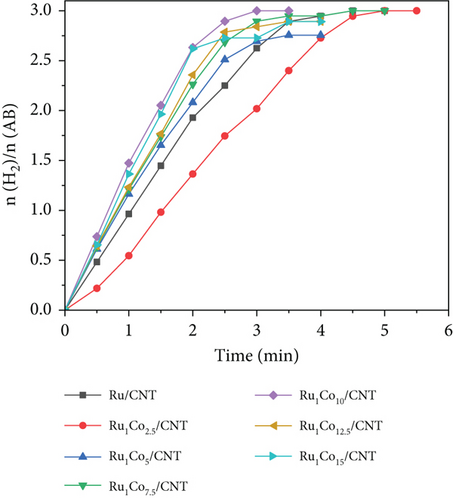
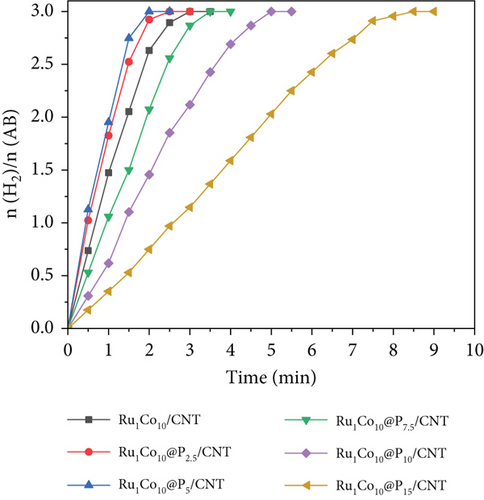
We synthesized several RuCo@P/CNT catalysts by adjusting the proportions of the three components and investigated the catalytic performance of each catalyst for AB hydrolysis separately. As illustrated in Figure 5(a), when the molar proportion of Ru to AB was maintained at 0.005, if no Co element was added, Ru/CNT catalyzed the total hydrolysis of AB in about 4.5 min. When the Co/Ru ratio was increased to 10, the decomposition of AB was completed in 3 min, and further increasing the Co/Ru ratio to 15 led to a prolonged reaction duration, indicating that the RuCo/CNT nanoparticles had a promoting effect on the hydrolysis of AB, while the optimal proportion of Co/Ru was 10. On this basis, the amount of phosphorus was varied by changing the amount of NaH2PO2 when P was selected for addition to the catalyst RuCo/CNT NPs. As revealed in Figure 5(b), the entire breakdown of AB through hydrolysis can be achieved within approximately 3 min by utilizing Ru1Co10/CNT as a catalyst while maintaining a consistent proportion of Ru to AB at 0.005, while the proportion of AB hydrolysis via catalysis was markedly enhanced subsequent to the introduction of P doping. Notably, the catalytic performance peaked when the P/Ru ratio was increased to 10, leading to the completion of the reaction in just 2 min, accompanied by a turnover frequency of 327.33 min-1. This TOF value surpasses numerous Ru-based and other noble metal catalysts outlined in Table 1. However, as the P content was further increased, and the catalytic activity experienced a decline. Finally, the Ru1Co10@P5/CNT catalyst exhibiting the highest catalytic activity was obtained. The mass loading of RuCo@P/CNT nanoparticles and on reduced CNT is 33.98 wt% which is calculated from the results of ICP-OES. In addition, the Ru content was determined to be 2.19 wt%, the Co content was determined to be 21.85 wt%, and the P content was determined to be 9.95 wt% by ICP-OES. The ICP-OES results showed that the content of Ru, Co, and P elements was 1 : 9.98 : 4.54, and the slightly lower P element ratio should be the decrease in purity caused by the dampness of NaH2PO2, with an error within an acceptable range.
| Catalyst | TOF (mol H2 m ol-1 M min-1) M = Pt, Ru, Pd, Ph |
Ea (kJ mol-1) | Ref. |
|---|---|---|---|
| Ru@f-CNTs | 764 | 35.68 | [41] |
| Rh/CNTs | 431 | 32 | [42] |
| Ru@Co/graphene | 344 | / | [43] |
| Ru@Ni/graphene | 339.5 | 36.59 | [44] |
| Ru(0)@MWCNT | 329 | 33 | [45] |
| Ru1Co10@P5/CNT | 327.33 | 36.77 | This study |
| Ru/g-C3N4 | 313 | 37.4 | [46] |
| Pt-Ru@PVP | 308 | 56.3 | [47] |
| Ru/TiO2 | 241 | 70 | [48] |
| Ru@SiO2 | 200 | 38.2 | [49] |
| Pd@Co@P/rGO | 127.57 | 39.05 | [11] |
| Ru@MIL-101 | 178 | 51.12 | [50] |
| Ru/SiO2-CoFe2O4 | 172 | 45.6 | [51] |
| RuCu/graphene | 135 | 30.39 | [52] |
| Ru@X-NW | 135 | 77 | [53] |
| Co | 44.2 | / | [54] |
| Co-P (at 30°C) | 10 | 22 | [55] |
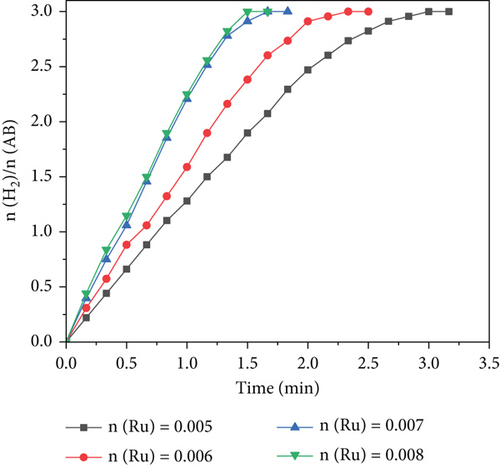
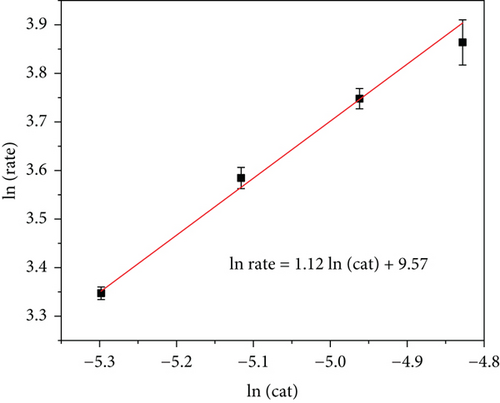
After identifying the RuCo@P/CNT nanoparticles that exhibited the most effective catalytic performance, further investigation was conducted to examine the reaction kinetics of the catalytic dehydrogenation process using the Ru1Co10@P5/CNT catalyst. Figure 6(a) presents the experimental outcomes obtained by varying the Ru concentrations (n(Ru) = 0.05, 0.06, 0.07, and 0.08). At a steady temperature of 25°C, 1 mmol of ammonia borane was added. The dehydrogenation rate constant (k) was calculated using the linear section of the curve. For a linear fit, we performed a logarithmic transformation of the catalyst concentration. As seen in Figure 6, the slope of the fitted curve was 1.12, indicating a first-order reaction mechanism for the hydrolysis of ammonia borane with the catalyst.
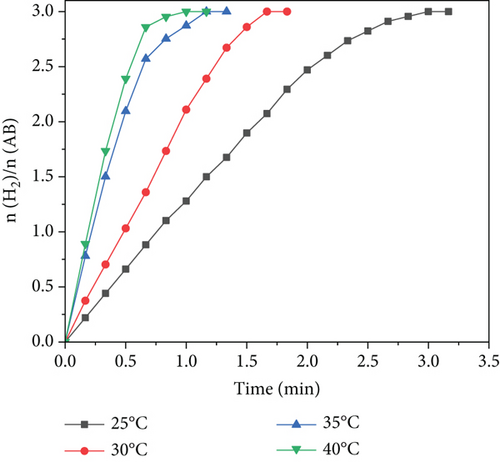
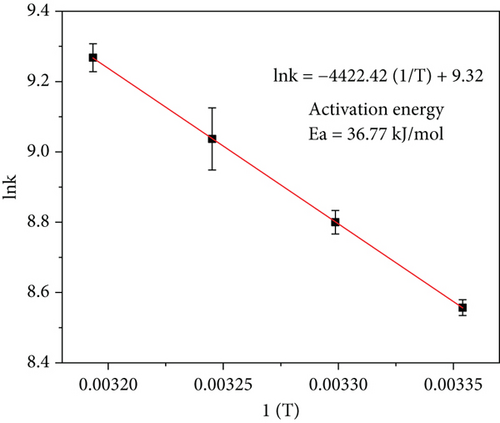
A suitable catalyst can effectively reduce the Ea of a chemical reaction, thus promoting the reaction products to reach the activated state and increasing the proportion of the reaction. Figure 7(a) shows the catalytic tests with Ru1Co10@P5/CNT catalyst at various temperatures (298, 303, 308, and 313 K). According to the Arrhenius formula, we must perform a logarithmic fit to the rate constant and the inverse of the temperature for the dehydrogenation reaction. By choosing the linear part of the experimental curve, we can calculate the rate constant k and obtain the fitting outcomes shown in Figure 7(b). The outcomes revealed that the Ea of Ru1Co10@P5/CNT catalyst was 36.77 kJ·mol-1, which was better than most of the reported ruthenium-containing catalysts (Table 1), indicating that our synthesized Ru1Co10@P5/CNT catalyst had suitable catalytic activities.
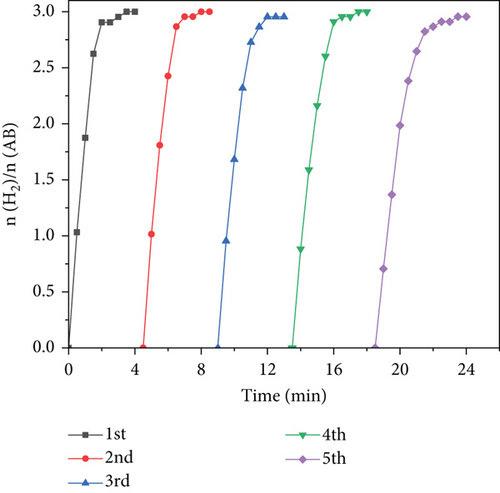
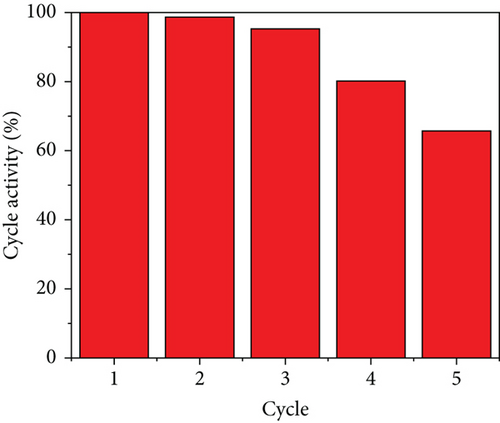
To decrease the frequency of catalyst replacements in actual practice, it is necessary to ensure a high degree of cycling stability of the catalyst. The cycling stability of Ru1Co10@P5/CNT in catalytic AB hydrolysis is shown in Figure 8. Ru1Co10@P5/CNT nanoparticles maintained 65.74% of the initial catalytic activity following the catalytic hydrolysis of ammonia borane for five consecutive cycles. In addition, the hydrogen conversion was also well maintained, indicating that Ru1Co10@P5/CNT nanoparticles can be cycled at least five times and possess good cycling performance.
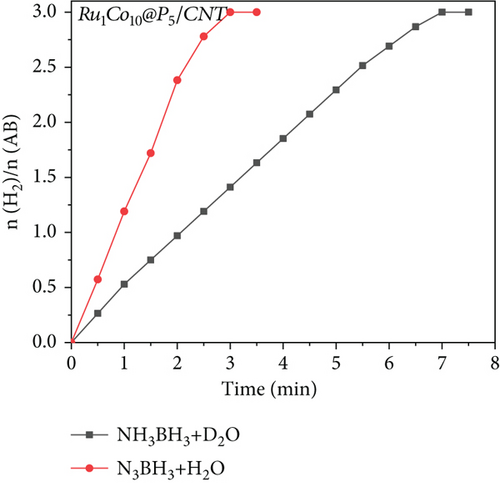
To further investigate the hydrolysis mechanism of Ru1Co10@P5/CNT-catalyzed ammonia borane, isotope experiments were conducted using D2O and H2O as reaction solutions, respectively (Figure 9). The dehydrogenation rate of ammonia borane in D2O was substantially lower than in H2O. This difference may be related to the higher molecular mass of D2O, which needs more energy to break chemical bonds. Indirect evidence from this phenomenon supports the primary role of this reaction in the O-H bond breakage of water. The linear portion of the corresponding reaction curves was computed to produce the reaction rate constant k, and the kinetic isotope effect value (kH2O/kD2O) was determined to be 2.61. This finding demonstrates the existence of a first-order kinetic isotope effect. It confirms that the synergistic catalytic breakage of the H-OH bond in water is the critical rate step in the hydrolysis of AB rather than the H-BH2NH3 bond. Combining the results, we can deduce the mechanism of Ru1Co10P5/CNT-catalyzed hydrolysis of ammonia borane [7, 37, 38], shown in Figure 10.
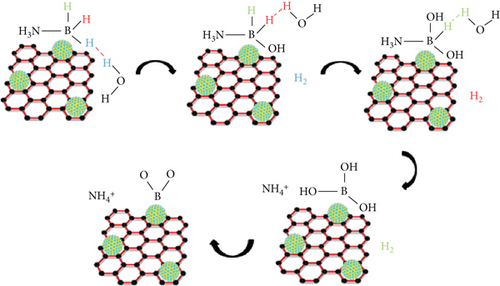
The surface of the RuCo@P nanoparticles is simultaneously bound to and activated in the earliest stages of the process when H2O molecules form the structure [H3NBH2H]∙∙∙H-OH [39] with AB. The B-H bond is initially cleaved during the activation process, while RuCo@P nanoparticles adsorb the H atoms in the B-H bond under synergistic effects. At the same time, the water molecule undergoes a continuous O-H break, in which the OH- replaces an H atom in the AB. Thus, NH3BH3 and H2O each provide an active H atom, which combines to form the product H2, while the reaction also produces the by-products BH(OH)2NH3 and B(OH)3 [40].
4. Conclusion
Summarily, transition metal phosphide-based core-shell Ru1Co10@P5/CNT catalysts were successfully synthesized in this study. We thoroughly investigated the catalytic activity, catalytic mechanism, and cyclic performance of the catalysts in the hydrolytic dehydrogenation of ammonia borane. The experimental outcomes revealed that NaBH4 was reduced by the hydrolysis of NaH2PO2, leading to the formation of RuCo core from Ru3+ ions and Co2+ ions and prompting H2PO2- reduction, which resulted in the growth of the shell. The Ru1Co10@P5/CNT catalyst exhibited an excellent conversion frequency (TOF) of 327.33 min-1 and activation energy of 36.77 kJ·mol-1, exhibiting excellent catalytic activity and cycling stability in the catalytic process. Moreover, few researchers have studied the catalytic effect of the combination of Ru, Co, and P on AB before this. The results indicate that Co doping can effectively improve the performance of Ru-based metal catalysts while reducing catalyst costs. The introduction of P alters the electronic structure between Ru and Co metal elements, making it easier for H to be adsorbed and desorbed on the surface of the nanoparticles, resulting in RuCo@P/CNT having superior performance compared to other Ru-based catalysts, and this simple synthesis method can be extended to other Ru-based bimetallic core-shell systems for wider applications.
This simple one-pot synthesis method can be used to synthesize other metal phosphide catalysts and further apply in the dehydrogenation of AB.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
We acknowledge the financial support of the Natural Science Foundation of Hubei Province (Grant No. 20231j0195), the National Natural Science Foundation of China (Grant No. 21805217), and the Fundamental Research Funds for the Central Universities (WUT: 2019IVB014 and 2021IVA014) for this research.
Open Research
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.




