Effect of Different Surfactants and Nanoparticles on Pore-Scale Oil Recovery Process Using Heterogeneous Micromodel
Abstract
The application of surface-active agents during oil recovery process is ubiquitous. It is essential to achieve a satisfying oil recovery rate with low dosage of surface-active substances in an environment-friendly manner. Despite the wide application of surface-active agents, the impact of individual and the combination of surface-active agents on the microscale multiphase flow and interfacial phenomenon have not been systematically investigated. In this work, idealized pore-throat network micromodels were employed as the surrogate of the porous media to study the influence of surface-active agents on the oil recovery involving nonionic, anionic, zwitterionic surfactants, and nanoparticles. Oil recovery efficiency and residual oil characteristic in different permeable regions were quantitatively analyzed. Anionic surfactants resulted in the highest oil recovery of 79% and were selected to formulate composite agents. The combination of anionic and zwitterionic surfactants resulted in better overall oil recovery which was up to 84%, yet complicated interfacial phenomenon was observed. To comprehend the complex interactions between crude oil and assorted surface-active agents, the impact of interfacial tension, wettability, and emulsification on oil-brine flow behaviors and final oil recovery was discussed providing an insight on the efficient and cost-effective application of surface-active agents on enhancing oil recovery.
1. Introduction
Fossil fuels nowadays still account for the majority of primary energy consumption worldwide. Various efforts have been made to mitigate the negative impacts of fossil fuel consumption including CO2 sequestration, utilization of environmentally friendly chemicals in oil production, and addressing subsidence issues [1, 2]. Waterflooding is regarded as one of the most widely applied methods during production operation featuring low-cost, convenient, and environmentally friendly. However, there still remains large quantity of residual oil after waterflooding, and the critical physical properties of the reservoir such as permeability, porosity, and wettability could be altered after long-term waterflooding that complicates the physical properties of the formation [3, 4, 5, 6]. Therefore, it is necessary to apply surface-active materials to reduce the interfacial tension between oil and brine and modify the physical properties of the formation, thus enhancing the sweep efficiency and the displacement efficiency [7].
Surfactants are the most common and representative surface-active materials that have long been the candidates for enhancing oil recovery. Surfactants can be categorized based upon its ionic type when dissociating in water, and they are nonionic, cationic, anionic, and zwitterionic, respectively. Nonionic surfactants typically do not dissociate in the aqueous solution and exist as ionic species and thus can be relatively stable and more environmental friendly [8]. Cationic surfactants are more capable of altering rock wettability especially for carbonates [9, 10]. Anionic surfactants are highly surface-active and capable of effectively reducing interfacial tension and improving wettability as well [11]. In addition, there are variety of sources for synthesizing anionic surfactant making it more economically sound [12]. Zwitterionic surfactants possess both positive and negative charges and therefore show good solubility and can achieve ultralow interfacial tension under relatively low concentration [13, 14, 15]. As for the application of enhancing oil recovery, a wide range of surfactants have been incorporated [2, 12, 16, 17, 18]. A variety of research has been conducted on the performance of surfactants under different conditions to examine the EOR potential and the underlying mechanism [7, 16, 19, 20]. It has been widely believed that the presence of surfactants aids in wettability alteration and oil mobilization [8, 21, 22]. However, due to the continuous development of reservoirs, fluid distribution and rock wettability have become more complicated such that single-type surfactants no longer meet the demand for enhancing oil recovery. As a result, combination of multiple surfactants and other surface-active materials was proposed and applied in the oilfield [23, 24]. Mixed-surfactant systems have demonstrated better capability of reducing interfacial tension (IFT) [25, 26, 27]. However, previous studies have shown that some of the synthetic surfactants applied in EOR demonstrated environmental and health hazards impacting water environment as well as micro-organisms [28]. Therefore, it is of great importance to reduce the dosage of these surfactants while maintaining the oil recovery efficiency. Benefiting from the rapid development of nanotechnology [29, 30, 31], nanoparticles have been applied in assorted research fields and specifically in the oil and gas industry to cooperate with surfactants to promote the oil displacement efficiency [32, 33, 34, 35, 36]. Assorted chemically-modified nanoparticles have also been proposed for EOR process [37, 38, 39]. Research showed that the addition of nanoparticles is beneficial to the IFT reduction and wettability alteration [38, 40]. It was also shown that the adsorption of surfactants was reduced with the presence of nanoparticles [41, 42]. Therefore, investigation on the synergistic effect and interplay of different combinations of surface-active agents is crucial to achieve higher oil recovery efficiency with as fewer surface-active agents as possible for the purpose of sustainable development. However, impact and synergy of various commonly used surface-active agents on oil recovery efficiency have rarely been examined systematically in pore-scale.
In order to investigate the oil displacement efficiency and mechanism of different materials, diverse experiments have been carried out including conventional core sample or sand pack flooding tests [19, 43]. Reservoir conditions can be better restored with these models, thus providing relatively effective evidence for field application. However, it is usually difficult to directly observe the dynamics and distribution of multiple fluids inside the model, therefore hindering a solid understanding of the underlying mechanism. Advanced scanning technologies have been applied in order to grasp the inner fluid distribution [44, 45, 46, 47]. Nevertheless, it is still challenging to achieve real-time observation of the oil recovery process. To better understand the fluid flow behaviors and interactions between oil and injected agents, micromodels have become prevalent in investigating the microscale phenomenon. Micromodels are the idealized physical models fabricated from silicon, glass, and polymers [48, 49, 50]. Varying pore-throat networks were designed including repeated patterns [50, 51, 52] and replication of realistic pore-throat topology [48, 53] which have been applied to serve for studying different oil recovery processes including surfactant flooding [54, 55], foam flooding [56, 57], polymer flooding [58], and smart waterflooding [50, 59].
Therefore, micromodels with patterned pore-throat network were adopted in this work to visualize the oil recovery process. Surfactants in this study were selected on the basis of wide application and accessibility. Systematic evaluation of these agents on the EOR performance was conducted. Specifically, the effect of surface-active agents’ composition and concentration and heterogeneity of the pore-throat network on oil recovery were examined with real-time monitoring using microscope. Characteristics of residual oil and interfacial phenomenon between oil and surface-active agents were analyzed. Subsequently, the underlying mechanisms of multiple interactions between crude oil and surface-active agents were discussed. As a consequence, this work provides an experimental reference for optimal selection and dosage of surface-active agents in heterogeneous micromodel. Mechanisms proposed in this study could also aid in a comprehensive understanding of the pore-scale interactions between crude oil and these chemicals. Ultimately, a more environmental friendly EOR approach can be developed while maintaining the production efficiency.
2. Materials and Methods
2.1. Materials
In this work, polydimethylsiloxane (PDMS) microfluidic devices were used and fabricated by standard rapid prototyping and soft lithology method [60, 61]. In brief, a silicon wafer with the designed pattern was fabricated photolithographically. PDMS was then cast against the wafer with raised posts creating an inverted replica. Holes were punched at the inlet and outlet of the PDMS device which was then bonded to a glass slide using oxygen plasma treatment to form a sealed microfluidic device. Microfluidic devices used in this work feature idealized pore-throat network generated by regular arrays of pillars. By varying the arrangement density and size of the pillars, two regions were generated as shown in Figure 1. High-permeable region comprises of pillars with a diameter of 240 μm and spacing of 60 μm. Low-permeable region comprises of pillars with a diameter of 100 μm and spacing of 20 μm.
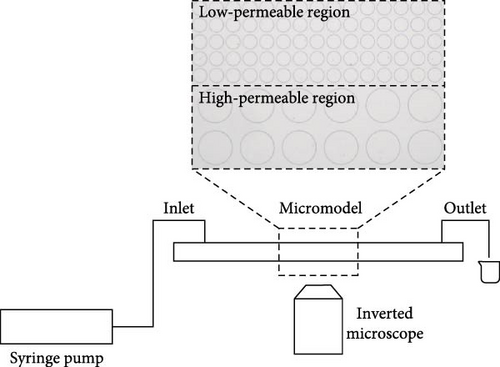
Formation water with 50,000 ppm salinity was prepared, and the detailed composition was shown in Table 1. Crude oil was used, and its composition was shown in Table 2. Surface-active agents involved in this work are as follows: Tween 80 (Tween), sodium 1-dodecanesulfonate (SDS), dodecyldimethylbetaine (BS-12), and neutral colloidal SiO2 solutions with average diameter of 20 nm (NP). All surfactant solutions were prepared using distilled water. The details of the applied agents are shown in Table 3.
| Total salinity (mg/L) | Concentration (mg/L) | |||
|---|---|---|---|---|
| NaCl | CaCl2 | MgCl2 | KCl | |
| 50,000 | 40,000 | 5,000 | 3,500 | 1500 |
| Asphaltenes (%) | Saturates (%) | Aromatics (%) | Resins (%) |
|---|---|---|---|
| 7.2 | 54.58 | 16.15 | 16.53 |
| No. | Agent name | Agent type | Concentration |
|---|---|---|---|
| 1 | Tween 80 (Tween) | Nonionic surfactant | 0.1 wt% |
| 2 | Tween 80 (Tween) | Nonionic surfactant | 0.3 wt% |
| 3 | Sodium 1-Dodecanesulfonate (SDS) | Anionic surfactant | 0.1 wt% |
| 4 | Sodium 1-Dodecanesulfonate (SDS) | Anionic surfactant | 0.3 wt% |
| 5 | Dodecyldimethylbetaine (BS-12) | Zwitterionic surfactant | 0.1 wt% |
| 6 | Dodecyldimethylbetaine (BS-12) | Zwitterionic surfactant | 0.3 wt% |
| 7 | Sodium 1-Dodecanesulfonate + Tween 80 (SDS + Tween) | Anionic + nonionic surfactant | 0.1 wt% (1 : 1 ratio) |
| 8 | Sodium 1-Dodecanesulfonate + Tween 80 (SDS + Tween) | Anionic + nonionic surfactant | 0.3 wt% (1 : 1 ratio) |
| 9 | Sodium 1-Dodecanesulfonate + Dodecyldimethylbetaine (SDS + BS-12) | Anionic + zwitterionic surfactant | 0.1 wt% (1 : 1 ratio) |
| 10 | Sodium 1-Dodecanesulfonate + Dodecyldimethylbetaine (SDS + BS-12) | Anionic + zwitterionic surfactant | 0.3 wt% (1 : 1 ratio) |
| 11 | Sodium 1-Dodecanesulfonate + SiO2 nanoparticles (SDS + NP) | Anionic surfactant + nanoparticles | 0.1 wt% + 0.1 wt% |
| 12 | Sodium 1-Dodecanesulfonate + SiO2 nanoparticles (SDS + NP) | Anionic surfactant + nanoparticles | 0.3 wt% + 0.1 wt% |
2.2. Methods
Experimental setup was displayed in Figure 1 consisting of syringe pump, microfluidic device, and inverted microscope with high-speed camera. Fresh PDMS devices as shown in Figure 2(a) were prepared before every experiment to ensure the consistency of the initial experimental conditions. Syringes filled with different fluids were connected to the microfluidic device via tubing and installed to the syringe pump. Formation water was first injected into the device through the pump at a rate of 5 μL/min until the void space inside the device was fully saturated to establish the initial water saturation which is corresponding to Figure 2(b). Crude oil was then injected to displace the indigenous water until its saturation was stabilized to establish initial oil saturation and connate water saturation. Figure 2(c) demonstrated the image taken after oil saturation process which was used to calculate the initial oil saturation afterward. Surface-active agent was then injected at a rate of 2 μL/min to simulate the oil recovery process and was terminated until oil saturation no longer changed. Figure 2(d) illustrated the last image taken during the experiment to obtain the residual oil saturation. The entire flooding process was observed using the microscope and recorded via the high-speed camera which was demonstrated in Figure 2. Experimental procedures were repeated with different surface-active agents. All experiments were conducted at room temperature and pressure. Interfacial tension was measured via spinning drop method (Kruss SITE 100).

3. Results and Discussion
3.1. Effect of Single Surfactant on Oil Recovery
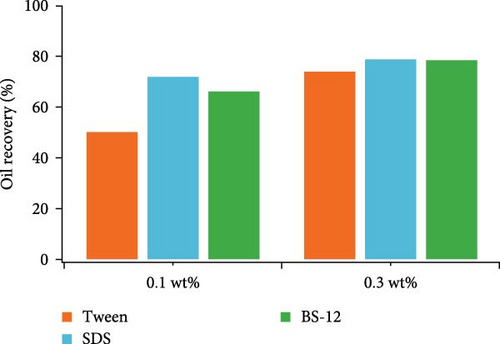
Single-type surfactant was used in the beginning to examine the effect of different surface-active agents on microscale residual oil pattern and oil recovery efficiency. Three different surfactants with two concentration levels (0.1 and 0.3 wt%) were used including nonionic, anionic, and zwitterionic surfactants. Fluid saturation change during the flooding process was shown in Table 4. Oil recovery efficiency of each agent was plotted in Figure 3. SDS and BS-12 clearly showed better oil displacement efficiency than Tween under two concentration levels. For SDS, it demonstrated good oil displacement ability at both concentration levels. For Tween and BS-12, oil recovery rate was clearly higher when the surfactant concentration increased especially for Tween.
| No. | Applied agent | Initial oil saturation (%) | Connate water saturation (%) | Residual oil saturation (%) |
|---|---|---|---|---|
| 1 | Tween 0.1 wt% | 96.30 | 3.70 | 49.49 |
| 2 | Tween 0.3 wt% | 94.44 | 5.56 | 25.62 |
| 3 | SDS 0.1 wt% | 93.71 | 6.29 | 27.64 |
| 4 | SDS 0.3 wt% | 94.35 | 5.65 | 20.79 |
| 5 | BS-12 0.1 wt% | 93.75 | 6.25 | 33.47 |
| 6 | BS-12 0.3 wt% | 94.16 | 5.84 | 21.08 |
In order to closely investigate the flow behaviors and oil displacement ability of different surfactants, oil saturation changes in both high- and low-permeable regions were calculated using ImageJ and plotted against time in Figure 4. For Tween 80, higher surfactant concentration aids in the removal of oil especially in the low-permeable region indicated by the big gap between two lines representing the oil saturation change by increasing Tween concentration. In high-permeable region, the difference in oil saturation is not as significant as that in low-permeable region. As for SDS, in high-permeable region, surfactants with both concentrations were capable of recovering the majority of crude oil indicated by the overlapped curves. SDS with higher concentration showed better performance on oil displacement in low-permeable region, and this discrepancy is not as high as Tween. For BS-12, increasing surfactant concentration favors higher oil recovery in both high- and low-permeable zones. Additionally, in low-permeable zone, oil recovery efficiency was higher at the early stage of the oil recovery process. Compared among the three different surfactants, SDS and BS-12 were clearly better at displacing oil in high-permeable region than Tween. In low-permeable region, the ability for the agents to displace oil was similar especially when the concentration of the agents was higher.
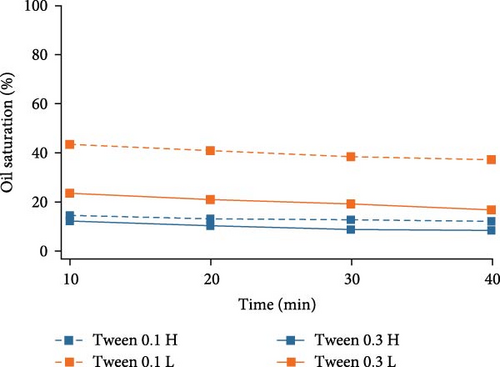
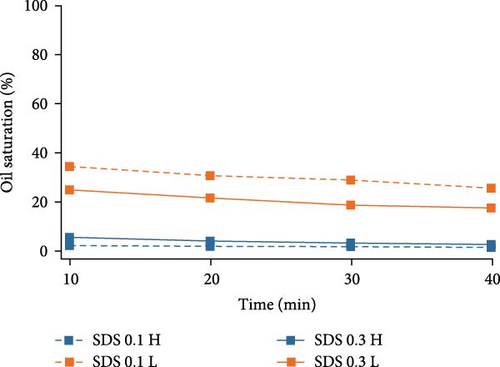
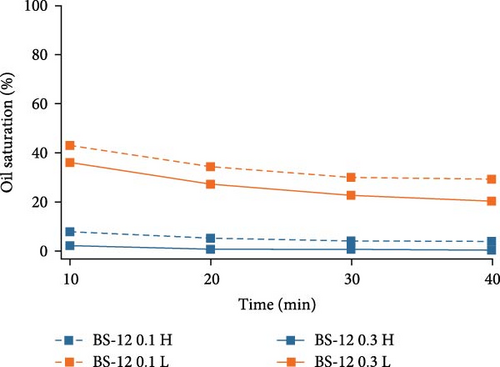
Overall, in high-permeable zone where pore-throat size is larger and the flow resistance is lower, both anionic and zwitterionic surfactants were able to recovery the majority of oil, and in this case, the surfactant concentration did not play an important role. In low-permeable zone where pore-throat size is smaller, higher surfactant concentration is essential in lowering the interfacial tension and displacing the crude oil. Table 5 showed the measured IFT of different surfactants. SDS and BS-12 showed lower IFT than Tween. The measured values mostly conform to the final oil recovery except that 0.1 wt% BS-12 showed the lowest IFT, yet the oil recovery was not the highest.
| No. | Surfactant | Concentration (wt%) | IFT (mN/m) |
|---|---|---|---|
| 1 | Tween | 0.1 | 2.000 |
| 2 | 0.3 | 0.402 | |
| 3 | SDS | 0.1 | 0.220 |
| 4 | 0.3 | 0.335 | |
| 5 | BS-12 | 0.1 | 0.149 |
| 6 | 0.3 | 0.370 | |
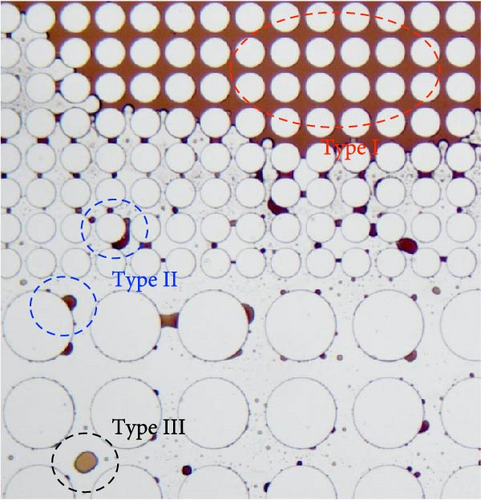
Subsequently, the residual oil characteristics were analyzed to better understand the oil displacement behaviors by different surfactants and provided an insight on the future EOR strategies. The observed residual oil was roughly categorized as three types based upon its shape as demonstrated in Figure 5. Type I is defined as the continuous residual oil, and its major characteristic is that it existed as interconnected oil phase and usually remained unswept. Type II is defined as the trapped residual oil. It was usually trapped in the throats that cannot be easily dislocated or clung to the post surface that was difficult to remove. Type III is defined as dispersed residual oil. It existed as dispersed and isolated oil drops in either pores or throats. The proportion of every residual oil type was analyzed using ImageJ and plotted in Figure 6.
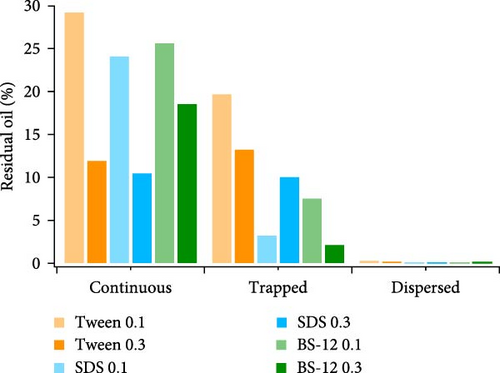
The majority of the residual oil can be classified as type I, and it was mostly observed in the low-permeable region for most experiments. Type II residual oil takes up the second large share, and the percentage of type III residual oil can be almost negligible compared with the others. Increasing surfactant concentration aids in decreasing type I residual oil especially for Tween and SDS. IFT between oil and brine is generally lower when the surfactant concentration increased. Wettability state could be improved as well by the addition of surfactant. Therefore, more oil phase could be emulsified and mobilized leading to higher oil recovery. For SDS, the percentage of type II residual oil increased when surfactant concentration is higher. It could attribute to the fact that when 0.3 wt% SDS was applied, the quantity of type I residual oil was the lowest and reached its capacity in displacing oil, therefore leaving a relatively large portion of trapped oil behind. However, the combination of type I and type II residual oil is still low compared with other. As for BS-12, increasing its concentration is less helpful in reducing the continuous crude oil compared with the others. However, type II residual oil decreased when surfactant concentration is higher. It was observed that the displacement front was more uniform when BS-12 was applied and less oil was left behind after the sweep. BS-12 solution first invaded the high-permeable region and displaced the indigenous oil and proceeded toward the low-permeable region exhibiting an approximately piston-like displacement behavior. Regarding BS-12 as the zwitterionic surfactant, both anionic and cationic heads were formed when dissolved in water. A more complicated and robust interfacial layer could be generated consisting of polar components in crude oil and the surfactant molecules which could be packed more tightly at the interface due to the electrostatic interactions [13, 62, 63]. A robust interfacial layer could be beneficial to the uniform displacement front. In addition, the relatively low IFT between BS-12 agent and oil also favors the displacement of oil in the micromodel.
Based on the above analysis, SDS showed better overall performance in displacing oil in both high- and low-permeable regions under two concentration levels. After comprehensive evaluation, SDS was selected to form composite oil displacement agents with other surface-active agents on account of wide accessibility, lower cost, and better performance under lower surfactant concentration.
3.2. Effect of Surface-Active Agent Combinations on Oil Recovery
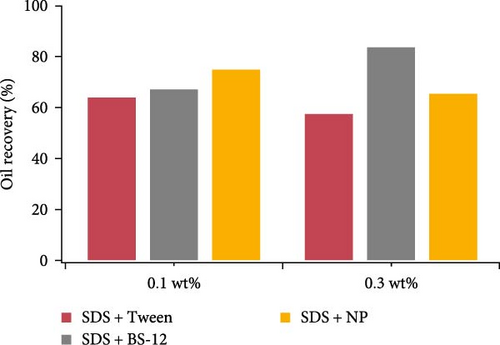
Three combinations of surface-active agents were examined including SDS with Tween, SDS with BS-12, and SDS with NP under two concentration levels (0.1 and 0.3 wt%). Figure 7 showed the overall oil recovery rate of these composite agents. Table 6 presents the fluid saturation change during the experiment. At lower concentration, SDS with NP showed better performance, and at higher concentration, SDS with BS-12 resulted in higher oil recovery. Among them, SDS with Tween resulted in relatively low oil recovery rate. Three different combinations showed different reactions to the change of concentration. For SDS with Tween, and SDS with NP, increasing concentration led to lower oil recovery. For SDS with BS-12, the trend was the opposite.
| No. | Applied agent | Initial oil saturation (%) | Connate water saturation (%) | Residual oil saturation (%) |
|---|---|---|---|---|
| 1 | SDS + Tween 0.1 wt% | 78.28 | 21.72 | 35.79 |
| 2 | SDS + Tween 0.3 wt% | 93.76 | 6.24 | 42.22 |
| 3 | SDS + BS-12 0.1 wt% | 92.72 | 7.28 | 32.52 |
| 4 | SDS + BS-12 0.3 wt% | 93.58 | 6.42 | 16 |
| 5 | 0.1 wt%SDS + NP | 94.96 | 5.04 | 24.78 |
| 6 | 0.3 wt%SDS + NP | 95.40 | 4.60 | 34.28 |
To further inspect their oil displacement behaviors, oil saturation variations with time in high- and low-permeable regions were calculated and plotted in Figure 8. Different from the single surfactant agent, the combination of different surface-active agents all demonstrated good oil displacement ability in high-permeable region for both concentration levels. Especially for SDS with BS-12 and SDS with NP, oil saturation in high-permeable region almost reached zero at the end of the flooding. However, their oil displacement abilities in low-permeable region varied. For SDS with Tween, varying concentration resulted in slight change in the oil saturation. As for SDS with BS-12, the impact of agent concentration on oil recovery was distinct. Increasing the concentration of the composite agents significantly improve its performance in low-permeable region. Additionally, oil recovery efficiency at early flooding period was improved at higher concentration level indicated by the greater slope of the curve. For SDS with NP, two lines representing different concentrations diverged and lower concentration ultimately achieved higher oil recovery rate in the low-permeable region which is discussed in Section 3.3.
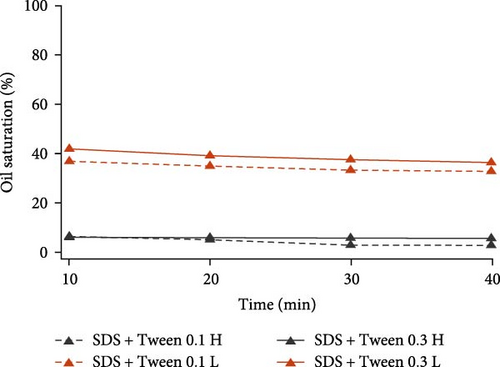
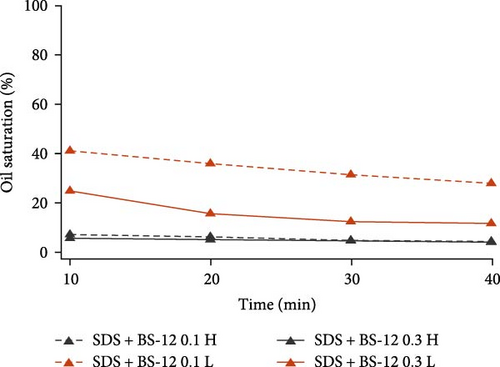
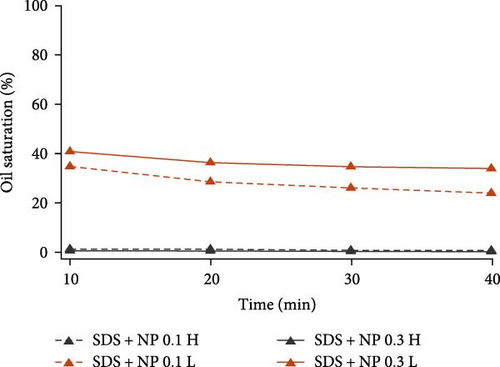
Overall, for the six composite agents, they were all capable of perfectly recovering oil in the high-permeable zone. The difference in the final oil recovery lies in the performance in low-permeable region. 0.3 wt% SDS with BS-12 showed the highest oil recovery efficiency in low-permeable region. To interpret the different performances of these composite agents, IFT values were measured and tabulated in Table 7. SDS with BS-12 showed lower IFT. For SDS with Tween, the IFT of their combination was even higher than individual SDS and Tween. For SDS with NP, higher concentration led to lower IFT; however, the oil recovery rate was lower than that with lower concentration implying that IFT is not the only factor influencing the oil recovery and there exists an optimal IFT value for different surface-active agents.
| No. | Solutions | Concentration (wt%) | IFT (mN/m) |
|---|---|---|---|
| 1 | SDS + Tween | 0.1 | 1.532 |
| 2 | 0.3 | 1.713 | |
| 3 | SDS + BS-12 | 0.1 | 0.133 |
| 4 | 0.3 | 0.164 | |
| 5 | SDS + NP | 0.1 | 0.804 |
| 6 | 0.3 | 0.312 | |
To comprehend the different oil displacement behaviors after different floodings, microscale residual oil characteristics were analyzed as well. The criteria for residual oil classification are the same as the one mentioned previously. As shown in Figure 9, type I residual oil still takes up the largest portion of residual oil; however, the quantity of type I residual oil is clearly lower than that of single-type surfactant flooding. The combination of different surface-active agents generally improved the sweep and displacement efficiency. Noteworthily, the increase of concentration for SDS with BS-12 greatly aids in the reduction of type I residual oil implying that the majority of the oil was swept by the agents. For SDS with NP, higher agent concentration led to slightly larger portion of interconnected oil remaining unswept. However, for SDS with Tween, increasing concentration had a negative impact on the recovery of type I residual oil. This combination also resulted in relatively large portion of type II and even type III residual oil. For SDS with BS-12, residual oil distribution and its response to the saturation change are similar to single SDS surfactant. Type II residual oil occupied the largest proportion; therefore, it would be relatively difficult to further enhance oil recovery. This could be accomplished by achieving lower IFT to dislodge the trapped oil. 0.1 wt% SDS with NP showed good performance in reducing type II residual oil. Specifically, type II residual oil is the smallest when this agent combination was applied implying its good ability in mobilizing and carrying the oil phase in the throats. Future work can be conducted on combining SDS and BS-12 with NP to reduce both type I and type II residual oil.
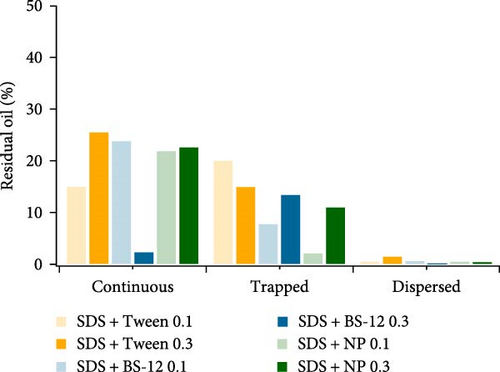
Residual oil characteristics as well as oil recovery inside heterogeneous porous media are a result of synergistic effect of fluid–fluid and fluid–solid interactions. The addition of surface-active agents could greatly impact both interactions by affecting properties such like IFT and wettability state. To closely examine the microscale two-phase flow behaviors and interfacial phenomenon, a discussion was provided afterward.
3.3. Impact of Surface-Active Agents on the Microscale Two-Phase Flow Behaviors and Interfacial Phenomenon
As shown previously, the application of different surface-active agents could lead to varied crude oil saturation and distribution, and it is the result of different oil–water flow behaviors and interfacial interactions. Therefore, microscale two-phase flow behaviors and crude oil–surface-active agent interfacial phenomenon were discussed in this part to comprehend the mechanism of oil recovery by various surface-active agents.
3.3.1. Impact of IFT
Reduction of IFT is generally considered as one of the major impacts that surface-active agents have on the oil recovery efficiency. However, it was also reported that lower IFT does not guarantee higher oil recovery and there exists an optimum range of IFT for different formations [64]. To reveal the relationship between the IFT of different surfactants and the combinations and the final oil recovery, oil recovery rate versus IFT values was plotted in Figure 10. Since the IFT values are very close, logarithm scale was applied to the horizontal axis for a better representation of the data points. In general, lower IFT resulted in higher oil recovery rate at either lower or higher concentration level of the surface-active agents. However, lower IFT did not guarantee higher oil recovery as a whole. Figure 10 is divided into three sections for a clearer comprehension. In the range of 0.164–0.804 mN/m of IFT, the oil recovery is between 65.7% and 79.2%. Higher oil recovery was achieved when the surfactant concentration was higher. When IFT is lower than 0.164 mN/m, the oil recovery efficiency is between 66.5% and 84.0%. The combination of 0.1 wt% SDS and BS-12 exhibited the lowest IFT; however, the highest oil recovery rate was achieved by the same combination with higher concentration. When the IFT is higher than 0.804 mN/m, it is clear that the corresponding oil recovery is lower and the agents used in these experiments always included Tween 80. Interestingly, the IFT of SDS with NP is not low compared with the others, yet the resulted oil recovery was relatively satisfying implying that NP play an important role during oil recovery process in other aspects other than lowering the IFT.

3.3.2. Impact of Wettability
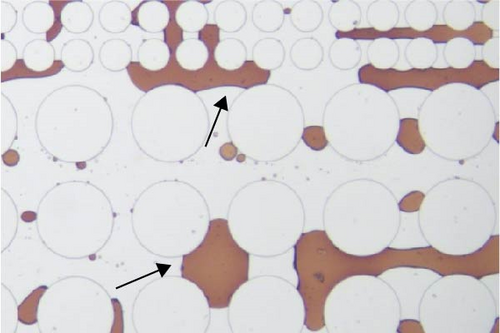
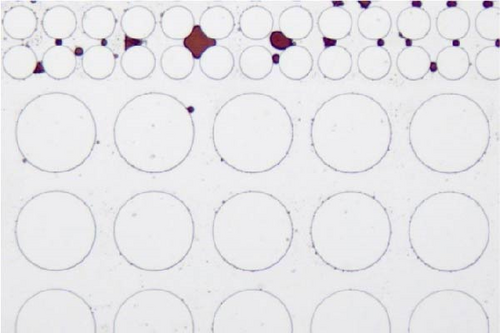
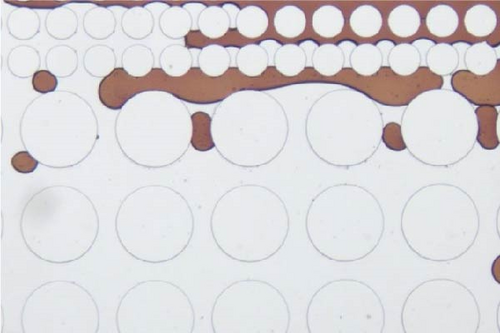
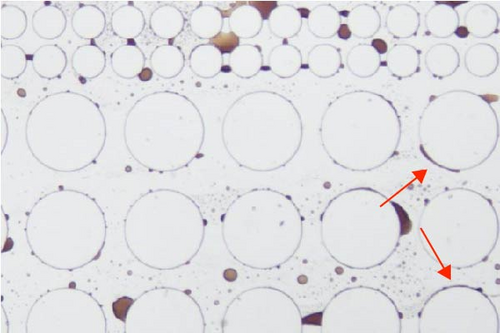
In this work, an idealized surrogate was adopted to study the oil recovery process, and its wettability state was not as complicated as the real reservoir rocks. However, it could still be observed that the wettability state was altered after the injection of surface-active agents. PDMS microfluidic devices used in this work were initially water-wet. After crude oil saturation, some polar components in the oil phase may cling to the solid surface rendering the device partially oil wet. The major cause for this attachment is that the polar components in the crude oil could adsorb onto the solid surface [65]. After surfactant flooding, different wettability state was achieved as demonstrated in Figure 11. It was found that a relatively less water-wet state was achieved after surfactant flooding using Tween as evidenced by the attachment of oil droplets on the post surface as well as the presented contact angle in Figure 11(a) illustrated with black arrows. When SDS and BS-12 were applied, respectively, it is clear that less oil was adhered to the solid surface and the wettability state was relatively uniform shown in Figures 11(b) and 11(c), respectively. In Figure 11(d), it was observed that the addition of SDS to Tween facilitated maintaining a more water-wet state. When surfactants contacted the oil phase, ionic components with better surface activity could interact with the adsorbed carboxylic groups and release the attached oil phase. The combination of SDS and BS-12 resulted in a more complicated wettability status as shown in Figure 11(e). Oil film and tiny droplets were attached on the solid surface demonstrated with red arrows which could be the consequence of emulsification process that will be discussed afterward. Concentration of these agent could also make a difference. Increasing the concentration of these surface-active agents favored achieving more uniform wettability state, respectively, and less oil attachment. Particularly, increasing concentration of SDS and BS-12 alleviated the film attachment, yet small-sized emulsions were still produced, and only minority of them clung to the post surface. Nevertheless, wettability alteration induced by surfactant injection benefited the removal of adsorbed crude oil as shown in Figure 12. Furthermore, the combination of SDS and NP resulted in a perfectly uniform wettability state as displayed in Figure 11(f). The addition of NP greatly facilitated the removal of the oil phase as the solution sweeping through the pores. When nanoparticles were added, wedge structure can be formed; therefore, oil phase was easily detached from the solid surface.

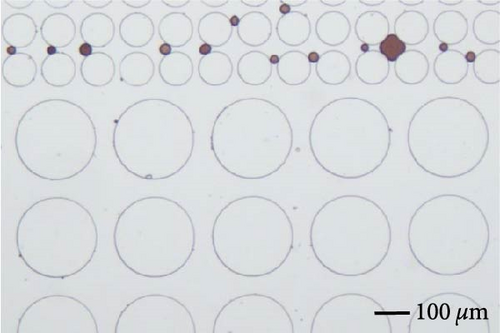

3.3.3. Impact of Emulsification
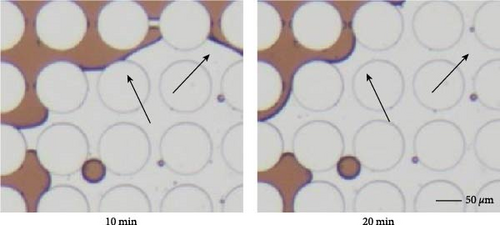
Emulsification is one of the most commonly observed phenomena when surface-active agents are applied. During the flooding, oil phase was the dispersed phase for most of the time, and it was emulsified by the invading surfactant solution and carried to the outlet. Continuous oil phase was disintegrated into individual droplets or slugs that potentially influenced the final oil recovery in different ways. Firstly, it is easier for the aqueous phase to transport smaller droplets especially within smaller pore throats. The size of the produced droplets was related to the IFT as shown in Figure 13. Dashed lines circled the emulsification behaviors. Lower IFT was achieved when the combination of SDS and BS-12 was applied, and, in this case, multiple emulsions were formed. A crowd of small droplets with radius less than 2 µm was formed after the displacement front. In the meantime, solubilization of crude oil was demonstrated in Figure 13 indicated by the lighter brown color and pointed out with black arrows. The concentration of the applied surfactant solution is above the critical micelle concentration enabling the oil solubilization. This complicated phase behavior was only observed when the combination of SDS and BS-12 was employed. Despite the complicated wettability status and phase behaviors, 0.3 wt% SDS and BS-12 resulted in the highest oil recovery rate among these experiments.
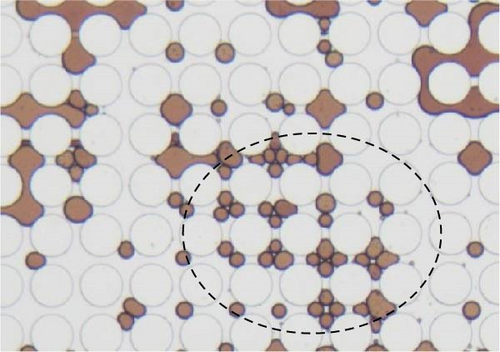
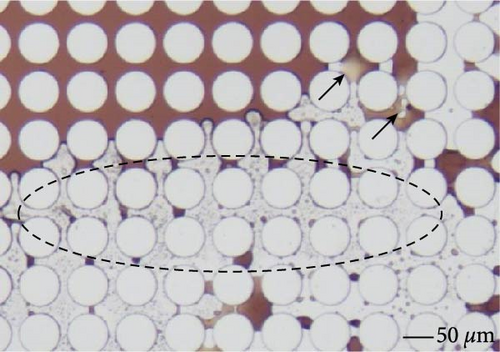
Figure 14 illustrated the oil detachment process using SDS with BS-12 and SDS with NP. For SDS with NP, it is evident that the existence and accumulation of nanoparticles at the post-oil–water interface generated the structural disjoining pressure facilitating peeling off crude oil from the solid surface as shown in Figure 14(b) which was indicated by black arrows in the figure. It is also possible that the addition of nanoparticles to the surfactant modified the viscoelastic behavior of the EOR agent such that it is easier to displace the oil phase [34, 66]. And an optimal ratio of surfactant to nanoparticles is necessary for this viscoelastic behavior [67]. This also possibly explains the observation in this work that 0.3 wt% SDS + NP resulted in lower oil recovery than that of 0.1 wt% SDS + NP. When the surfactant solution consisting of SDS and BS-12 flows through the throats, a film was seemingly formed on the post surface circled with dashed lines in Figure 14(a). Yet after a period of time, oil was recovered, and the post surface was free of crude oil. The film was generated during the dynamic contact between this specific surfactant solutions and crude oil which might also consist of small-sized emulsions. The generation of such film could be beneficial to both sweep and displacement efficiency and therefore resulted in higher final oil recovery.
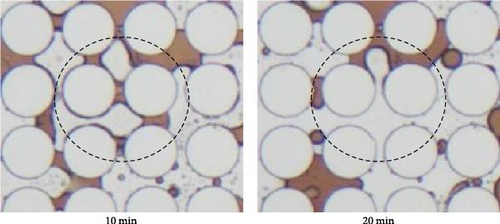
To summarize, the application of different surface-active agents could lead to varying oil recovery rate which was regarded as a result of varying IFT, wettability state, and emulsification process. Specifically, there is an optimal range for IFT to achieve higher oil recovery. A more uniform and hydrophilic wettability state was preferred and could be achieved by application of SDS and BS-12 as well as the combination of SDS + Tween and SDS + NP. Noteworthily, the combination of SDS and BS-12 resulted in a complicated wettability state yet higher oil recovery. They could possibly attribute to the strong emulsification and oil solubilization. Hence, a combination of proper IFT, uniform wettability state, and strong emulsification could possibly lead to higher oil recovery. However, in this work, two-dimensional idealized pore-throat network was employed as a surrogate of the oil reservoir which cannot fully represent the realistic porous media. Moreover, improvements can be made toward a better emulation of reservoir condition (i.e., high temperature and pressure).
4. Conclusions
Micromodels consisting of idealized pore-throat network were applied to study the impact of surface-active agents on oil recovery. Single surfactants and composite oil displacement agents were examined, respectively. The results showed that SDS demonstrated better performance, and the combination of SDS and BS-12 with 0.3 wt% concentration resulted in the highest oil recovery. To investigate the mechanism of oil recovery process with different surface-active agents, the impact of IFT, wettability, and emulsification was discussed. Oil recovery was not solely dependent on lowering IFT, but instead, an optimal range of IFT exists. As for the wettability state, SDS and BS-12 were better at maintaining a water-wet state than Tween individually. The combination of SDS and BS-12 resulted in a complicated wettability state. A film was seemingly formed on the post surface which was presumably beneficial to achieve higher oil recovery.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no. 52174028 and 51934005), the Innovation Capability Support Program of Shaanxi (program no. 2023KJXX–051), and the Scientific Research Program Funded by Shaanxi Provincial Science and Technology Department (2023-JC-QN-0432).
Open Research
Data Availability
Data available upon request.




