The Usefulness of Atopic Dermatitis Control Tool in Upadacitinib Treatment for Patients with Atopic Dermatitis
Abstract
Background. Atopic dermatitis control tool (ADCT) is a patient-reported measure to assess disease control of atopic dermatitis (AD), consisting of the severity of symptoms, itch duration, bother, sleep, daily activities, and mood/emotions. Objectives. We evaluated the alterations of total and individual ADCT item scores during treatment with Janus kinase 1 inhibitor upadacitinib in AD patients. Methods. Forty-seven patients aged ≥12 years with moderate-to-severe AD were treated with oral upadacitinib 15 mg/day plus topical corticosteroids. Total and individual ADCT item scores, eczema area, and severity index (EASI) and peak pruritus numerical rating scale (PP-NRS) were evaluated. Results. Before treatment, total ADCT correlated with EASI and PP-NRS. The median percent reduction at months 1, 3, and 6 of upadacitinib treatment was 80%, 76.2%, and 67.4% in total ADCT, 75.28%, 85.06%, and 81.73% in EASI, 66.67%, 75%, and 75% in PP-NRS, respectively, and percent reduction of total ADCT correlated with that of EASI and PP-NRS except for no correlations with that of EASI at month 1. The percent reduction of ADCT no.4 was the highest among the 6 items. Conclusions. These results suggest that changes in ADCT reflect the therapeutic effects of upadacitinib and that ADCT can be a treatment target for upadacitinib therapy. Upadacitinib might preferentially improve sleep disturbance.
1. Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by eczema and severe itching. Poor disease control in AD can lead to persistent itch, sleep disturbances, depression, anxiety, and impaired daily activities [1–4]. To evaluate the long-term control of AD, the atopic dermatitis control tool (ADCT) has been designed [5, 6]. It is reported that ADCT correlates with eczema area and severity index (EASI) and patient-oriented eczema measure or itch numerical rating scale (PP-NRS) and is considered as a useful instrument in daily practice [7]. Furthermore, a recent study showed that ADCT can be used as an instrument for evaluating long-term disease control with dupilumab therapy [8]. However, how ADCT is altered during various treatments for AD has not been precisely studied. Especially, the transition of individual ADCT item scores during the treatments has not been examined.
Recently, a variety of treatment modalities for AD has been developed; biologics or small-molecule medicines such as inhibitors of Janus kinase (JAK) or phosphodiesterase. Upadacitinib is an oral JAK1 inhibitor approved for patients with moderate-to-severe AD in Japan [9–11]. We have recently shown that upadacitinib in real-world practice was well-tolerated by AD patients and gave therapeutic effects comparable to those in clinical trials [12–14]. In this study, we evaluated how total and individual ADCT item scores are altered during upadacitinib treatment in patients with AD, and if the alterations may reflect the therapeutic effects of upadacitinib to see if ADCT can be a treatment target for upadacitinib therapy.
2. Materials and Methods
2.1. Study Design and Data Collection
Forty-seven patients (≧12 years of age) with moderate-to-severe AD were treated with oral upadacitinib 15 mg/day plus medium to strongest classes of topical corticosteroids twice daily from September 2021 to December 2022. Medical records were analyzed retrospectively. Patients provided written informed consent.
This study was conducted in accordance with the Declaration of Helsinki (2004) and was approved by the Ethics Committee of Chiba Hokusoh Hospital, Nippon Medical School. These patients were diagnosed with AD according to the Japanese Guidelines for Atopic Dermatitis 2021 [15] and were assessed as having moderate-to-severe AD (EASI ≥16 or a head-and-neck EASI ≥2.4). The patients’ sex, age, body mass index (BMI), disease duration, and presence or absence of prior dupilumab treatment were recorded (Table 1).
| Sex, n (%) | |
| Male | 35 (74.5) |
| Female | 12 (25.5) |
| Age (years)a | 38.23 ± 17.66 |
| <18 years | 11 (23.4) |
| Body mass index (kg/m2)b | 24.4 [21.02–27.28] |
| Disease duration (years)a | 26.17 ± 15.32 |
| Prior dupilumab treatment | 11 (23.4) |
| EASIa | 22.18 ± 9.97 |
| PP-NRSb | 8 [7–10] |
| Total ADCTa | 14.32 ± 6.33 |
| ADCT no. 1: overall severity of symptomsb | 3 [2–4] |
| ADCT no. 2: days with intense episodes of itchingb | 2 [1–4] |
| ADCT no. 3: intensity of bothersomenessb | 3 [2–3.5] |
| ADCT no. 4: problem with sleepb | 1 [1–4] |
| ADCT no. 5: impact on daily activitiesb | 2 [2-3] |
| ADCT no. 6: impact on mood or emotionsb | 2 [2-3] |
- aData provided as the mean ± standard deviation. bData provided as the median (interquartile range). EASI, eczema area, and severity index; ADCT, atopic dermatitis control tool; PP-NRS, peak pruritus numerical rating scale.
2.2. Measurement of ADCT and Other Clinical Indexes
The ADCT is a patient-reported composite measure to assess the control of AD and to foster patient-physician communication around disease control. The symptoms and impacts of AD over the recent 1 week are evaluated using ADCT on six items: no. 1 (overall severity of symptoms), no. 2 (days with intense episodes of itching), no. 3 (intensity of bothersomeness), no. 4 (problem with sleep), no. 5 (impact on daily activities), and no. 6 (impact on mood or emotions), with scores 0 to 4 for each item, totally from 0 to 24 [5]. A total ADCT score of ≧7 is identified as uncontrolled AD. The patients reported scores of total ADCT and ADCT no. 1–6 [5] and peak pruritus NRS (PP-NRS) at months 0 (baseline), 1, 3, and 6 of upadacitinib treatment [16]. We calculated the proportion of patients who achieved total ADCT of <7 points in the patients with baseline total ADCT of ≥7 points (ADCT response) at months 1, 3, and 6 [17]. We calculated the proportion of patients achieving 0 or 1 point with ≥2 points of reduction from baseline in each ADCT item (ADCT 0/1 for no. 1–6). The PP-NRS is a patient self-reported item designed to measure peak pruritus over the previous 24 h based on a scale of 0–10, with 0 being “no itch” and 10 being “worst itch imaginable.” The proportion of patients whose PP-NRS improved by at least 4 points from baseline (PP-NRS response) was calculated.
EASI is calculated by assessing four body regions for erythema, edema/papulation, excoriation, or lichenification. Each of these four signs is given a score from 0 to 3, with 0 indicating no involvement and 3 indicating severe involvement. These scores are added up for all four body regions, resulting in a total EASI score. The EASI was assessed at months 0, 1, 3, and 6 of treatment. The proportion of patients whose EASI was reduced from baseline by at least 75% (EASI 75) was calculated at months 1, 3, and 6.
The database of this study partially overlapped with that of our previous study [12]; the data of total ADCT, EASI, and PP-NRS of 31 patients at months 0, 1, and 3 of upadacitinib treatment are common, while these sorts of data of the same patients at month 6, the same patients’ data of individual ADCT item scores, and all sorts of data of 16 newly registered patients are only included in the database of this study. We performed a novel analysis and presented novel results based on the presented database.
2.3. Statistical Analysis
All statistical analyses were performed using EZR (Jichi Medical School, Saitama Medical Center). The Shapiro–Wilk test was used to assess the normality of the data distribution. Results were expressed as mean ± standard deviations for variables with a normal distribution, and medians (interquartile ranges) for variables with a nonparametric distribution. Differences between months 0, 1, 3, and 6 were analyzed by repeated measures analysis of variance for normally distributed variables and by the Friedman’s test for variables with nonparametric distribution. Bonferroni correction was used for post hoc analysis. The correlation between the variables was analyzed by Spearman’s correlation coefficient. Statistical significance was set at p < 0.05.
3. Results
3.1. Demographics of Patients with AD
Forty-seven Japanese AD patients (35 males and 12 females) were enrolled in the study (Table 1). The patients were 36 adults (≧18 years) and 11 adolescents (<18 years).
3.2. Correlations of Baseline Total or Individual ADCT Item Scores with Baseline EASI and PP-NRS
Baseline (month 0) total ADCT, ADCT item no. 1 to 6 scores, EASI, and PP-NRS are shown in Table 1. Baseline total ADCT and 6 individual ADCT item scores positively correlated with baseline EASI and PP-NRS (Table 2). The results indicate that total and individual ADCT item scores can reflect the status of long-term disease control.
| Rho | p | |
|---|---|---|
| Correlations of EASI with total or item ADCT | ||
| EASI vs total ADCT | 0.55 | 0.0000631 ∗∗ |
| EASI vs no. 1: symptoms | 0.651 | 0.000000733 ∗∗ |
| EASI vs no. 2: itching | 0.384 | 0.00779 ∗∗ |
| EASI vs no. 3: bother | 0.444 | 0.00178 ∗∗ |
| EASI vs no. 4: sleep | 0.375 | 0.00949 ∗∗ |
| EASI vs no. 5: daily activities | 0.467 | 0.000933 ∗∗ |
| EASI vs no. 6: mood/emotions | 0.47 | 0.000862 ∗∗ |
| Correlations of PP-NRS with total or item ADCT | ||
| PP-NRS vs total ADCT | 0.603 | 0.00000718 ∗∗ |
| PP-NRS vs no. 1: symptoms | 0.514 | 0.000219 ∗∗ |
| PP-NRS vs no. 2: itching | 0.408 | 0.0044 ∗∗ |
| PP-NRS vs no. 3: bother | 0.545 | 0.000074 ∗∗ |
| PP-NRS vs no. 4: sleep | 0.534 | 0.00011 ∗∗ |
| PP-NRS vs no. 5: daily activities | 0.556 | 0.0000503 ∗∗ |
| PP-NRS vs no. 6: mood/emotions | 0.515 | 0.000209 ∗∗ |
- Correlations between variables were examined using Spearman’s correlation coefficients. ∗∗Statistically significant at p < 0.01. vs, versus.
3.3. The Improvement of Total and Individual ADCT Item Scores by Upadacitinib and Their Correlations
Total ADCT and ADCT no. 1–6 scores greatly reduced at month 1 of upadacitinib treatment (Supplemental Table 1). The reduced levels were maintained at months 3 and 6.
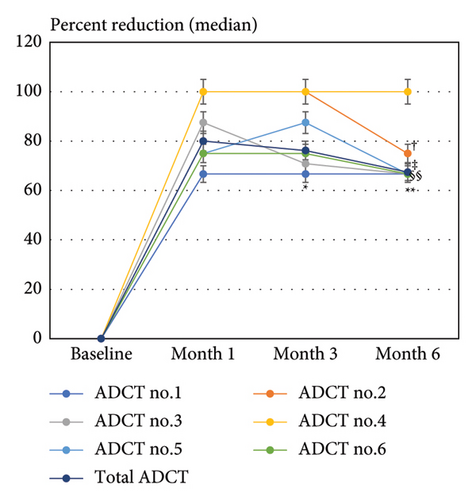
The median percent reduction of total ADCT score at months 1, 3, and 6 was 80%, 76.2%, and 67.4%, respectively. Similar patterns were observed in the individual ADCT item scores, with the highest reduction seen in item no. 4 (sleep), reaching 100% at all three time points (Figure 1(a)). The percent reduction of ADCT no. 4 was significantly higher than that of ADCT no. 1 (symptoms) at months 3 and 6 and that of ADCT no. 2 (itch), no. 5 (daily activities), and no. 6 (mood/emotions) at month 6.
The percent reduction of total ADCT score positively correlated with those of all 6 individual ADCT item scores at months 1, 3, and 6 (Table 3). The percent reductions of 6 individual ADCT item scores mostly positively correlated with each other at months 1, 3, and 6. However, there were no significant correlations in several combinations; no. 1 (symptoms) versus (vs) no. 4 (sleep) and no. 2 (itch) vs no. 4 at months 1, 3, and 6; no. 1 vs no. 2, or no. 1 vs no. 5 (daily activities), no. 2 vs no. 6 (mood/emotions), and no. 3 (bother) vs no. 4 at month 1; no. 4 vs no. 6 at month 3 (Table 3).
| Month 1 | Month 3 | Month 6 | ||||
|---|---|---|---|---|---|---|
| Rho | p | Rho | p | Rho | p | |
| Item-to-total correlation | ||||||
| ADCT no. 1: symptoms | 0.678 | 0.000000162 ∗∗ | 0.852 | 0.000000000000031 ∗∗ | 0.834 | 0.000000000000647 ∗∗ |
| ADCT no. 2: itch | 0.529 | 0.00032 ∗∗ | 0.745 | 0.0000000149 ∗∗ | 0.604 | 0.0000286 ∗∗ |
| ADCT no. 3: bother | 0.722 | 0.0000000145 ∗∗ | 0.831 | 0.0000000000009 ∗∗ | 0.79 | 0.000000000114 ∗∗ |
| ADCT no. 4: sleep | 0.514 | 0.00135 ∗∗ | 0.436 | 0.00779 ∗∗ | 0.483 | 0.0033 ∗∗ |
| ADCT no. 5: daily activities | 0.598 | 0.0000112 ∗∗ | 0.736 | 0.00000000557 ∗∗ | 0.776 | 0.000000000375 ∗∗ |
| ADCT no. 6: mood/emotions | 0.797 | 0.000000000167 ∗∗ | 0.861 | 0.000000000000134 ∗∗ | 0.875 | 0.0000000000000336 ∗∗ |
| Item-to-item correlation | ||||||
| No. 1 vs no. 2 | 0.294 | 0.0588 | 0.592 | 0.000036 ∗∗ | 0.548 | 0.000206 ∗∗ |
| No. 1 vs no. 3 | 0.316 | 0.0322 ∗ | 0.675 | 0.000000271 ∗∗ | 0.66 | 0.000000816 ∗∗ |
| No. 1 vs no. 4 | 0.284 | 0.0936 | 0.0578 | 0.738 | 0.194 | 0.263 |
| No. 1 vs no. 5 | 0.123 | 0.415 | 0.531 | 0.000147 ∗∗ | 0.482 | 0.000796 ∗∗ |
| No. 1 vs no. 6 | 0.456 | 0.00213 ∗∗ | 0.637 | 0.0000043 ∗∗ | 0.698 | 0.000000271 ∗∗ |
| No. 2 vs no. 3 | 0.44 | 0.00352 ∗∗ | 0.635 | 0.00000629 ∗∗ | 0.527 | 0.000403 ∗∗ |
| No. 2 vs no. 4 | 0.219 | 0.22 | 0.312 | 0.0771 | 0.205 | 0.26 |
| No. 2 vs no. 5 | 0.736 | 0.0142 ∗ | 0.506 | 0.000623 ∗∗ | 0.415 | 0.00691 ∗∗ |
| No. 2 vs no. 6 | 0.255 | 0.104 | 0.683 | 0.000000611 ∗∗ | 0.411 | 0.00753 ∗∗ |
| No. 3 vs no. 4 | 0.293 | 0.083 | 0.353 | 0.035 ∗ | 0.35 | 0.0394 ∗ |
| No. 3 vs no. 5 | 0.568 | 0.0000383 ∗∗ | 0.466 | 0.00109 ∗∗ | 0.592 | 0.0000187 ∗∗ |
| No. 3 vs no. 6 | 0.488 | 0.0009 ∗∗ | 0.795 | 0.000000000196 ∗∗ | 0.603 | 0.0000237 ∗∗ |
| No. 4 vs no. 5 | 0.371 | 0.0257 ∗ | 0.404 | 0.0146 ∗ | 0.376 | 0.026 ∗ |
| No. 4 vs no. 6 | 0.539 | 0.00101 ∗∗ | 0.32 | 0.0646 | 0.391 | 0.0243 ∗ |
| No. 5 vs no. 6 | 0.667 | 0.00000103 ∗∗ | 0.696 | 0.000000215 ∗∗ | 0.85 | 0.0000000000011 ∗∗ |
- Correlations between variables were examined using Spearman’s correlation coefficients. ∗Statistically significant at p < 0.05. ∗∗Statistically significant at p < 0.01. ADCT, atopic dermatitis control tool.
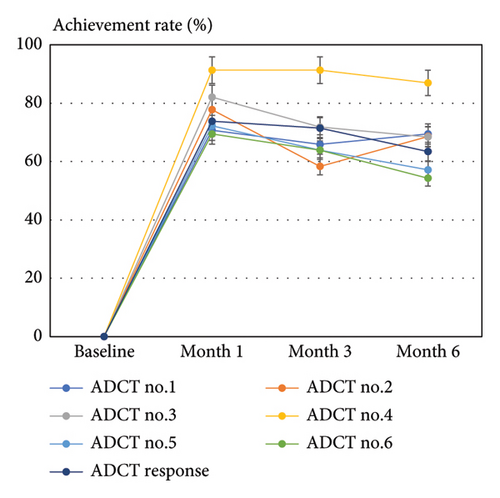
The achievement rates of total ADCT response (ADCT <7) at months 1, 3, and 6 were median 73.8%, 71.4%, and 63.4%, respectively. The achievement rates of ADCT 0/1 for items no. 1–6 closely mirrored those of the total ADCT response, with the highest rate observed for item no. 4 at all time points (Figure 1(b)), reaching 91.3%, 91.3%, and 86.96% at months 1, 3, and 6, respectively.
3.4. The Improvements of EASI, PP-NRS, and Their Correlations with That of Total and Individual ADCT Scores
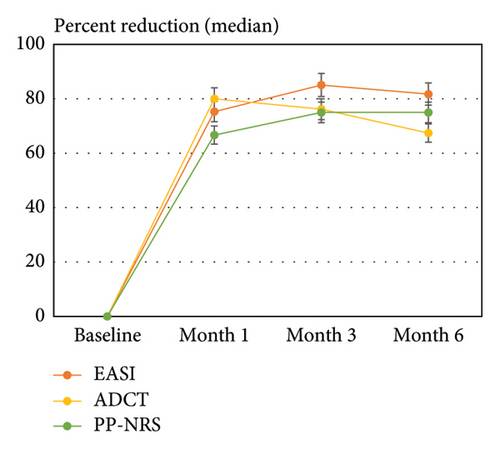
We then evaluated the improvement of EASI and PP-NRS by upadacitinib treatment. The EASI and PP-NRS were greatly reduced at month 1 of upadacitinib treatment (Supplemental Table 1). The reduced levels were maintained at months 3 and 6. The median percent reductions at months 1, 3, and 6 were 75.3%, 85.1%, and 81.7% for EASI and 66.7%, 75%, and 75% for PP-NRS, respectively, as compared with 80%, 76.2%, and 67.4% for total ADCT (Figure 1(c)). Thus, the peak of percent reduction in ADCT (month 1) was slightly ahead of that in EASI and PP-NRS (month 3). The percent reduction of total ADCT significantly correlated with that of EASI and PP-NRS except for no correlations with EASI at month 1 (Table 4). Similarly to the percent reductions of total ADCT, the percent reductions of 6 individual ADCT item scores mostly correlated with those of EASI at months 3 and 6 or with those of PP-NRS at months 1, 3, and 6 while without significant correlations with that of EASI at month 1 except for item no. 6 (Table 4). There were no significant correlations in several combinations of percent reductions; EASI versus ADCT no. 4 and no. 5 at months 1, 3, 6; EASI versus ADCT no. 6 at month 6; PP-NRS versus ADCT no. 4 at months 3 and 6; PP-NRS versus ADCT no.5 at month 1.
| Month 1 | Month 3 | Month 6 | |||||
|---|---|---|---|---|---|---|---|
| Rho | p | Rho | p | Rho | p | ||
| EASI | Total ADCT | 0.263 | 0.0736 | 0.574 | 0.0000244 ∗∗ | 0.48 | 0.000726 ∗∗ |
| ADCT no. 1: symptoms | 0.219 | 0.138 | 0.57 | 0.0000293 ∗∗ | 0.499 | 0.000418 ∗∗ | |
| ADCT no. 2: itch | 0.0819 | 0.606 | 0.52 | 0.000411 ∗∗ | 0.297 | 0.0594 | |
| ADCT no. 3: bother | 0.219 | 0.144 | 0.533 | 0.000139 ∗∗ | 0.413 | 0.00484 ∗∗ | |
| ADCT no. 4: sleep | 0.144 | 0.401 | −0.0412 | 0.811 | −0.0195 | 0.912 | |
| ADCT no. 5: daily activities | 0.249 | 0.0958 | 0.237 | 0.113 | 0.262 | 0.0825 | |
| ADCT no. 6: mood/emotions | 0.349 | 0.0219 ∗ | 0.336 | 0.0274 ∗ | 0.292 | 0.0606 | |
| PP-NRS | Total ADCT | 0.659 | 0.000000473 ∗∗ | 0.753 | 0.00000000102 ∗∗ | 0.693 | 0.0000000932 ∗∗ |
| ADCT no. 1: symptoms | 0.632 | 0.00000185 ∗∗ | 0.698 | 0.0000000498 ∗∗ | 0.612 | 0.00000618 ∗∗ | |
| ADCT no. 2: itch | 0.241 | 0.125 | 0.59 | 0.0000385 ∗∗ | 0.436 | 0.00438 ∗∗ | |
| ADCT no. 3: bother | 0.372 | 0.0108 ∗ | 0.813 | 6.79e − 12 ∗∗ | 0.596 | 0.0000158 ∗∗ | |
| ADCT no. 4: sleep | 0.394 | 0.0175 ∗ | 0.156 | 0.363 | 0.283 | 0.0999 | |
| ADCT no. 5: daily activities | 0.288 | 0.0525 | 0.468 | 0.00104 ∗∗ | 0.528 | 0.00019 ∗∗ | |
| ADCT no. 6: mood/emotions | 0.668 | 0.000000986 ∗∗ | 0.711 | 0.0000000896 ∗∗ | 0.653 | 0.00000272 ∗∗ | |
- Correlations between variables were examined using Spearman’s correlation coefficients. ∗Statistically significant at p < 0.05. ∗∗Statistically significant at p < 0.01.
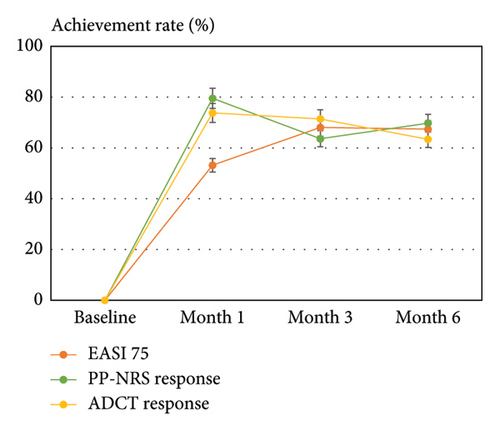
We then evaluated the achievement rates of EASI 75 and PP-NRS response (PP-NRS <4) with that of total ADCT response (ADCT <7) after upadacitinib treatment. The achievement rate at months 1, 3, and 6 was 53.2%, 68.09%, and 67.4% in EASI 75 and 79.6%, 63.6%, and 69.8% in PP-NRS response, respectively, as compared with 73.8%, 71.4%, and 63.4% in total ADCT response, respectively (Figure 1(d)). Thus, the peak achievement rate of PP-NRS and ADCT responses (month 1) preceded that of EASI 75 (month 3).
4. Discussion
The baseline scores of total ADCT were correlated with baseline EASI and PP-NRS. Furthermore, the percent reductions in both total ADCT and individual ADCT items showed correlations with those of EASI and PP-NRS. These findings suggest that ADCT can serve as an indicator of long-term disease control and its improvement reflects the therapeutic effects of upadacitinib. The ADCT items cover symptoms, itching, bother, sleep, daily activities, and mood/emotions, with items 3–6 closely related to quality of life (QOL). Thus, the total ADCT score encompasses symptoms, itch, and QOL. Notably, the peak improvement of total ADCT occurred slightly earlier (month 1) than that in EASI (month 3). This time lag in upadacitinib treatment was consistent with our previous study involving a smaller number of patients [12]. The time lag may contribute to the lack of significant correlation between percent reductions in EASI and total or individual ADCT items at month 1 (Table 4).
There is a time lag between the improvement of ADCT and EASI in upadacitinib treatment, which could be caused by two possible reasons. First, upadacitinib may rapidly alleviate itch compared to clinical signs reflecting inflammation and skin barrier disruption. By blocking JAK1-mediated signals from pruritogenic cytokines, upadacitinib directly targets sensory neurons and provides quick relief from itch. However, resolving skin inflammation and restoring the skin barrier may require more time due to the complex mechanism involving multiple cytokines. Second, ADCT, which encompasses symptoms, itch, and QOL, may be more responsive to treatment compared to EASI, which focuses solely on symptoms and lacks itch and QOL items. The improvement of ADCT, particularly of ADCT no. 1 (symptoms) and no. 2 (itch), may synergistically improve QOL-related items (bother, sleep, daily activities, and mood/emotions) in ADCT. Further investigation is needed to examine the time course of EASI and ADCT in other AD treatments to determine if a similar time lag exists.
The percent reduction of total ADCT correlated with those of all 6 ADCT item scores at months 1, 3, and 6 of upadacitinib treatment, indicating that 6 individual ADCT items equally contributed to disease control status and treatment responses. ADCT no. 4 (sleep) showed the highest percent reduction and achievement rate of ADCT 0/1 after treatment (Figures 1(a) and 1(b)). However, its reduction did not correlate with ADCT no. 1 (symptoms) or ADCT no. 2 (itch) during upadacitinib treatment (Table 3). The percent reduction of ADCT no. 4 had a weak correlation with that of PP-NRS at month 1 but did not generally correlate with those of EASI or PP-NRS (Table 4). These findings suggest that upadacitinib may specifically improve sleep disturbance in AD through mechanisms independent of symptoms or itch. Possible mechanisms include circadian rhythm restoration, melatonin secretion, or improvements in skin permeability or blood flow [18]. Patients with sleep disturbance may benefit from upadacitinib treatment. Further research is needed to determine if this effect is specific to upadacitinib or common among other JAK inhibitors. Comparing improvements in individual ADCT item scores after treatment can clarify the target points of individual treatments and characterize suitable patients for individual treatments of AD.
This study has several limitations. First, the sample number is small and patients are limited to Japanese. Second, the sex ratio of patients was rather biased for male preponderance (74%). Third, this study was performed on the treatment of 15 mg/day of upadacitinib. The usefulness of ADCT should further be examined in 30 mg/day of upadacitinib. Fourth, ADCT and other clinical indexes were not examined in the earlier phases (1 or 2 weeks) or for a longer duration (1 or 2 years) of upadacitinib treatment in this study. Fifth, this study also has limitations regarding the validation of ADCT assessment in patients under the age of 18. The accuracy of ADCT assessment in patients under the age of 18 may be compromised. It is possible that the assessment of ADCT in underage patients could be influenced by developmental or physiological factors. Therefore, the results of ADCT in underage patients should be interpreted with caution. Lastly, this study did not include factors such as sex and age as covariates in the analysis, which may have affected the correlation between ADCT and existing evaluation methods (EASI and PP-NRS). Future studies should consider these factors in their analyses. In addition, the p values reported in the tables are based on raw data and are not adjusted p values. Therefore, caution is needed in interpreting the results. Furthermore, it is necessary to conduct a detailed examination of the significant advantages of ADCT compared to existing metrics such as EASI and PP-NRS in future studies.
5. Conclusions
In conclusion, baseline total ADCT correlated with baseline individual ADCT item scores and with baseline EASI and PP-NRS. The percent reduction of total ADCT mostly correlated with those of EASI and PP-NRS and with individual ADCT item scores after upadacitinib treatment. The peak of improvement of total ADCT by upadacitinib (month 1) slightly preceded that of EASI (month 3). Among 6 ADCT items, the improvement of no. 4 (sleep) was the highest and independent from those of symptoms or itch. These results suggest that the transition of ADCT reflects the therapeutic effects of upadacitinib and that ADCT can be a treatment target for upadacitinib. Upadacitinib might preferentially improve sleep disturbance among 6 ADCT items.
Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Nippon Medical School Chiba Hokusoh Hospital (protocol code H-2022-945 and approval on February 10, 2022).
Conflicts of Interest
H. S. received a lecture fee and research cost from AbbVie GK. T. H. and N. K. received lecture fees from AbbVie GK. E. F. has no conflicts of interest to declare.
Open Research
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.