The Synergetic Roles of Stromal Vascular Fraction (SVF) and Extracellular Matrix (ECM) on Fat Graft Retention in Nude Mice
Abstract
Background. Both stromal vascular fraction (SVF) and extracellular matrix (ECM) are of great concern to adipogenesis and angiogenesis. SVF and ECM are rich in adipose tissue and may provide structural and biochemical support and form a microenvironment for free granular fat. The present study was to investigate whether SVF-gel, a mixture of SVF and ECM harvested by mechanical emulsification, could improve the long-term volume retention of fat grafts. Methods. Human SVF-gel of different percentages was mixed with microfat. According to the percentages of SVF-gel into microfat, 4 groups were included in the study; they are the microfat group, 10% gel group, 30% gel group, and gel group. The fat grafts were transplanted in the subcutaneous layer on each flank in nude mice. The sample volume was measured to evaluate the fat retention rate 90 days post-transplantation. Tissue integrity, collagen content, numbers of viable adipocytes, and density of blood vessels were examined by further detection. Results. The retention volume rates in the 30% gel group and gel group were significantly higher than the microfat group and the 10% gel group (p < 0.05). Equivalent fat integrity was observed in the four groups. Higher collagen volume, enhanced mRNA expression of VEGF, TNF-α and adiponectin, more CD31-positive blood vessels, and more regenerative adipocytes were observed in the 30% gel group and the gel group. The fat tissue in the 30% gel group showed similar structures as the normal fat tissue, while almost of the tissue in the gel group exhibited as fibrous tissue. Conclusion. SVF-gel could improve fat graft retention while it came to a certain ratio into microfat via proangiogenic effect and fat regeneration which may be provided by SVF and ECM synergistically. SVF-gel-assisted fat grafting is a promising strategy to be used in clinical operations.
1. Introduction
Autologous fat grafting (AFT) is a widely applied technique to achieve soft tissue volume augmentation and contour improvement and has obtained great attention. Autologous fat is easy to harvest and innately biocompatible, representing the ideal filler in plastic surgery [1]. The principles of fat harvest, processing, and injection have reached broad consensus. However, the retention rate of mass volume engraftment remains suboptimal due to dynamic changes of various components during grafting [2, 3]. Nowadays, surgeons advocate in-need volume injection than overcorrection. The ensuing volume loss leads some patients to undergo repeated fat-grafting procedures in 3–6 months after the initial one, increasing healthcare expenditures while diminishing patient satisfaction.
Apoptosis of mature adipocytes, especially those located in the center of the fat particles, leads to eventual loss of graft volume after transplantation. Whereas adipose tissue-derived stem cells (ASCs) are more likely to survive the physical stress and hypoxic result of transplantation. They thereafter play a pivotal role in adipose tissue regeneration through adipogenic and vascular differentiation, as well as paracrine of angiogenic, antiapoptotic, and antioxidative factors [4–6]. These observations are rationale behind cell-assisted lipotransfer. Therefore, in recent years, some studies have been carried out promoting fat graft retention with advanced cell therapies including ASCs and stromal vascular fraction (SVF) cells [7, 8]. However, it is time consuming and laborious to isolate SVFs and culture ASCs, accompanied by some safety concerns [9].
Mechanical shear force created by the liposuction system cuts the intact adipose tissue into small particles with broken ECM fibers and detached adipose lobules [10, 11]. After fat grafting, these free adipose particles turn into an integrated tissue by adipose extracellular matrix (ECM) reconstruction. Generally, a prompt ECM reconstruction process occurs and completes in the first week in the surviving and regenerative areas, and in the necrotic zone, there was no complete ECM framework [12]. It means ECM reconstruction participates into fat survival directly. After adding porcine acellular dermal matrix into fat grafts, adipocyte necrosis in the central area of the grafts reduced while tissue regeneration was increased by increasing ECM genes expression levels [13]. It may be beneficial to add ECM in grafted fat to improve fat retention.
In 2017, Yao et al. disposed nanofat and obtained an injectable mixture by mechanical emulsification, which was rich in native ECM and SVFs, named SVF-gel [14]. The SVF cell density (>4 × 105 cells/ml) in the gel is twice that of the microfat [14]. Moreover, recent research studies have demonstrated that SVF-gel could improve ischemic flap survival, remodel hypertrophic scars, and accelerate wound healing for their angiogenic potential and shed lights on rescuing varied ischemic conditions [15–17]. Either ECM or SVF transplantation could promote fat survival by building an angiogenic and anti-inflammatory microenvironment [13, 18, 19]. SVF and protective ECM component in the SVF-gel may synergistically contribute to tissue regeneration, making it an optimal option for cytotherapy. What is more, the process of mechanical emulsification circumvents the collagenase-related safety concerns and cell culture limitations. However, the effects of combined ECM and SVF-assisted fat graft still remain unclear.
Therefore, it seems a promising workable application to add SVF-gel into fat grafts to observe the synergetic roles of SVF and ECM on increasing volume retention. In the present study, we mixed SVF-gel into standard Coleman microfat with different ratios and aimed to evaluate its effects on fat retention, to investigate the underlying mechanisms, and to establish a new SVF-gel-assisted fat graft strategy for future clinical application.
2. Materials and Methods
2.1. Fat Source and Patient Information
All adipose tissues were obtained from abdomen or inner thigh of 8 healthy Chinese female patients who underwent cosmetic liposuction (mean age ± SD, 30.37 ± 4.6 years; range, 24–37 years). Patients with metabolic diseases were excluded. All patients signed the informed consent, and the study was approved by the Ethic Committee of Shanghai Ninth People’s Hospital. Each experiment was performed with lipoaspirate from a single patient.
2.2. Fat Preparation
Lipoaspirate was harvested via a standard 3-mm multiport cannula (Tulip Medical Products, San Diego, California, USA) with sharp side holes of 1 mm in diameter under tumescent anesthesia. Then, the fat was centrifuged at 1200 g for 3 min, and the liquid portion was discarded. The fat in lower 1/2 layer in each syringe was transferred into a new syringe and processed with cotton pad infiltration and works as microfat. The other part of lipoaspirate was prepared for SVF-gel as described previously [16]. In brief, the adipose tissue was mechanically emulsified by shifting between two 10-ml syringes connected by a female-to-female Luer-Lok connector with an internal diameter of 2.4 mm for 1 min (10 mL/s) and turned into an emulsion. The emulsified Coleman fat was filtered through a nylon mesh of 500 μm pore size and centrifuged at 2000 g for 3 min. The sticky substance under the oil layer was defined as SVF-gel.
2.3. Animals and Fat-Grafting Model
All animal experiments were approved by Shanghai Ninth People’s Hospital Institutional Animal Care and Use Committee and performed according to the guidelines of the National Health and Medical Research Council (China). Female nude mice (aged 6–8 weeks) were housed in individual cages with a 12 h light/dark cycle and provided with standard food and water. According to the percentages of SVF-gel mixed into microfat, four groups were included in the study, namely, microfat group, 90% microfat + 10% SVF-gel (10% gel group), 70% microfat + 30% SVF-gel (30% gel group), and SVF-gel group (gel group). Eighty nude mice were divided into 4 groups averagely and randomly, and 0.3 ml fat was injected into a spherical shape in the subcutaneous layer on each flank using a 1.0 mm blunt infiltration cannula.
2.4. Sample Collection and General Measurement
The animals in each group were sacrificed at day 7, 14, and 90 after transplantation. At sacrifice, the grafts were harvested and carefully separated from the surrounding tissue. The samples at day 90 were photographed, and their volumes were immediately measured using the liquid overflow method. The graft was put into a syringe filled with normal saline and the graft volume was recorded as the increase of the liquid volume. The volume retention rate was calculated as the increased volume (ml)/0.3 ml (the original volume of fat graft) ∗ 100%.
2.5. RNA Isolation and Real-Time Polymerase Chain Reaction (RT-PCR)
The fat tissue was quickly excised, immediately frozen in liquid nitrogen, and stored at −80°C. The total RNA was extracted from 50 mg of the frozen tissue using the RNeasy Lipid Tissue Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany) and a Rotor-Gene 3000 Real-Time PCR Detection System (Corbett Research, Sydney, Australia) was used to amplify cDNA. GAPDH was used as the reference gene. Expression levels were calculated using the 2−∆∆Ct method.
2.6. ELISA Assay
An enzyme-linked immunoabsorbent assay (ELISA) was applied for the detection of the collagen type 1α1 (COL1A1). Specifically, skin tissue (50 mg) from each sample was harvested and homogenated in 0.3 mL tissue protein extraction reagent and 3 μL phenylmethylsulfonyl fluoride (Sigma-Aldrich). The tissue supernatant was collected to detect hydroxyproline using an ELISA kit according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN). Optical density values were examined on a microplate reader (Denley Dragon, Thermo Labsystems, Helsinki, Finland).
2.7. Histological Staining
Tissue samples were fixed in 4% paraformaldehyde and embedded in paraffin. The samples were then sectioned and stained with haematoxylin-eosin (H&E) to evaluate fibrosis and fat integrity. Fat integrity was evaluated as described by Hu et al. [20]. For immunohistological staining, paraffin-embedded tissue sections were incubated with rabbit anti-CD31 and antiperilipin antibodies (Abcam, Cambridge, UK), followed by incubation with a horseradish peroxidase-conjugated secondary antibody (Dako Glostrup, Denmark) or Alexa Fluor 488-conjugated secondary antibodies (Invitrogen, California, USA). The number of CD31-positive blood vessels was calculated. Five random fields were selected from each sample. All measurements were performed with Image-Pro Plus software (Media Cybernetics; Silver Spring, MD, USA).
2.8. Masson’s Trichrome Staining
Sections were deparaffinized in xylene, rehydrated in a graded ethanol series, and postfixed in Bouin’s fixative for 1 h at 55°C. The nuclei and collagen were sequentially stained with equal volumes of ferric chloride solution and alcoholic hematoxylin/trichrome solution, respectively. Total collagen volume was reported as the percentage of the total tissue area positively stained with aniline blue as determined using Image J software.
2.9. Statistical Analysis
Data are presented as the mean ± standard deviation. Differences between the groups were analyzed using one-way analysis of variance (ANOVA) or nonparametric tests. Differences between groups were evaluated using one-way analyses of variance followed by Bonferroni post-test. Statistical analyses were performed using IBM SPSS software (SPSS Statistics V22, IBM Corporation, USA). Values of p < 0.05 were considered statistically significant.
3. Results
3.1. SVF-Gel Improved Fat Graft Survival
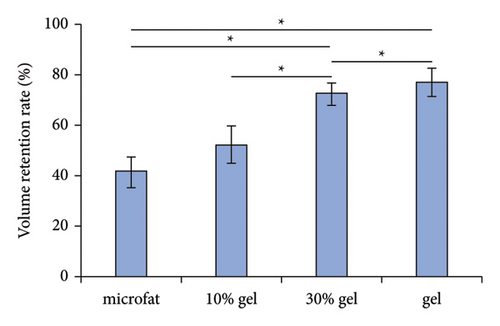
At 90 days post-transplantation, the grafts were spherical and easy to extract. Representative grafts in each group are shown in Figure 1(a). The fat retention volume of each fat graft was measured, and the results showed that both the volume retention rates in the 30% gel group (72.8 ± 4.3%) and the gel group (76.65 ± 5.4%) were much higher than the microfat group (52.1 ± 7.3%) and the 10% gel group (41.3 ± 6.6%), p < 0.05. No significant difference was observed either between the microfat group and the 10% group or between the 30% gel group and the gel group, p > 0.05 (Figure 1(b)). The results indicated a beneficial effect of SVF-gel supplementation on improving graft retention while its percentage achieved up to 30%.
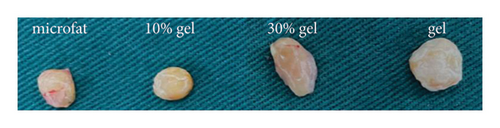
3.2. Histological Evaluation of the Grafts
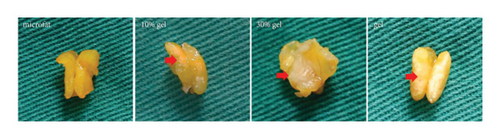
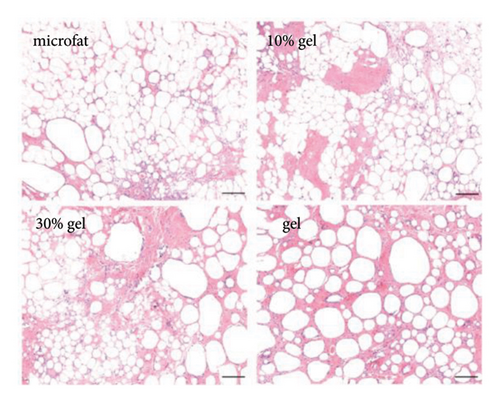
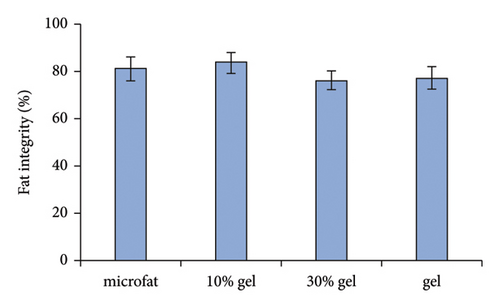
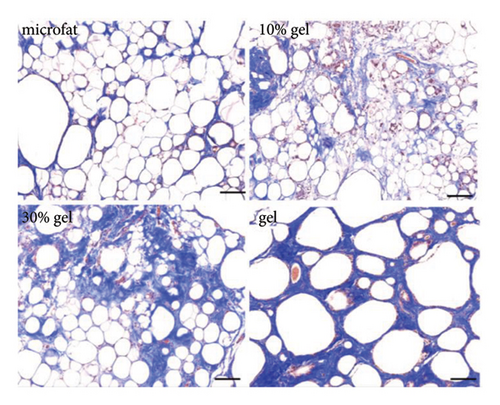
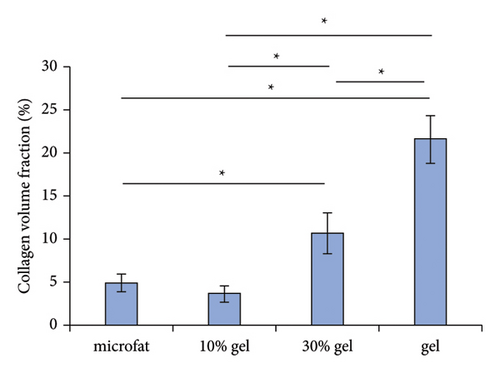
White fibrous constituents were found in the 10% gel group, the 30% gel group, and the gel group when dissecting the fat globules harvested 90 days post-transplantation, and its content increased conspicuously along with the proportion of SVF-gel added into the microfat. The fatty portion in samples of microfat group showed gold, oil-rich appearance. Whereas in the gel groups, more fibrous tissues instead of normal fatty structure were found (Figure 2(a)). Statistical analysis derived from H&E staining showed equivalent fat integrity area in the four groups, p > 0.05 (Figures 2(b) and 2(c)). Conformance to the results of H&E, the quantities of total collagen volume in the 30% gel group (22.7 ± 2.4%) and gel group (21.6 ± 2.8%) were much more than that in the microfat group (4.9 ± 0.9%) and the 10% gel group (3.6 ± 1.0%), demonstrated by Masson’s Trichrome staining, p < 0.05. In addition, there was also significant difference between the 30% gel group and the gel group in the formation of collagen tissue, p < 0.05 (Figures 2(d) and 2(e)).
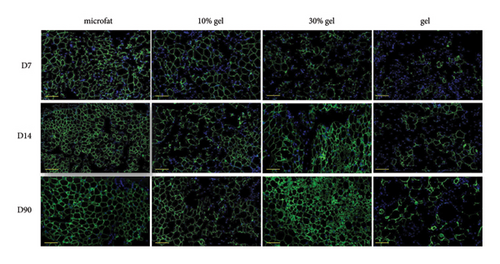
3.3. Viable Adipocytes and Collagen 1A1 Content in the Grafts
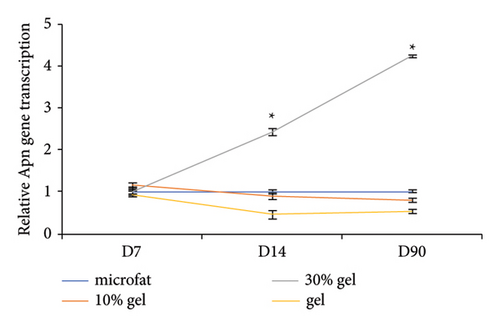
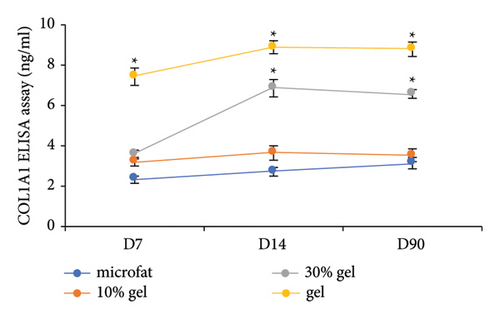
The adipocyte diameter was approximately 90–110 μm [20]. The results of antiperilipin staining showed there were more small adipocytes observed in the 30% gel group at days 14 and 90 (Figure 3). Adiponectin is an adipocyte-specific factor, and its expression was positively correlated with the numbers of adipocytes [21]. As for the mRNA expression of Apn, no significant difference was observed among the four groups at day 7. At days 14 and 90, mRNA expression of Apn was significantly higher in the 30% gel group than that in the other three groups (p < 0.05, Figure 3(b)). The size of adipocytes and Apn expression suggested that fat regeneration was active in the grafts added with 30% gel. Type I collagen is the major component in adipose ECM [22]. Higher COL1A1 content was found in the gel group from day 7 to 90. In the 30% gel group, the COL1A1 content increased at day 14 and remained at the high level until day 90. No significant difference of the COL1A1 content was observed between the 10% gel group and the microfat group at all the time points (p > 0.05, Figure 3(c)).
3.4. Proangiogenic Effect of SVF-Gel In Vivo
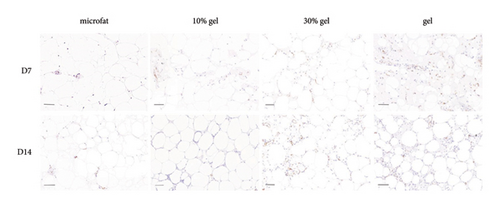
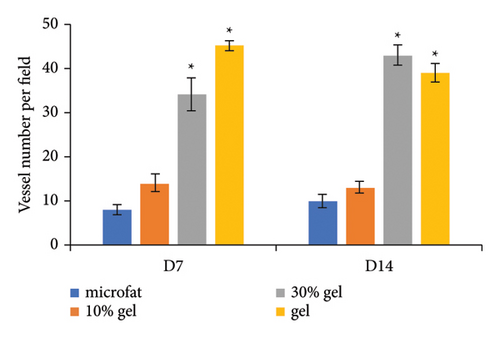
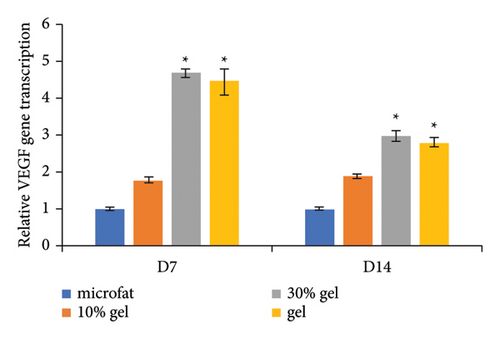
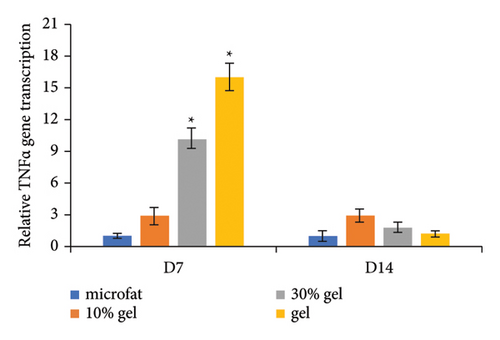
Anti-CD31 staining at days 7 and 14 post-transplantation demonstrated that the numbers of CD31-positive blood vessels per high power field in the 30% gel group (33.7 ± 3.8 and 42.6 ± 2.2, respectively) and the gel group (44.8 ± 1.1 and 37.9 ± 2.1, respectively) were much higher than that of the microfat group (8.2 ± 1.0 and 10.1 ± 1.6, respectively) and the 10% gel group (14 ± 1.9 and 13 ± 1.3, respectively), p < 0.05. No significant difference was observed between the microfat group and the 10% gel group, p > 0.05 (Figures 4(a) and 4(b)). The RNA expression level of the angiogenic factor VEGF in each group was consistent with the number of CD31-positive vessels (Figure 4(c)). The expression of inflammatory factor TNF-α in the 30% gel group and the gel group peaked on day 7 and decreased sharply thereafter (Figure 4(d)).
4. Discussion
In this study, SVF-gel was mixed into microfat with different percentages to be transplanted into nude mice. A higher volume retention in the SVF-gel-treated group when SVF-gel amount achieved a certain ratio to microfat was found after grafting. SVF-gel provided a better ECM framework and enhanced the expression of VEGF and TNF-α in the grafted fat, leading to increased vascularization and adipocyte regeneration, both of which are of great importance in long-term volume retention of the fat grafts.
It is supposed that graft survival and fat regeneration coexist in the grafted fat. After fat grafting, mature adipocytes are particularly susceptible to hypoxia-induced apoptosis due to their high cytoplasmic oxygen consumption, and fat regeneration derived from stem cell differentiation plays a vital role in volume retention [4]. Therefore, it is beneficial to protect vulnerable adipocytes and promote fat regeneration to obtain an optimal retention.
It has been shown that timely revascularization can minimize the inevitable degenerative changes induced by hypoxia, and is crucial for fat survival and regeneration [4]. Therefore, it is beneficial to reestablish fat grafts blood supply as early as possible in order to protect vulnerable adipocytes and promote fat regeneration to obtain an optimal retention. Various factors attribute to the revascularization. ASCs are the key component in SVF-gel. Besides ASCs differentiating into endothelial cells and secreting angiogenic factors, ASCs can induce angiogenesis through the dynamic reassembly of endothelial cells, which are one of the components in SVFs [23]. We found more CD31-positive blood vessels in the 30% gel group than the microfat group in this study, indicating that the gel enhances fat graft survival partially through the stimulation of new vessel formation, and this effect is likely attributed to the presence of well-known proangiogenic factor VEGF in the grafts. In a previous study, SVF-gel increased vessel numbers around the wound and enhanced formation of fine branches extended into the ischemic area by increasing the expression of the angiogenic factors VEGF and basic fibroblast growth factor (bFGF), whereas in the SVF group, vessels were limited in the skin surrounding the wound [16]. In the process of promoting flap survival, except increasing the expression of VEGF and bFGF, the cells in SVF-gel were directly engrafted as vessel component [17]. It has been demonstrated nanofat, which is the intermediate product of harvesting SVF-gel, contains a large number of cytokines and growth factors including VEGF, bFGF, and insulin-like growth factor 1 (IGF-1) and exerts antiapoptotic effects on adipocytes and proangiogenic effects on grafted fat to increase fat graft survival [24].
During the process of tissue remodeling after fat grafting, numerous macrophages infiltrated in the graft, and scavenged oil drop, secreted factors and influenced blood-derived stem cell infiltration, enhancing tissue revascularization [12, 25]. The expression of TNF-α is a key response of inflammatory response. In our study, the TNF-α expression in the 30% gel group and the gel group is much higher than the other 2 groups at day 7 and declined to a similar level at day 14. While injecting SVF-gel intradermally in nude mouse wound healing model, the gel induced intense inflammatory response in the early stage (days 2 and 4) and recruited various cells to the recipient area, leading to accelerated wound-healing process. The inflammatory response declined at a late stage (day 14) of wound healing till the control level [16]. These consistent results indicated that SVF-gel could regulate microenvironment dynamically according to the different features in each stage, and both ECM and ASCs may participate in the regulation.
The ultimate therapeutic potential of ASCs or SVFs appears to be limited while injected in isolation without ECM protection at the recipient site due to poor cell retention [26–28]. A previous study which used the cell tracing method showed little SVF cells from SVF suspension remained in the injection site, while numerous SVF-gel-derived cells existed in the injection site, and the results showed that the adipose ECM niche in SVF-gel provided physical protection to SVF cells in it [17]. It suggests the SVF-gel itself is an ideal mixture of stem cells and ECM and could be used as a better alternative for cytotherapy. The scanning electron microscope analysis revealed that ECM integrity in SVF-gel was similar as fresh adipose tissue [14]. Native ECM is much superior to synthetic materials mimicking ECM properties because of its biocompatibility and inherent biological functions, while working as carrier scaffolds [16]. The adipose ECM contains a variety of key protein modulators of cell functions and signaling proteins associated with adipogenesis, forming a reservoir of biologically active factors [29]. What is more, Feng et al. found that the SVF-gel could preserve cellular viability after cryopreservation and showed great regenerative potential in wound healing [30]. All these results suggest that the rich ECM in SVF-gel provides the small adipose particles with structural and biochemical support and forms a microenvironment in favor of adipocyte survival and regeneration.
The harvested fat in the gel group was rich in fibrous tissue in our study because there is no adipose tissue in the subcutaneous layer in nude mice. Without the microenvironment of adipose tissue, it is hard to induce adipogenic differentiation of the cells in SVF-gel. While mixing microfat and the gel, the microenvironment formed by microfat tissue itself could stimulate the ASCs in SVF-gel differentiate into adipocytes, demonstrated by the higher Apn expression in the 30% gel group at days 14 and 90 and higher numbers of adipocytes with less diameter. These results indirectly show that fat regeneration, not fat survival, plays a prior role in volume retention.
There are some advantages of SVF-gel-assisted fat grafting. First, there are no ethical issues associated with the clinical applications of SVF-gel. Second, the SVF-gel supports the tissue framework and improves local SVF delivery to enhance tissue neovascularization and regeneration. Third, adipose tissue with high content of fibrosis tissue is harvested to provide more strong support for tissue defect under the premise of saving the features of fat tissue. In clinic, SVF-gel-based fat grafting could be filled in the areas that require higher levels of structural support, such as nasal dorsum, chin, and retaining ligament. However, a standard SVF-gel-based fat grafting scheme is required. For example, the ratio between transplanted SVF-gel volume and fat grafting should be developed. Further studies are needed to detect the synergetic roles of SVF and ECM on fat survival and regeneration, including cell apoptosis of grafted fat tissue and stem cell differentiation into adipocytes. A comparative study about the roles of SVF alone and combined transplantation of SVF and ECM on fat volume retention is worth exploring. In addition, more clinical cases and clinical trials are needed to support efficacy, side effect profiles, and long-term outcomes.
5. Conclusions
In conclusion, the present study proves that SVF-gel is a good biomaterial carrying abundant SVFs and can exert a promoting effect on fat retention, which is likely attributed to a favorable neovascularization and increased collagen accumulation and tissue regeneration.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
Lingling Sheng and Ziyou Yu contributed equally to the work.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (82272295, 82302822), Shanghai Municipal Key Clinical Specialty (shslczdzk00901), and Shanghai Sailing Program (21YF1424000).
Open Research
Data Availability
The data used to support the findings of this study are available on request to the corresponding author.