Protein Kinase Inhibitors Indicated for Lung Cancer: Pharmacodynamics, Pharmacokinetics, Adverse Drug Reactions, and Evaluation in Clinical Trials
Abstract
WhatIs Known and Objective. Anti-EGFR (epidermal growth factor receptor) drugs are indicated for non-small-cell lung cancer (NSCLC). This review summarises the information available to date on the first anti-EGFRs granted market authorisation: erlotinib TARCEVA®, gefitinib IRESSA®, afatinib GIOTRIF®, dacomitinib VIZIMPRO®, and osimertinib TAGRISSO®. Methods. A literature search was conducted in the PubMed database including studies published in English using the terms gefitinib, erlotinib, afatinib, dacomitinib, and osimertinib. Furthermore, bibliographies of selected references were also studied for relevant articles. Clinical trial (CT) data were extracted from clinicaltrials.gov (ongoing trials and adverse events (AEs)). Assessment of AEs for these drugs was conducted using global pharmacovigilance data from VigiBase®. Results and Discussion. Erlotinib and gefitinib are first-generation anti-EGFR drugs, able to bind competitively and reversibly to the ATP- (adenosine triphosphate-) binding site of the EGFR exon 19 and exon 21 mutations. Afatinib and dacomitinib are second-generation anti-EGFRs able to bind covalently and irreversibly to the ATP site and inhibit EGFR and HER (human epidermal growth factor receptor) such as HER2 and HER4 enzyme activity. Osimertinib is a third-generation PKI and overcomes the EGFR T790M gatekeeper mutation through covalent binding at the ATP site. Medical interactions with these drugs are reported, notably with cytochrome P450 inducers or inhibitors. The most reported AEs in CTs are cutaneous reactions and gastrointestinal disorders. The occurrence of cutaneous reactions is less reported with third generation than with first- and second-generation anti-EGFR drugs. These results are consistent with the results from the VigiBase® global pharmacovigilance database. What Is New and Conclusion. This review summarises current knowledge regarding five anti-EGFRs in the literature. The third-generation anti-EGFR appears to be more effective than the first and second generations and is indicated as first-line therapy. This trial is registered with NCT01523587, NCT01466660, NCT01774721, NCT01360554, and NCT02296125.
1. What Is Known and Objective
Lung cancer has been one of the most commonly diagnosed deadly cancers in the last several decades [1]. Approximatively 2.1 million lung cancers were diagnosed in 2018. The incidence of this disease is lower for women than men because the consumption of tobacco differs according to sex [1]. In 2018, this cancer was associated with 1.8 million deaths worldwide. There are two different types of lung cancer: NSCLC and small-cell lung cancer which are reported in 85% and 15% of patients, respectively [2]. In addition to tobacco consumption, other reported risk factors were asbestos, radon, arsenic, chromium, nickel and vinyl chloride exposure, and also particulate matter 2.5 (PM2.5) [3]. Different treatment types exist, including surgery, radiotherapy, immunotherapy, and targeted therapy [4]. However, even though the drugs under discussion are preselected as targeted therapies, the mechanism of action of such drugs is not lung tumour specific. Therefore, in practice, these drugs have a profile of sometimes complex AEs associated with their ability to bind to proteins of interest (on-target) and also to other proteins (off-target) [5, 6]. The current aim of medical research is to develop personalised medicine. In oncology, the objective is to bring to market target therapies to decrease the occurrence of AEs. Several PKIs (protein kinase inhibitors) have been developed. In the last twenty years, around 450 PKIs have been studied in clinical trials [7]. Various PKIs are indicated for the treatment of lung cancer and target ALK (activin receptor-like kinase) or EGFRs in particular. EGFR mutation is less associated with smoking condition [8, 9]. Five main EGFR targeting PKIs (erlotinib, gefitinib, afatinib, dacomitinib, and osimertinib) have received marketing authorisation from the EMA (European Medicines Agency) and the FDA (Food and Drug Administration) [10, 11]. Globally, the most frequent resistance mechanism with first-generation PKIs is the onset of 790 mutation on exon 20 [12, 13]. Therefore, a second generation is developed to target this mutation. However, during clinical trials, this generation of drugs was not fully active against these mutations. Therefore, a third generation is developed to overcome EGFRs with T790M mutation [14, 15]. In clinical trials, these drugs are associated with better outcomes than standard therapy [16].
These drugs have been studied in various clinical trials and a number of studies have been published. Our aim in this review is to collate and summarise the information available on these five EGFR inhibitors.
2. Methods
A literature search was conducted in the PubMed database, for the years 1993–2024, on English-language studies published that contain any of the following terms: erlotinib, gefitinib, afatinib, dacomitinib, and osimertinib. The bibliography of the articles in question was also studied. The studies included in this review were limited to those providing the most recent available human data. We selected only studies published in in peer-reviewed journals.
Clinical trials relative to the five PKIs of interest were identified from the ClinicalTrials website: https://www.clinicaltrials.gov. For each clinical trial, several data points were collected such as clinical development phase, clinical trial status (active not yet recruiting, completed, recruiting, suspended, terminated or unknown), results availability, estimated number of enrolled patients, and cancer location. We selected five clinical trials which compared the efficacy of PKIs and presented their results. We also examined AEs reported in these clinical trials for all PKIs combined: first-generation PKI, afatinib, dacomitinib, and osimertinib. We chose to identify AEs in ClinicalTrials because they are all referenced with a MedDRA classification. In our AE analysis, a distinction was made between severity levels. Serious AEs were events such as death, life-threatening events, and/or events requiring patient hospitalisation or extension of current hospitalisation. AEs resulting in significant incapacity, a substantial interference with normal living, or those associated with a congenital malformation were also classed as serious. AEs not associated with death or that were not life-threatening or did not require hospitalisation were classed as serious AEs if the patient was at risk of or actually required surgical intervention to prevent one of the results listed above [17]. We listed the most frequent non-serious and serious AEs identified in the five clinical trials selected. When presenting the AEs reported in the clinical trial, we added the patient number for whom an AE was reported in each drug arm. For the four drug arms, we calculated the proportion of patients for whom each AE was reported out of all patients receiving the drug (expressed as a percentage).
Then, we identified AEs reported in VigiBase®, which is the global pharmacovigilance database, of the five PKIs of interest in our study. This database contains information on the patient (age, gender, and medical history), the reporter, the country, suspect and concomitant medications (name, dosage, start, and end dates), and AEs (description and severity). The ten most common SOCs (system organ classes) were presented for each PKI.
3. Results
3.1. Pharmacological Action
Within the ERBB group, consisting of EGFR (ERBB1), HER2 (ERBB2), HER3 (ERBB3), and HER4 (ERBB4), there is a wide range of receptor protein kinases, each playing a distinct role in cell signaling pathways associated with cellular processes such as growth, survival, adhesion, migration, and differentiation. Physiological pathways of action include RASRAF, MAPK (mitogen-activated protein kinase), PI3K (phosphatidylinositol 3-kinase)/AKT, and JAK/STAT (Janus kinase/signal transducer and transcription activator) [18]. Affinity profiles for protein kinases differ for erlotinib, gefitinib, and afatinib (Figure 1). Little data on protein kinases are available for dacomitinib and osimertinib.

The proportion of EGFR mutation differs according to ethnicity. In the Southeast Asian population, this mutation is present for 40–60% patients with NSCLC but concerns only 10 to 15% of Caucasian patients [11, 20, 21]. The mutation is located between the 18 to 21 exons which encode for the ATP binding pocket in the intracellular protein kinase domain [18]. The mutation/deletion is mainly located on exon 19 in 60% of patients [11, 20, 21]. It can also be located on exon 21 (30% of patients). This mutation permits the stimulation of tyrosine residues that give rise to a tumour profile that is dependent on EGFR activity for its development. EGFR inhibition is associated with tumour cell division decrease and death of cells overexpressing EGFR [18]. The overexpression of EGFR can also lead to the development of other types of cancer including head and neck, prostate, breast, ovarian, and colon [22].
The anti-EGFR treatments can be categorised according to their generations. Erlotinib and gefitinib are first-generation anti-EGFR drugs. The first-generation PKI drugs bind competitively and reversibly to the ATP-binding site [23]. Afatinib and dacomitinib are second-generation anti-EGFRs. This second-generation PKI binds covalently and irreversibly to the ATP site and inhibits EGFR and HER such as HER2 and HER4 enzyme activity [24, 25]. Osimertinib is a third-generation PKI [18]. This generation overcomes EGFR with T790M mutation and inhibition is irreversible on the ATP site [13, 14]. This generation is also 200-fold less potent in inhibiting wild-type EGFR.
3.2. Resistance Mechanisms
3.2.1. First-Generation PKIs: Erlotinib and Gefitinib
The first-generation PKIs are active on the EGFR exon 19 deletion and on EGFR L858R mutation. The sensitivity of first-generation anti-EGFR treatments is around 70% for patients with EGFR mutation. However, within 1 or 2 years, these patients will develop an acquired resistance [15, 26].
There are three resistance mechanisms: target modification, signaling pathway bypass, and histological modification of the tumour [13, 27]. For target modification, two resistance mechanisms are reported. These are defined as primary and secondary mutations.
Primary resistance is reported for 10–20% of NSCLC patients. Several primary resistance mechanisms are reported such as T790 exon 20 mutation, hepatocyte growth factor overexpression, T854A exon 21 mutation, and D761Y and L747S exon 19 mutation. Other resistance mechanisms are reported including de novo HER2 and MET amplification [11].
Overall, the most frequent resistance mechanism (49% of patients) is the onset of 790 mutation on exon 20 [12]. It is the substitution between threonine to methionine. The side chain becomes bulky. Thus, EGFR therapy cannot interact with the ATP site [23]. The second most frequent resistance mechanism is MET amplification. This concerns 20% of patients who receive a first-generation PKI [12].
3.2.2. Second-Generation PKIs: Afatinib and Dacomitinib
This generation is initially developed to inhibit EGFR with T790 or T854 mutations and HER2 amplification. However, during clinical studies, this type of drug is not fully active against these mutations. The majority of patients developed a resistance to this treatment, with around 50–60% presenting T790 mutation. Other mutations are reported such as HER2 and MET amplification and also phenotypic and genotypic lung cancer modifications [12, 28]. C797S, TP53, PTEN, and PKHD1 mutations are also reported [29, 30].
3.2.3. Third-Generation PKI: Osimertinib
Resistance to osimertinib may be due, for example, to the C797S mutation in exon 20 of EGFR or to loss of the T790 mutation. Other EGFR mutations can occur such as I796, L792, L718, L844, and G719. Resistance mechanisms can be EGFR independent such as MET amplification, HER2 amplification, and PI3K activation. Other resistance processes involved in NSCLC are reported: cell cycle alteration, oncogenic fusion, and histologic and phenotypic modification of the cancer [13, 31–34]. This type of mutation may be associated with the emergence of other mutations such as Kirsten rat sarcoma viral oncogene homologue or MET amplification.
3.2.4. Therapeutics for Patients with Resistance
In the case of T790 mutation development with first- or second-generation anti-EGFR agents, treatment with osimertinib may be prescribed.
In the absence of a T790 mutation, early anti-EGFR therapy may be prescribed. For mutations such as L718Q or L844V in the absence of a T790M mutation, treatment with gefitinib and afatinib is possible [13]. In the case of a C797S mutation in exon 20 of EGFR, prescription of a fourth anti-EGFR drug such as LS-106, BLU-945, BLU-701, EAI045, and JBJ-09-063 may be considered. Then, in the event of MET amplification, a combination of crizotinib and anti-EGFR may be prescribed [35]. Furthermore, if resistance is associated with HER2 amplification, anti-EGFR can be combined with the anti-HER2 ADC trastuzumab emtansine (TDM1).
Finally, for 30–50% of patients, the mechanism of resistance is unknown. In these cases, chemotherapy such as etoposide, platinum, or pemetrexed-based agents, an immune checkpoint inhibitor (ICI), or antiangiogenic therapy may be prescribed [36, 37].
3.3. Pharmacokinetics of EGFR Blockers
Pharmacokinetic parameters differ for each of the PKIs of interest (Table 1). Overall, PKIs have a high bioavailability and distribution volume. These drugs are mainly eliminated in feces with a half-life varying from 36 to 63 hours.
| Pharmacokinetic parameters and units | Erlotinib | Gefitinib | Afatinib | Dacomitinib | Osimertinib |
|---|---|---|---|---|---|
| Bioavailability | 59 | 50 | NA | 80 | 70 |
| AUCt | 27 (ng/h/mL) | 258 (ng/h/mL) | 631 (ng/h/mL) | 1171 (ng/h/mL) | 9 570 (nmol/h/L) |
| Cmax | 1521 (ng/mL) | 101 (ng/mL) | 38 (ng/mL) | 21.51 (ng/mL) | 550.4 (nmol/L) |
| Tmax(h) | 4 | 4 | 3 | 8 | 4 |
| T1/2,h(h) | 36 | 52 | 37 | 63 | 48.6 |
| CL/F (L/H) | 4.5 | 46 | 1070 | 27.06 | 17.7 |
| Vz/F (L) | 232 | 1700 | 2870 | 2415 | 1216 |
| Protein binding (%) | 95 | 90 | 95 | 98 | 95 |
- AUC: area under the curve, Cmax: peak concentration, CL: clearance, F: bioavailability, T1/2: half-life, Tmax: time to peak drug concentration, and Vz: distribution volume.
3.3.1. Absorption
Erlotinib, gefitinib, and osimertinib’s absolute bioavailability is estimated to be 59, 50, and 70 percent, respectively. For the PKIs of interest, Tmax (time to peak drug concentration) is between 3 and 4 hours. However, Tmax for dacomitinib is estimated to be 8 hours. Peak concentration differs with each drug. For example, this parameter is estimated to be 1,521 ng/mL for erlotinib. However, for gefitinib, it is 101 ng/mL [10]. The absolute bioavailability of dacomitinib is around 80%, ranging between 65% and 100% [38]. The absolute bioavailability of afatinib is unknown.
3.3.2. Distribution
The distribution volume differs according to the PKI. Afatinib has a high distribution volume of approximately 2,870 L. For gefitinib and osimertinib, the distribution volume is estimated to be around 1,216 and 1,700 L, respectively. Finally, for erlotinib, the distribution volume is around 232 L. Dacomitinib distribution volume is estimated to be 3,310 L [41]. Protein binding is estimated to be between 90 and 95% for the PKIs of interest [10].
3.3.3. Elimination
Erlotinib is principally eliminated in feces (83%) and in low amounts in urine (8%) [42]. The elimination half-life for the drug is around 36 hours. The elimination half-life for gefitinib is 52 hours. This drug is mainly eliminated in feces and in low amounts in urine (7%) [43]. The elimination half-life for afatinib is estimated to be around 37 hours. The elimination half-life for osimertinib is estimated to be 48.6 hours and is eliminated in feces (68%) and in urine (14%) [44]. Dacomitinib is mainly eliminated in feces and a small percentage (5%) in urine [45]. The elimination half-life for dacomitinib is estimated to be 63 hours [46].
3.3.4. Variation Factors
Erlotinib and afatinib absorption can be affected when administered with food. Food can delay gastric emptying and modify the bioavailability of erlotinib and osimertinib. These drugs can be administered one hour before meals or two hours after eating [10, 47].
The pharmacokinetics of gefitinib, dacomitinib, and osimertinib are not affected when administered with food [10, 48].
The anti-EGFRs of interest are metabolised by CYP2D6 [49, 50]. As a result, metabolisation may be modified if the patient is a poor CYP2D6 metaboliser. The proportion of poor CYP2D6 metabolisers is around 5 to 10% in the Caucasian population and is a rarity for Asian people. For sub-Saharan African and Afro-American patients, this proportion is variable. For CYP2D6 intermediate metabolisers, the proportion is around 10 to 15% for Caucasians, over 50% for Asians, and around 30% for sub-Saharan African and African-American populations [51]. For CYP2D6 ultra-rapid metabolisers, the proportion is around 6.4% for Caucasians, around 2% for Asians, and less than 1% for sub-Saharan African and African-American populations [52, 53]. Other patients are considered extensive metabolisers.
The AUC (area under the curve) of gefitinib is higher for poor vs. extensive metabolisers. However, dosage adjustment is not required in this case [54]. The AUC of gefitinib metabolites is higher for intermediate vs. extensive metabolisers. However, this increased AUC is not associated with any increases in adverse reactions [55]. The AUC of gefitinib is around 39% lower for ultra-rapid vs. extensive metabolisers. However, the clinical consequence of this decrease is limited [39]. Similar pharmacokinetic parameters are reported for patients with different CYP2D6 metabolisation profiles for dacomitinib. However, for its metabolite PF-05199265, the AUC and peak exposure are higher for extensive vs. intermediate CYP2D6 metabolisers [56]. Few other studies are conducted to assess the effect of differences in CYP2D6 metabolisation on the pharmacokinetic parameters of PKIs.
3.4. Drug Interactions
Overall, anti-EGFR PKI absorption can be affected by antiacid drug administration. Then, their metabolism can be affected by concomitant administration of drugs which inhibit or induce cytochrome such as CYP 3A4, 2C8, or 2D6.
Anti-EGFR PKIs can be affected by concomitant antacid administration, such as proton pump inhibitors (PPIs) and histamine H2-receptor antagonists [57]. This can affect the solubility of anti-EGFRs with a pKa value of less than 4-5. The concomitant administration of these drugs can lead to a decrease in anti-EGFR bioavailability [51]. This interaction is particularly demonstrated for erlotinib and gefitinib. Afatinib is soluble at a pH of between 1 and 7.5. However, potential interaction is also identified for afatinib [58]. Dacomitinib bioavailability is decreased by the concomitant administration of a PPI [48, 59, 60]. Finally, for osimertinib, impact of interaction is controversial [47, 61].
Interactions between anti-EGFR drugs and other medications can be explained by inducer and inhibitor cytochrome metabolism (Table 2). The cytochrome metabolisation profile varies with different anti-EGFRs [51, 61, 62].
| 3A4 | 3A5 | 2D6 | 1A1 | 1A2 | 1B1 | 2C8 | 2C9 | 2C19 | 2E1 | May inhibit | May induce | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Erlotinib | +++ | +++ | + | + | ++ | + | + | + | − | − |
|
|
| Gefitinib | +++ | ++ | +++ | ++ | + | − | − | − | − | − |
|
− |
| Afatinib | − | − | − | − | − | − | − | − | − | − | − | − |
| Dacomitinib | + | − | +++ | − | − | − | − | + | − | − |
|
CYP1A2, CYP2B6 CYP3A4 |
| Osimertinib | +++ | +++ | − | − | − | − | − | − | − | − | − | CYP3A4 |
Erlotinib metabolism is mainly mediated by CYP3A4 and 3A5. Thus, erlotinib metabolism can be increased by co-administering a CYP3A4 inducer. For example, erlotinib exposure is around 69% for rifampicin. Co-administration of ketoconazole and ciprofloxacin increases erlotinib metabolism by 86 and 39%, respectively. If the co-administration of a potent CYP3A4 inhibitor is unavoidable and if AEs occur, the erlotinib dosage can be decreased to 50 mg. Erlotinib also inhibits CYP3A4, CYP2C8, and CYP1A1. More cases of AEs are reported when erlotinib is co-administered with CYP3A4 and CYP2C8 substrates such as phenytoin and simvastatin [51, 63–65].
Gefitinib is mainly metabolised by CYP3A4 and CYP2D6 and less by CYP3A5, CYP1A1, and CYP1A2 [50]. However, it is metabolised more by CYP3A4 than by CYP2D6. Thus, gefitinib metabolisation is influenced by a CYP3A4 inducer or inhibitor. For example, the co-administration of itraconazole with this PKI is characterised by an increase in the AUC of around 78%. Also, gefitinib administration with rifampicin or phenytoin decreases the PKI AUC to 83% and 47%, respectively. When gefitinib is administered with a CYP3A4 inducer, the PKI dosage can be increased to 500 mg per day. The administration of a CYP2D6 inducer can also inhibit gefitinib metabolisation. However, this interaction is less studied. Finally, gefitinib can increase the exposure of drugs metabolised by CYP2D6. For example, in the case of metoprolol and gefitinib co-administration, exposure is increased by around 35% [43, 51, 54].
Afatinib is metabolised less by cytochromes. Consequently, its exposure is not influenced by concomitant administration of cytochrome-inhibiting and cytochrome-inducing drugs [51].
Dacomitinib is mainly metabolised by CYP2D6. This drug is also less metabolised less by other cytochromes such as CYP 3A4 and CYP 2C9. In vitro, dacomitinib is a minor inhibitor of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP3A4, and CYP3A5. It is also a minor CYP1A2, CYP2B6, and CYP3A4 inducer [38]. In the case of concomitant administration with dacomitinib, the AUC of a CYP2D6 substrate may increase. For example, compared with dextromethorphan alone, the AUC of dextromethorphan co-administered with dacomitinib increase by around 955% [66]. Similarly, in the case of concomitant administration of dacomitinib and trazodone, exposure to both drugs increases [67].
Finally, osimertinib is mainly metabolised by CYP3A4 and CYP3A5 and may induce CYP3A4. In pharmacokinetic studies, no clinically significant interaction is demonstrated between osimertinib and itraconazole, a potent CYP3A4 inhibitor. However, osimertinib absorption at around 2 hours is higher and AUC of PKI is increased. Finally, with the co-administration of rifampicin, a potent CYP3A4 inducer, the AUC of the PKI decreases by around 78%. The co-administration of a cytochrome inducer with osimertinib must be avoided if possible. If co-administration is unavoidable, osimertinib dosage should be increased to 160 mg per day, and a standard dosage (80 mg/day) may be prescribed only 3 weeks after the end of cytochrome inducer treatment [51].
Another interaction mechanism concerns UGT1A1 (UDP (uridine diphosphate)-glucuronosyltransferase). This catalyses glucuronic acid conjugation to endogenous and exogenous substances. UGT1A1 is responsible for bilirubin elimination and prevents its accumulation. The co-administration of a different UGT inhibitor can cause AEs such as hyperbilirubinemia and hepatotoxicity. Erlotinib is also a potent UGT1A1 inhibitor. Patients with low UGT1A1 expression can develop a high bilirubin serum concentration [51, 68]. Gefitinib is a UGT inhibitor, particularly of UGT1A1, UGT1A7, UGT1A9, and UGT2B7. However, this drug is unlikely to cause clinically significant drug interactions. Dacomitinib is also a minor inhibitor of UGT1A4, UGT1A6, UGT1A9, UGT2B7, and UGT2B15 [38].
Drug interactions can occur with PKI transport in blood circulation. Different transporters participate in PKI metabolism, notably efflux transporters such as P-gp (P-glycoprotein) and ABC (ATP-binding cassette) subfamily B. Erlotinib is a potent inhibitor of BCRP (breast cancer resistance protein) and P-gp. Interaction can occur with co-administration of a P-gp inhibitor such as cyclosporine or verapamil. Gefitinib inhibits P-gp activity. As a result, gefitinib can inhibit transporter activity and cause clinically significant drug interactions. Afatinib is a substrate and a P-gp inhibitor. As a result, afatinib metabolism can increase with the co-administration of a P-gp inhibitor such as ritonavir. Conversely, in the case of co-administration with a potent P-gp inducer, afatinib exposure can decrease. For example, rifampicin co-administration decreases afatinib exposure by around 34%. With P-gp inhibitor co-administration, afatinib dosage can be reduced by 10 mg. If afatinib is co-administered with a P-gp inducer, its dosage can be increased by 10 mg. Afatinib is also a substrate and a BCRP inhibitor. It can increase the bioavailability of BCRP substrates such as rosuvastatin and sulfasalazine [51]. Dacomitinib is also a minor P-gp and BCRP inhibitor [38]. Finally, osimertinib is a potent BCRP inhibitor and can increase exposure to its substrates. For example, it increases the AUC of rosuvastatin by around 35% [51].
Other significant drug interactions are reported. For example, an increase in international normalised ratio is reported with erlotinib and gefitinib when co-administered with warfarin [69–71].
3.5. Dosage Adjustment and Methods of Administration
One of the advantages of these anti-EGFR PKIs is that they can be orally administered [72]. The recommended daily dose of erlotinib is 150 mg. No change in dose is recommended in the event of mild or moderate renal or hepatic failure. However, erlotinib prescription is not recommended for patients with severe renal or hepatic failure [72].
The recommended daily dosage for gefitinib is 250 mg. No change in dose is recommended for mild or moderate renal or hepatic failure [73].
The recommended daily dose of afatinib is 40 mg. An increase of this dose to 50 mg is possible if the patient does not present any AEs. No change in dose is recommended for patients with renal failure. Again, no change in dose is recommended in the case of mild or moderate hepatic failure. However, afatinib prescription is not recommended in the case of severe hepatic failure. Nevertheless, under FDA recommendations, afatinib may be prescribed for patients with severe hepatic failure [74, 75].
The recommended daily dose of dacomitinib is 45 mg. Dacomitinib can be administered to patients with mild and moderate renal or hepatic failure. For patients with severe hepatic failure, a dose of 30 mg per day is recommended [38, 76]. No change in dose is recommended for patients with mild or moderate renal failure. Limited data are available for patients with severe renal failure [38].
The daily dose of osimertinib is 80 mg. No change in dose is recommended for patients with renal failure. Also, no change in dose is recommended for patients with mild or moderate hepatic failure. The administration of osimertinib is not recommended in cases of severe hepatic failure. However, in the literature, a minimal correlation has been suggested between renal osimertinib clearance and renal function [77].
Several cases reported the safe administration of the PKIs of interest for patients undergoing dialysis [78–81].
Anti-EGFR PKI efficacy can be decreased by being a smoker and can cause treatment resistance. Smoking increases oxidative stress with an imbalance in reactive oxygen species and is linked to abnormal EGFR activation. Cigarette smoke can also induce CYP1A1 and 1A2 and increase erlotinib catabolism and clearance. For smokers, erlotinib clearance can increase by around 24% in comparison with non-smokers. Moreover, smokers required a dose of 300 mg to obtain the same area under the curve value obtained with the recommended dose administered to non-smokers. According to the FDA, erlotinib dosage should be increased to 300 mg per day. This interaction is less studied for gefitinib, afatinib, dacomitinib, and osimertinib [63, 82, 83].
3.6. Clinical Development of Anti-EGFR Treatment
Information concerning ongoing clinical trials with the PKIs of interest is summarised in Tables 3, 4, 5, 6, and 7.
| Phase (number of clinical trials, expected number of patients included) | Status (number of clinical trials, expected number of patients included) | Study results (number of clinical trials, expected number of patients included) | Condition (number of clinical trials, expected number of patients included) |
|---|---|---|---|
|
|
|
|
- Information in the table is presented as follows: number of clinical trials and expected number of patients included.
| Phase (number of clinical trials, expected number of patients included) | Status (number of clinical trials, expected number of patients included) | Study results (number of clinical trials, expected number of patients included) | Condition (number of clinical trials, expected number of patients included) |
|---|---|---|---|
|
|
|
|
- Information in the table is presented as follows: number of clinical trials and expected number of patients included.
| Phase (number of clinical trials, expected number of patients included) | Status (number of clinical trials, expected number of patients included) | Study results (number of clinical trials, expected number of patients included) | Condition (number of clinical trials, expected number of patients included) |
|---|---|---|---|
|
|
|
|
- Information in the table is presented as follows: number of clinical trials and expected number of patients included.
| Phase (number of clinical trials, expected number of patients included) | Status (number of clinical trials, expected number of patients included) | Study results (number of clinical trials, expected number of patients included) | Condition (number of clinical trials, expected number of patients included) |
|---|---|---|---|
|
|
|
|
- Information in the table is presented as follows: number of clinical trials and expected number of patients included.
| Phase (number of clinical trials, expected number of patients included) | Status (number of clinical trials, expected number of patients included) | Study results (number of clinical trials, expected number of patients included) | Condition (number of clinical trials, expected number of patients included) |
|---|---|---|---|
|
|
|
|
- Information in the table is presented as follows: number of clinical trials and expected number of patients included.
The different results of clinical trials evaluating erlotinib, gefitinib, afatinib, dacomitinib, and osimertinib are presented in Table 8. Clinical trials are referenced with varying degrees of progress (extracted in January 2023 from clinicaltrials.gov).
| Name of clinical trial | LUX-Lung 8 (NCT01523587) | LUX-Lung 7 (NCT01466660) | ARCHER 1050 (NCT01774721) | ARCHER 1009 (NCT01360554) | FLAURA (NCT02296125) |
|---|---|---|---|---|---|
| Design |
|
|
|
|
|
| Pathology | NSCLC | Lung adenocarcinoma | Advanced NSCLC | NSCLC | NSCLC |
| Treatment | Afatinib vs. erlotinib | Afatinib vs. gefitinib | Dacomitinib vs. gefitinib | Dacomitinib vs. erlotinib | Osimertinib vs. gefitinib/erlotinib |
| Number of patients | 398 vs. 397 | 160 vs. 159 | 227 vs. 225 | 439 vs. 439 | 279 vs. 277 |
| Progression-free survival | 2.6 vs. 1.9 months (HR: 0.8; 95% CI: [0.3; 1.0], p = 0.01) | 12.8 vs. 11.2 months (HR: 0.8; 95% CI: [0.7; 1.0], p = 0.09) | 14.7 vs. 9.2 months (HR: 0.6; 95% CI: [0.5; 0.7], p < 0.0001) | 2.6 vs. 2.5 months (HR: 0.9; 95% CI: [0.8; 1.1], p = 0.20) | 18.9 vs. 10.2 months (HR: 0.5; 95% CI: [0.4; 0.6], p < 0.01) |
| Overall survival | 7.82 vs. 6.77 months (HR: 0.8; 95% CI: [0.7; 0.97], p = 0.02) | 27.9 vs. 24.5 months (HR: 0.9; 95% CI: [0.7; 1.1], p = 0.02) | NA | 7.9 vs. 8.3 months (HR: 1.0; 95% CI: [0.9; 1.2], p = 0.64) | NA |
| Number of participants with objective response | 22 vs. 11 (HR: 2.1; 95% CI: [1.0; 4.3], p = 0.0551) | 79.4% vs. 74.8% (HR: 1.3; 95% CI: [0.8; 2.2], p = 0.32) | 74.9% vs. 71.6% (p = 0.19) | NA | 76.7% vs. 69.0% (HR: 1.5; 95% CI: [1.0; 2.2], p = 0.04) |
- CI: confidence interval, EGFR: epidermal growth factor receptor, HR: hazard ratio, NSCLC: non-small-cell lung cancer, and PKI: protein kinase inhibitor.
In this review, the main clinical trials comparing these drugs are presented. The clinical trials presented here are the first to compare the efficacy of EGFR inhibitors with each other which made it possible to constitute the marketing authorisation files. The LUX-Lung 7 (phase 2) and LUX-Lung 8 (phase 3) clinical trials compared the efficacy of afatinib with that of erlotinib and gefitinib. The AURA 2 clinical trial (phase 2) presented the efficacy of osimertinib following treatment with erlotinib and gefitinib. The progression-free survival rate increased with afatinib in comparison with erlotinib. Also, overall survival significantly increased with afatinib compared with first-generation anti-EGFRs. In the case of dacomitinib, the overall survival rate increased significantly compared with erlotinib in recent phase III clinical trials. However, the increase in the overall survival rate was not significant when dacomitinib was compared with erlotinib. Finally, the FLAURA clinical trial, a phase III study, demonstrated an increase in progression-free survival and a greater number of participants with an objective response for those exposed to third-generation anti-EGFR PKIs compared to those exposed to first-generation anti-EGFR PKIs [84].
As a result, osimertinib is currently indicated as first-line treatment for EGFR-mutated non-small-cell lung cancer.
3.7. Principal AEs
In the clinical trials under study, 1,431 patients were included in the gefitinib or erlotinib arms, 552 in the afatinib arm, 674 in the dacomitinib arm, and 279 in the osimertinib arm.
Only serious AEs with a frequency greater than 1% (all trials combined) are presented in Table 9. The full list of adverse events recorded during these trials can be found in Table S1 (supplementary material).
| Total gefitinib/erlotinib N = 1 431 patients (%) | Total afatinib N = 552 patients (%) | Total dacomitinib N = 674 patients (%) | Total osimertinib N = 279 patients (%) | |
|---|---|---|---|---|
| Disease progression | 60 (4, 2) | 0 (0, 0) | 62 (9, 2) | 0 (0, 0) |
| Dyspnoea | 49 (3, 4) | 14 (2, 5) | 9 (1, 3) | 2 (0, 7) |
| Pneumonia | 45 (3, 1) | 33 (6, 0) | 17 (2, 5) | 9 (3, 2) |
| Malignant neoplasm progression | 20 (1, 4) | 30 (5, 4) | 0 (0, 0) | 0 (0, 0) |
| Diarrhoea | 20 (1, 4) | 29 (5, 3) | 26 (3, 9) | 3 (1, 1) |
| General physical health deterioration | 21 (1, 5) | 12 (2, 2) | 4 (0, 6) | 0 (0, 0) |
| Respiratory failure | 20 (1, 4) | 3 (0, 5) | 6 (0, 9) | 0 (0, 0) |
| Haemoptysis | 17 (1, 2) | 5 (0, 9) | 7 (1, 0) | 0 (0, 0) |
| Vomiting | 17 (1, 2) | 5 (0, 9) | 6 (0, 9) | 1 (0, 4) |
| Pulmonary embolism | 14 (1, 0) | 16 (2, 9) | 5 (0, 7) | 4 (1, 4) |
| Dehydration | 11 (0, 8) | 15 (2, 7) | 14 (2, 1) | 0 (0, 0) |
| Pleural effusion | 15 (1, 0) | 12 (2, 2) | 7 (1, 0) | 3 (1, 1) |
| Interstitial lung disease | 12 (0, 8) | 5 (0, 9) | 3 (0, 4) | 4 (1, 4) |
| Sepsis | 12 (0, 8) | 10 (1, 8) | 2 (0, 3) | 3 (1, 1) |
| Renal failure acute | 1 (0, 1) | 9 (1, 6) | 0 (0, 0) | 0 (0, 0) |
| Pyrexia | 8 (0, 6) | 4 (0, 7) | 6 (0, 9) | 3 (1, 1) |
| Back pain | 4 (0, 3) | 6 (1, 1) | 1 (0, 1) | 1 (0, 4) |
| Chronic obstructive pulmonary disease | 5 (0, 3) | 6 (1, 1) | 1 (0, 1) | 0 (0, 0) |
Table 10 sets out the non-serious AEs reported in the five clinical trials on clinicaltrials.gov. Only AEs with a frequency of more than 10% (all trials combined) are presented. All the AEs noted during these trials can be found in Table S2 (supplementary material).
| Total gefitinib/erlotinib N = 1 431 patients (%) | Total afatinib N = 552 patients (%) | Total dacomitinib N = 674 patients (%) | Total osimertinib N = 279 patients (%) | |
|---|---|---|---|---|
| Diarrhoea | 764 (53, 4) | 427 (77, 4) | 531 (78, 8) | 167 (59, 9) |
| Rash | 517 (36, 1) | 296 (53, 6) | 261 (38, 7) | 17 (6, 1) |
| Dermatitis acneiform | 378 (26, 4) | 72 (13, 0) | 196 (29, 1) | 73 (26, 2) |
| Decreased appetite | 374 (26, 1) | 139 (25, 2) | 213 (31, 6) | 66 (23, 7) |
| Dry skin | 325 (22, 7) | 88 (15, 9) | 150 (22, 3) | 92 (33, 0) |
| Nausea | 293 (20, 5) | 124 (22, 5) | 138 (20, 5) | 55 (19, 7) |
| Cough | 283 (19, 8) | 113 (20, 5) | 106 (15, 7) | 60 (21, 5) |
| Fatigue | 244 (17, 1) | 99 (17, 9) | 105 (15, 6) | 45 (16, 1) |
| Paronychia | 219 (15, 3) | 130 (23, 6) | 243 (36, 1) | 89 (31, 9) |
| Alanine aminotransferase increased | 230 (16, 1) | 19 (3, 4) | 68 (10, 1) | 19 (6, 8) |
| Dyspnoea | 227 (15, 9) | 102 (18, 5) | 108 (16, 0) | 42 (15, 1) |
| Pruritus | 223 (15, 6) | 78 (14, 1) | 99 (14, 7) | 50 (17, 9) |
| Aspartate aminotransferase increased | 212 (14, 8) | 15 (2, 7) | 64 (9, 5) | 28 (10, 0) |
| Constipation | 193 (13, 5) | 72 (13, 0) | 77 (11, 4) | 51 (18, 3) |
| Stomatitis | 192 (13, 4) | 117 (21, 2) | 186 (27, 6) | 82 (29, 4) |
| Vomiting | 188 (13, 1) | 79 (14, 3) | 93 (13, 8) | 41 (14, 7) |
| Asthenia | 169 (11, 8) | 82 (14, 9) | 98 (14, 5) | 27 (9, 7) |
| Weight decreased | 161 (11, 3) | 56 (10, 1) | 136 (20, 2) | 20 (7, 2) |
| Back pain | 153 (10, 7) | 44 (8, 0) | 64 (9, 5) | 36 (12, 9) |
| Anaemia | 134 (9, 4) | 45 (8, 2) | 67 (9, 9) | 44 (15, 8) |
| Alopecia | 109 (7, 6) | 19 (3, 4) | 71 (10, 5) | 22 (7, 9) |
| Pyrexia | 109 (7, 6) | 54 (9, 8) | 66 (9, 8) | 32 (11, 5) |
| Insomnia | 105 (7, 3) | 33 (6, 0) | 47 (7, 0) | 31 (11, 1) |
| Chest pain | 102 (7, 1) | 34 (6, 2) | 43 (6, 4) | 0 (0, 0) |
| Musculoskeletal pain | 101 (7, 1) | 34 (6, 2) | 48 (7, 1) | 28 (10, 0) |
| Headache | 88 (6, 1) | 14 (2, 5) | 36 (5, 3) | 39 (14, 0) |
| Mucosal inflammation | 69 (4, 8) | 82 (14, 9) | 88 (13, 1) | 0 (0, 0) |
| Maculopapular rash | 85 (5, 9) | 0 (0, 0) | 51 (7, 6) | 38 (13, 6) |
| Upper respiratory tract infection | 84 (5, 9) | 16 (2, 9) | 54 (8, 0) | 36 (12, 9) |
| Conjunctivitis | 55 (3, 8) | 14 (2, 5) | 78 (11, 6) | 18 (6, 5) |
| Electrocardiogram QT prolonged | 12 (0, 8) | 0 (0, 0) | 0 (0, 0) | 28 (10, 0) |
3.7.1. Cutaneous Reaction
Cutaneous reactions are reported for all anti-EGFR PKIs. However, third-generation drugs are less linked to such reactions than first- and second-generation drugs. This can be explained by the more selective profile of third-generation PKIs. This cutaneous AE is rarely serious or fatal, but it is frequent. Severe cutaneous reaction may require dosage modification or discontinuation of the PKI [85].
Several studies have reported a relationship between tumour response and overall survival and rash occurrence [86–88].
(1) First- and Second-Generation Anti-EGFR Treatments. Within the first two weeks following PKI initiation, rashes or pruritus can appear. This is characterised by acneiform eruptions together with inflamed papules and pustules. Furthermore, serious and/or haemorrhagic crusting is reported. After the first one or two months of treatment, the occurrence of dry skin/pruritus, fissures, stomatitis and mucositis, facial hirsutism, and eyelash trichomegaly is reported. After 6 to 8 weeks, nail changes are a possible AE. Finally, after two or three months, the occurrence of alopecia has been reported in the literature [85, 89].
First- and second-generation drugs inhibit wild-type EGFR. However, this receptor type is expressed in epidermal basal cells in hair follicles, the sweat and sebaceous glands, and also in periungual tissue. These drug types inhibit proliferation, migration, and differentiation of these cells and consequently are associated with impaired skin integrity with an inflammatory mechanism [85, 90].
(2) Third-Generation Anti-EGFR Treatments. In clinical trials, among patients exposed to osimertinib, different cutaneous AEs are reported: rash, dry skin, and paronychia. Few reactions are reported with a severity grade greater than or equal to 3. For dry skin and paronychia, no reactions with a severity grade greater than or equal to 3 were reported [85].
(3) Management of Cutaneous AEs. In the case of rash, pruritus, or paronychia, treatment such as a topical corticosteroid lotion or solution or anti-inflammatory drugs can be prescribed. For pruritus, oral antihistamines or gamma-aminobutyric acid agonists can be prescribed. To treat xerosis or dry skin, moisturising creams or lotions containing urea, colloidal oatmeal, zinc oxide, and salicylic acid and exfoliants can be administered to the patient [90]. Minocycline, doxycycline, or cefadroxil may be prescribed in the event of skin reactions to PCIs, such as paronychia [90–92]. If the patient presents an intolerable or severe reaction, the PKI may be discontinued or the dose reduced. If the reaction is assessed as severity 4, the drug should be stopped immediately without attempting to reduce the dosage [84]. After dose reduction or discontinuation, PKI may be administered if the skin reaction is resolved or reduced to grade 2. On reintroduction, the dose of PKI may be reduced to 50% [84, 93].
3.7.2. Interstitial Lung Disease (ILD)
This is a rare but a severe and potentially fatal AE associated with anti-EGFR PKIs [94, 95]. It occurs in approximately 1–3% of patients treated with an anti-EGFR PKI. The occurrence of this AE is higher for osimertinib than gefitinib. However, the proportion of ILD with a grade superior or equal to 3 is the same [96]. Different risk factors are associated with this AE occurrence: male sex, smoking, a clinical history of pulmonary fibrosis, poor performance status, previous radiotherapy, and treatment with a PD-1 inhibitor. Also, a clinical history of interstitial pneumonia is a risk factor for developing this AE [97].
This AE is managed by the prescription of high dosage and prolonged administration of corticosteroid or immunosuppressive drugs. The anti-EGFR must be discontinued. However, some case studies report a possible re-challenging with the same or another anti-EGFR after this AE [98–100].
The pharmacological mechanism for this AE is unknown. However, the anti-EGFR can decrease EGFR phosphorylation and regenerative epithelial proliferation. It can lead to the development of pulmonary fibrosis. As a result, the risk of ILD can be exacerbated [95].
3.7.3. Gastrointestinal Disease
Different gastrointestinal diseases are reported in the literature with these drugs. Diarrhoea is the most frequent AE with anti-EGFRs [101, 102]. However, few cases of rare but severe gastrointestinal perforations are reported [103, 104].
(1) Diarrhoea. Diarrhoea frequency varies according to the anti-EGFR PKI. In several studies, for erlotinib and gefitinib, it is estimated, respectively, at around 27–69% and 18–68%. For afatinib, between 87 and 95% patients had this AE. For osimertinib, diarrhoea prevalence is estimated to be 41% [101]. The prevalence of a grade 3 diarrhoea reaction is estimated for erlotinib, gefitinib, afatinib, and osimertinib, respectively, to be around 1–25%, 1–12%, 5–17%, and 1% [101]. For dacomitinib, diarrhoea occurrence is observed in 97.5% of patients but only 12.5% had grade 3 or 4 reactions [105]. The prevalence of grade 3 and 4 diarrhoea is lower with osimertinib than with erlotinib, gefitinib, or afatinib. This AE mainly occurs in the first weeks following initiation of PKI treatment [101, 106].
The pharmacological mechanism of this AE is unknown. EGFRs are also located in the gastrointestinal tract in the basolateral membranes of epithelial cells. They regulate ion transport. EGFRs also play a role in intestinal epithelial chloride secretion, which is linked to passive water movement. Because EGFR inhibition can dysregulate ion transport, chloride secretion can increase and cause secretory diarrhoea [101]. In case of diarrhoea, drugs such as loperamide and racecadotril can be prescribed. Non-pharmacological management strategies, including dietary change, fluid intake, and probiotics, may also be prescribed. In cases of diarrhoeas with a grade superior to 1 not improved by symptomatic treatment, PKI should be discontinued until the AE reaches grade 1. PKI should be discontinued permanently in cases of grade 1 diarrhoea, despite 14 days of PKI discontinuation [106, 107].
(2) Gastrointestinal Perforation. Some cases of gastrointestinal perforation are reported with erlotinib. Risk factors associated with this AE occurrence are concomitant administration of anti-angiogenic agents, corticosteroids, nonsteroidal anti-inflammatory drugs, and taxane-based chemotherapy. Prior peptic ulceration and diverticular disease are also risk factors for developing this AE. The pharmacological mechanism is unknown. However, anti-EGFR PKIs can reduce vascular endothelial growth factor expression and have an anti-angiogenic effect. This can cause local ischemia and inadequate vascular perfusion of the gastrointestinal tract. The treatment for this AE is surgical, but in most of the cases reported in the literature, this AE resulted in death [104, 108].
3.8. AEs in VigiBase®, the Global Pharmacovigilance Database
Data were extracted from VigiBase® on 11 January 2023. There were 76,891 cases and 145,120 AEs reported with the PKIs of interest. Among them, 41,448, 9,243, 11,354, 504, and 14,162 patients, respectively, received erlotinib, gefitinib, afatinib, dacomitinib, and osimertinib. There were 78,854 reported AEs with erlotinib, 15,606 with gefitinib, 26,353 with afatinib, 1,078 with dacomitinib, and 22,549 with osimertinib (Figures 2, 3, 4, 5, and 6).
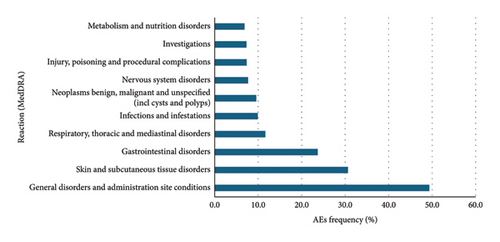
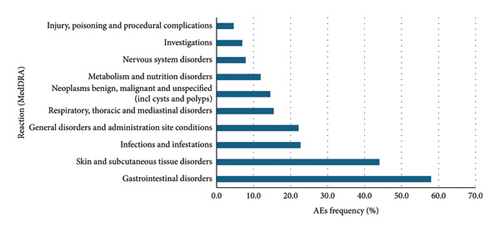

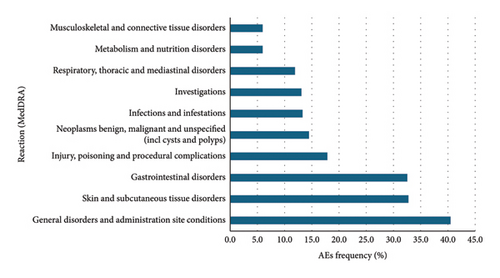
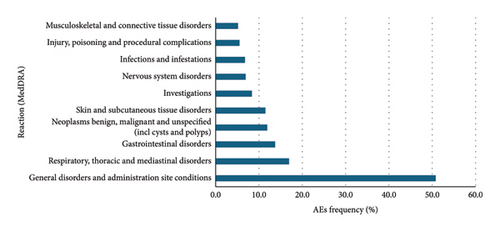
Overall, for the PKIs of interest for our study, the male-female distribution was 54.3-40.1% (with 5.6% no data). For erlotinib, gefitinib, afatinib, and osimertinib, the median age was between 65 and 74 years. For dacomitinib, the median age was between 45 and 64 years. For erlotinib, the male-female distribution was 50.2-45.1% (with 4.7% no data). For gefitinib, the male-female distribution was 59.6-35.3% (with 5.0% no data). For afatinib, the male-female distribution was 59.0-37.6% (with 3.5% no data). For dacomitinib, the male-female distribution was 43.1-49.4% (with 7.5% no data). For osimertinib, the male-female distribution was 59.3-30.0% (with 10.7% no data). Figures represent the 10 most represented SOCs for AEs. Percentages are for the number of SOC AEs out of the total number of AEs.
These results are consistent with those found in the clinical trials. Gastrointestinal, cutaneous, and subcutaneous tissue adverse events were more frequently reported than other AEs as is the case in the clinical trials. It is noteworthy that cutaneous and subcutaneous reactions are among the three most reported adverse reactions to osimertinib, as in the clinical trials. This type of reaction, such as the occurrence of a rash or acneiform dermatitis, is less reported with third-generation PKIs in clinical trials.
3.9. Regulatory Status of Anti-EGFR PKIs
Erlotinib was approved by the FDA on 18 November, 2004, and by the EMA on 19 September, 2005. The brand name is TARCEVA® [72, 109].
Gefitinib was approved by the FDA on 5 May, 2003, and by the EMA on 24 June, 2009. The brand name is IRESSA® [73, 110].
Afatinib was approved by the FDA on 12 July, 2013, and by the EMA on September 25, 2013. The brand name is GIOTRIF® [111, 112].
Dacomitinib was approved by the FDA on 27 September, 2018, and by the EMA on April 2, 2019. The brand name is VIZIMPRO® [24, 40].
Osimertinib was approved by the FDA on 13 November, 2015, and by the EMA on 2 February, 2016. The brand name is TAGRISSO® [113].
3.10. Indications
These drugs are indicated for the treatment of NSCLC, with EGFR mutation. Erlotinib is indicated for maintenance treatment for patients with stable disease following first-line chemotherapy. Erlotinib is also indicated for locally advanced and metastatic lung cancer, following the failure of first-line chemotherapy. Erlotinib is also indicated for pancreatic cancer [114].
Gefitinib is indicated for the treatment of locally advanced and metastatic lung cancer [115].
Afatinib is indicated for the treatment of locally advanced and metastatic lung cancer. Afatinib is also indicated for epidermoid cancer for the treatment of locally advanced and metastatic lung cancer that has progressed during or following treatment with platinum chemotherapy [112].
Dacomitinib is indicated as a monotherapy for the treatment of locally advanced or metastatic NSCLC with EGFR mutations [38].
Osimertinib is indicated as an adjuvant treatment following complete tumour resection in stage IB–IIIA NSCLC. It is also indicated for the treatment of locally advanced and metastatic lung cancer with or without the EGFR T790 mutation [116].
4. What Is New and Conclusion
EGFR inhibitors are an alternative to standard chemotherapy for the treatment of lung cancer with this mutation. The third-generation anti-EGFR appears to be more effective than the first and second generations and is indicated as first-line therapy. Overall, anti-EGFRs have a good safety profile. Third-generation anti-EGFRs appear to be less associated with adverse events such as skin reactions, due to their pharmacological mechanism, than other generations. However, a majority of patients develop resistance to these treatments. In the event of resistance, another generation of anti-EGFR may be prescribed, or a PKI targeting another receptor, chemotherapy, or ICI may be prescribed concomitantly, depending on the mechanism of resistance of the anti-EGFR [35].
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
Open-access funding was provided and organised by COUPERIN CY23.
Open Research
Data Availability
Data are openly available in a public repository that issues datasets with DOIs.




