Ferroptosis-Related LncRNA BCRP3 Promotes the Proliferation and Migration of Prostate Cancer
Signature for Predicting Biochemical Recurrence
Abstract
Background: Long noncoding RNAs (lncRNAs) are reported that play an important role in regulating tumorigenesis. This study aims to develop a ferroptosis-related lncRNA gene signature for predicting biochemical recurrence of prostate cancer (PCa) and prove a functional lncRNA BCRP3 as a promotor toward PCa progression.
Methods: The ferroptosis-related lncRNA were identified by interoperating the databases of The Cancer Genome Atlas (TCGA) and FerrDb. A predictive model for biochemical recurrence of PCa was established based on the LASSO Cox regression. Kaplan–Meier cures were applied in the measurement of the impact on patients’ survival caused by key lncRNAs. Meanwhile, the molecular assays of CCK8, EdU, wound healing, and Transwell were conducted to evaluate the tumor-suppressive effect of BCRP3 in vitro.
Results: This study presented that 17 differentially expressed ferroptosis-related lncRNAs were confirmed and further screened out 9 key lncRNAs for the establishment of risk model. The nomogram involved the risk model was also proved with an excellent predictive performance toward 1-, 3-, and 5-year survival with the area of curves (AUC) as 0.77, 0.79, and 0.65 separately. Notably, the lncRNA BCRP3 was significantly correlated with the survival of PCa patients based on multivariate Cox regression. Additionally, downregulation of BCRP3 effectively inhibited the proliferation, migration, and invasion of PC3 cells which further proved its anti-tumor function.
Conclusion: We developed a ferroptosis-related lncRNA risk model for predicting biochemical recurrence, which assisting in the clinical therapy of patients with PCa.
1. Introduction
Prostate cancer (PCa) is the second most common cancer among men worldwide, with an incidence ranging from 6.3 to 83.4 per 100,000 men in various regions [1]. In 2020, it is estimated that there are nearly 1.4 million new cases and 375,000 deaths worldwide [1]. According to statistics from the GLOBOCAN database, new cases in Asia accounted for 26.2% of the global cases in 2020, and deaths accounted for 32.1% of them [2]. For some patients, prostate cancer has metastasized, especially bone metastases, at the time of initial diagnosis, which portends a poorer prognosis [3]. Patients with metastatic hormone-sensitive prostate cancer (mHSPC) and castration-resistant prostate cancer (CRPC) remain at substantial risk and succumb to the disease despite treatment) [4]. Therefore, the identification of various regulators involved in the development and progression of prostate cancer and the study of their molecular mechanisms are of particular scientific interest for the development of novel therapeutic targets.
Ferroptosis as a new strategy for cancer treatment has attracted increasing attention in recent years. Ferroptosis is an iron-dependent form of regulated cell death, distinct from apoptosis, necrosis, and autophagy, characterized by accumulation of cellular lipid peroxidation (LPO) to lethal levels with iron accumulation [5–7]. Cell membrane transporters such as transferrin, lactotransferrin, etc., being inhibited may initiate the extrinsic pathway inducing iron death. Intracellular antioxidant enzymes, such as glutathione peroxidase (GPX4), blocked may initiate the intrinsic pathway to induce ferroptosis. Ferroptosis is also a breakthrough in cancer treatment, and the unique metabolism and mutations of cancer cells make some of these cells more sensitive to ferroptosis [8]. Therefore, the combination of ferroptosis inducers and conventional therapy may also have great potential in cancer treatment.
Long noncoding RNA (lncRNA) are noncoding RNA greater than 200 nucleotides in length. Certain lncRNA may act as important regulators in tumorigenesis and development, and affect the biological process of ferroptosis by binding to DNA, RNA, and proteins, thereby affecting the growth of cancer cells [9, 10]. The detailed molecular mechanism may be that it affects protein interaction, or inhibits the translation process, or regulates miRNA as a competitive endogenous RNA (ceRNA), which is thought to bind with microRNA to inactivate it, thereby affecting epigenetic modification and affecting the malignant phenotype of cancer [11–13]. There are various studies had confirmed that lncRNA could function as the predictive model for prostate cancer [14–16]. To better understand the mechanism, we tried to identifie the functions of ferroptosis-related lncRNA, combines experiments with bioinformatics, and develops prognostic models. Based on the in silico analyses, we conducted a predictive model including 15 lncRNAs for survival of PCa patients, which functioned well in predicting 1-, 3-, and 5-year survival. In terms of functional analysis of lncRNA, small interfering RNAs (siRNAs) were utilized to inhibit the expression of BCRP3, revealing its function in vitro. Our study will contribute to assessing patient outcomes, providing new therapeutic targets, and further translating into clinical applications.
2. Methods and Materials
2.1. Data Collection
RNA-seq count data and TPM (Transcripts per Kilobase of exon model per Million mapped reads) data were obtained from TCGA database (https://genome-cancer.ucsc.edu/). There were 501 PCa and 52 normal tissues’ transcriptome data obtained with the count data for expression analysis and TPM data for regression analysis. The ferroptosis related dates were identified from the FerrDb database (https://www.zhounan.org/ferrdb/current/), including 259 genes consisted of 150 drivers, 123 markers and 109 suppressors. Meanwhile, there were 423 clinical samples gained from TCGA database with the characteristics including survival time, recurrence, age, Gleason score, TNM stage, and PSA-value.
2.2. Exploration of lncRNAs
The eligible lncRNAs were screened out by the correlation analysis of expression data. Based on the selection criteria of absolute logFC > 1.5 and adjust p value < 0.05, we firstly obtained the differentially expressed lncRNAs (DELs) between PCa and normal tissues through the R software (DESeq2 package). Ferroptosis related genes were identified from the database of FerrDb (https://www.zhounan.org/ferrdb/current/). There were 259 ferroptosis related genes (150 driver genes, 123 marker genes, and 109 suppressor genes) were identified. Besides, the ferroptosis related lncRNAs (FRLs) were identified by the Pearson’s correlation analysis among the patients with biochemical recurrence with the absolute correlation coefficient > 0.50 and p < 0.05. Finally, the eligible lncRNAs (ferroptosis related differentially expressed lncRNAs, FRDELs) were selected from the intersection between DELs and FRLs, which were considered as the basis of predictive model.
2.3. Predictive Model Construction and Validation
The total 423 clinical samples were considered as the train set to generate the predictive model. Meanwhile, 254 patients were randomly selected through R software to form the test set for the validation of predictive model. Based on the clinical characteristics of train set, a risk model was conducted for survival prediction based on LASSO regression analysis through the R software (glmnet package). The selected FRDELs were performed to obtain the risk score, which would divide the patients into two groups (High vs. Low) among the train or test sets. The predictive performances were evaluated using the R software (“survminer” and “survival” packages) and visualized by Kaplan–Meier curves. Additionally, Cox regression analysis was conducted for the determination of risk factors toward PCa survival. Subsequently, a nomogram was established to predict 1-, 3-, and 5-year PSA-free survival of PCa patients on the basis of results from cox regression analysis. Simultaneous, the calibration curve and ROC curve were performed to assess the predictive performance of nomogram.
2.4. Independent Prognostic Analysis
The univariate and multivariate Cox regression analyses were further conducted to assess the independent prognostic lncRNAs among the selected FRDELs. Meanwhile, the relationships between lncRNAs and clinical characteristics were evaluated, including TNM-stage and Gleason score. And the predictive performances of lncRNAs expression toward 3- and 5-years survival were also assessed by the Kaplan–Meier curves.
2.5. Cell Culture and Transfection
The human cell line PC3, DU145 and LNCaP was brought from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in RMPI 1640 (Gibco, USA) added with 10% Fetal Bovine Serum (FBS, Hyclone, USA) and 1% Penicillin/Streptomycin (P/S, YEASEN, China) at 37°C in 5% CO2. Small interfering RNAs of BRCP3 (si-BRCP3) was obtained from RIBOBIO (China) and transfected in the cells through the jetPRIME (YEASEN, China). The detailed sequence of si-BRCP3 is “CTGTGAGTCTATCTATGCA”.
2.6. RNA Extraction and Quantitative Real-Time PCR
Total RNA was extracted using the Trizol reagent (Invitrogen, USA) with the RNA concentration measured by a Nanodrop 2000 spectrophotometer (Thermo Scientific, USA). After cDNA synthesis, the expression of BCRP3 was detected by RT-qPCR with the primers specific for BCRP3 (Sangon Biotech, Shanghai, China). And GAPDH was considered as a control to calculate relative RNA expression. Detailed primer sequences are listed in the Supporting Information (Supporting Information Table S1).
2.7. CCK8 Assay
A proliferation assay was performed using cell counting kit-8 (YEASEN, China). Cells were firstly transfected with si-BCRP3 to knockdown the BRCP3 expression. After 12 h of transfection, the cells were digested into cell suspension and further numbered for next step. Total numbers of two thousand cells were plated into each well of a 96-well plate with 100 μL cell cultured medium in each well. After 24 h, the CCK-8 medium (10 μL) was added to the well and cultured for 1 h. Subsequently, the absorbance in each well was measured at 450 nm to determine the cell viability. This step was conducted in day 2, 3, 4, and 5.
2.8. EdU Analysis for Cell Proliferation
Cells were cultured in 24-well plates after the transfection with si-BCRP3 and treated with 10 μM EdU (EpiZyme, China) for 2 h at 37°C in 5% CO2. Pretreated cells were fixed by the 4% paraformaldehyde for the next stage according to the protocol. After washing with PBS/0.3% BSA, the cells were incubated with Alexa Fluor 555 and DAPI in the dark. Then the EdU results were visualized through Leica DM6 B upright microscope system (Leica, Germany).
2.9. Colony Formation
A colony formation assay was performed to evaluate cell proliferation. Cells were firstly transfected with si-BCRP3 to knockdown the BRCP3 expression. After 12 h of transfection, the cells were digested into cell suspension and further numbered for next step. According to a density gradient of 1000 cells per well, inoculate the cells into a 6 well plate. Each well is filled with complete culture medium up to 2000 μL. Incubate the cells in a 5% CO2 and 37°C incubator for 14 days. When visible clones appear in the culture dish, terminate the culture. Discard the culture medium and carefully soak twice with PBS. The colonies were further fixed and stained for photography after air drying. Count the number of clones directly for the quantification.
2.10. Wound Healing
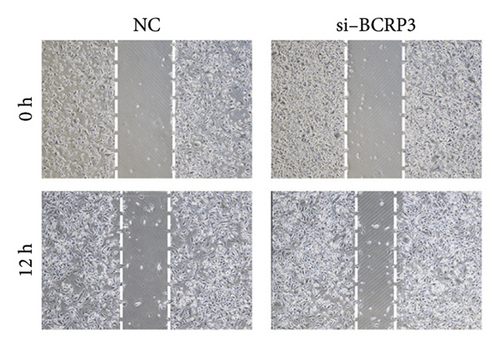
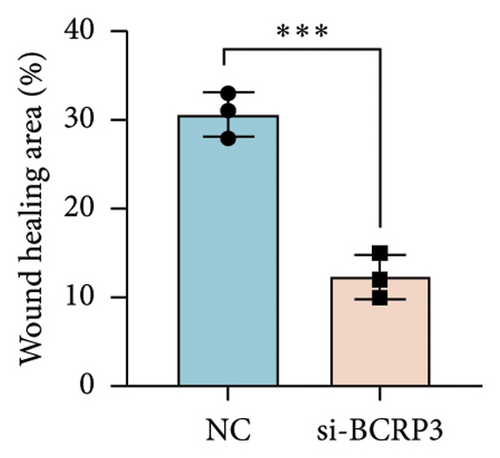
Wound healing experiments were conducted to assess the migration ability of cells. Cells were firstly transfected with si-BCRP3 to knockdown the BRCP3 expression. After 12 h of transfection, the cells were digested into cell suspension and further seeded into the 6 well plate. When the cell fusion rate has reached 90%, use the pipette tip of the pipette gun to make a scratch on the cell layer. The tip should be vertical and not tilted. After scratching, the cells were washed with PBS three times to remove the shed cells, and then added 1640 culture medium. Select the same field of view for photography at 0 h and 24 h time points. The wound healing rate was determined through the Image J software.
2.11. Transwell Assay
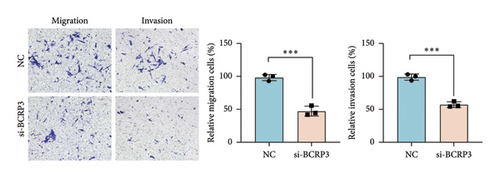
The invasion and migration abilities were further measured through the Transwell assay. Cells were firstly transfected with si-BCRP3 to knockdown the BRCP3 expression. After 12 h of transfection, the cells were digested into cell suspension and further numbered for next step. For the Transwell assay, 5^104 cells in 200 μL serum-free medium were cultured in the upper chambers with (invasion) or without (migration) matrigel, and 500 μL medium with 10% FBS was lorded in the lower chambers. Incubate the cells in a 5% CO2 and 37°C incubator for 24 h. After the incubation, the chamber was removed and the remaining cells were extracted from the chamber. The upper surface of the upper chamber was gently rubbed with a cotton swab. The lower surface of the small chamber membrane was washed through PBS, and further fixed and stained for photography.
2.12. Statistical Analysis
All the analyses were performed using R (v.3.6.3). Wilcoxon test or Student’s t-test were conducted to investigate the relationship between clinical features and expression of the lncRNAs. Kaplan–Meier analysis was used for the evaluation of TCGA patient PSA-free survival rates. The results were considered statistically significant with a p value < 0.05. All the data are presented as mean ± SD, and significant difference was determined based on the results of t-test.
3. Results
3.1. Development of the Risk Model Through FRDELs
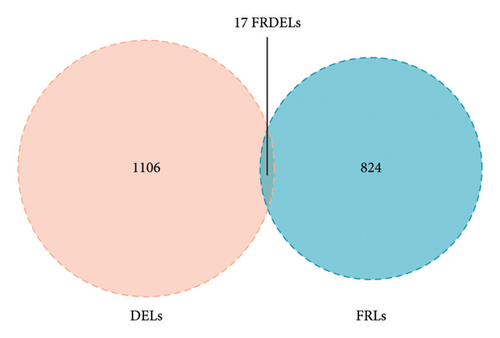
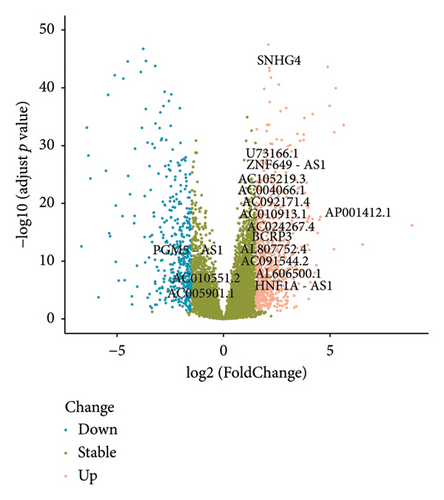
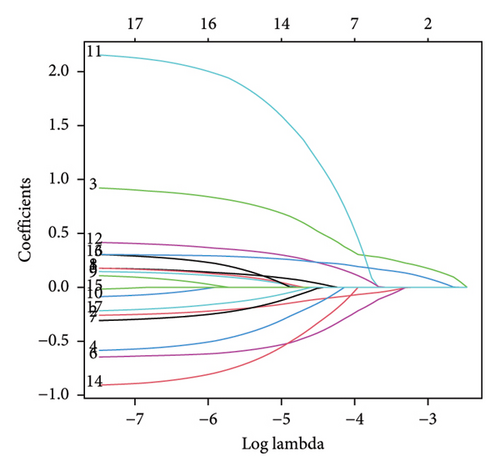
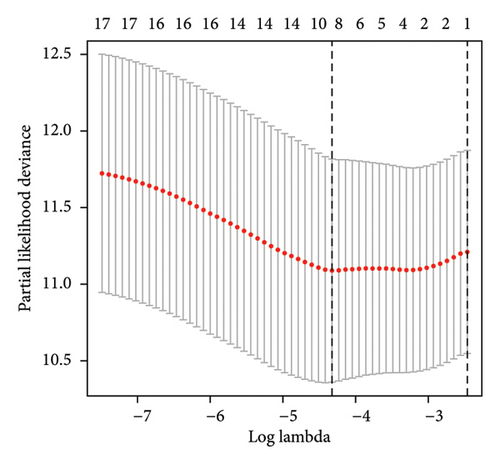
A number of 1123 DELs were selected from the DEGs through the selection criteria of absolute logFC > 1.5 and adjust p value < 0.05. Meanwhile, there were 841 FRLs chosen in this study based on the criteria of absolute correlation coefficient > 0.50 and p < 0.05 (Table S2). And only 17 eligible FRDELs were included in the next analyses after the intersection (Figure 1(a)), which was presented in Figure 1(b). Then, the relationship between FRDELs and biochemical recurrence of PCa was evaluated. Based on the 423 clinical samples (Table 1), the LASSO regression analysis was performed to determine the eligible FRDELs in the risk model (Figure 1(c), 1(d)). It was exhibited that ZNF649.AS1, U73166.1, SNHG4, PGM5.AS1, BCRP3, AP001412.1, AL807752.4, AC005901.1, and AC004066.1 were included in the establishment of the risk model. We further visualized the relationship between risk score of the model and biochemical recurrence of PCa both in the train set (Figure 1(e)) and test set (Figure 1(f)). It was founded that high risk score was related with the recurrence of PCa and the cut-off values of the two sets were 1.86 (train set) and 1.852 (test set). Besides, the relationships between separately lncRNA and patients’ PSA-free survival were further presented in the Figure S1.
| Characteristics | OS patients (N = 423) | No. (%) |
|---|---|---|
| Age | ||
| ≤ 60 | 188 | 44.44 |
| > 60 | 235 | 55.56 |
| psa_value | 412 (0.91 ± 3.81) | |
| Gleason_score | ||
| 6 | 37 | 8.75 |
| 7 | 197 | 46.57 |
| 8 | 56 | 13.24 |
| 9 | 119 | 28.13 |
| 10 | 3 | 0.71 |
| Unknown | 11 | 2.60 |
| Tumor | ||
| T2 | 151 | 35.70 |
| T3 | 249 | 58.87 |
| T4 | 23 | 5.44 |
| Node | ||
| N0 | 292 | 69.03 |
| N1 | 68 | 16.08 |
| Nx | 63 | 14.89 |
| Biochemical recurrence | ||
| Yes | 70 | 83.45 |
| No | 353 | 16.55 |
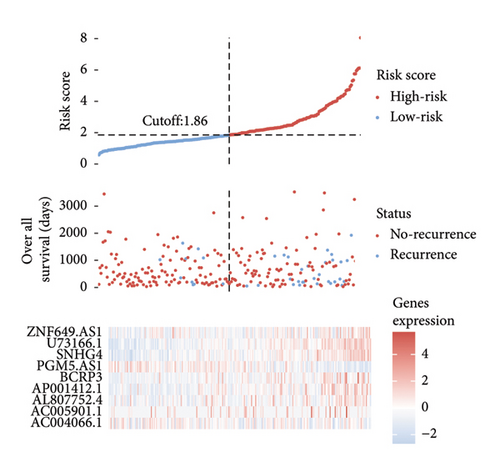
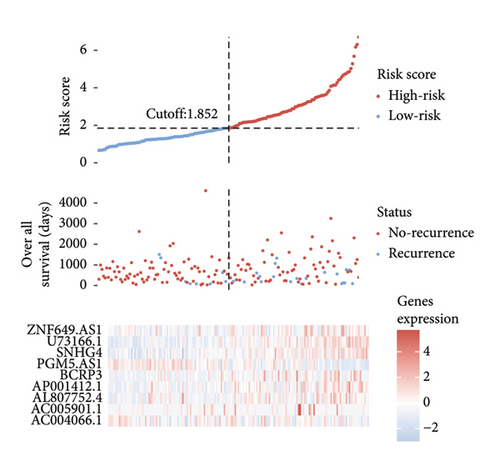
3.2. Validation of the Risk Model
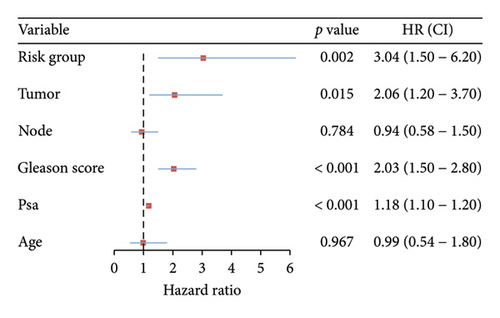
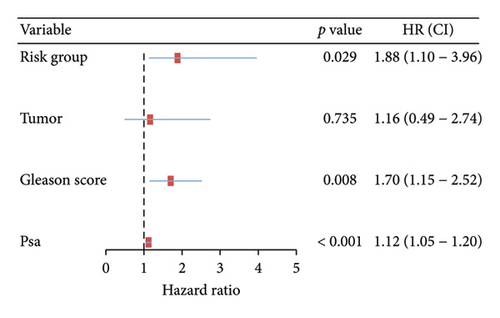
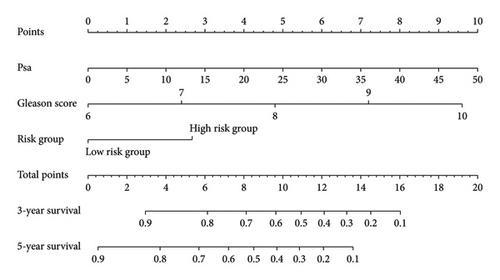
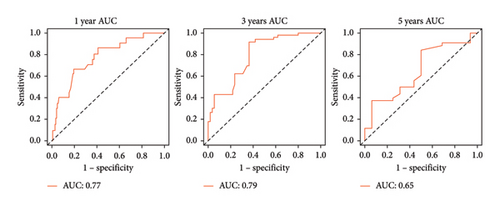
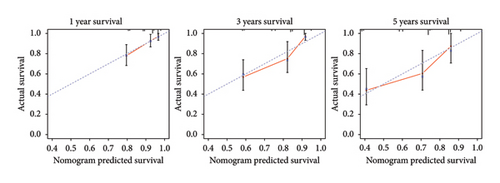
The survival curves of PCa patients were conducted on the basis of the risk model in the two sets (Figures 2(a) and 2(b)). It was noteworthy that the high-risk score group was closely related with the short PSA-free survival time with the two p values as 0.0013 and 0.054, which presenting the excellent predictive performances of this risk model. The Cox regression analyses were further performed to assess the risk factors of biochemical recurrence (Figures 2(c) and 2(d)). It was proved that our risk model was a significantly risk factor of biochemical recurrence in PCa patients both in the univariate and multivariate regression results (p < 0.001 and p = 0.013). Based on the multivariate Cox regression results, a nomogram for predicting 1-, 3-, and 5-year PSA-free survival was established with the factors of risk model, PSA, and Gleason score (Figure 3(a)). All the corresponded points of each factor added in the nomogram will match the detailed rate of 1-, 3-, and 5-year PSA-free survival, which would determine the clinical outcomes of PCa patients. Additionally, the ROC curves and calibration curves were also conducted to measure the predictive function of the nomogram (Figures 3(b) and 3(c)). The area of curves (AUC) was 0.77 (1-year), 0.79 (3-year), and 0.65 (5-year) separately, combined the great fitness of calibration curves, presented the accurate predicting performances.
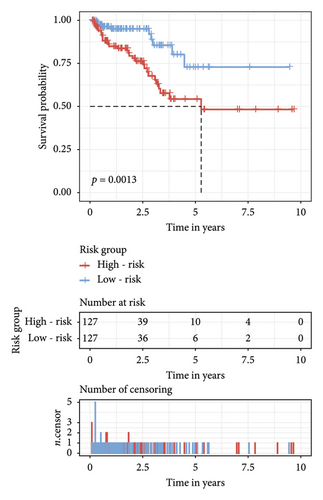
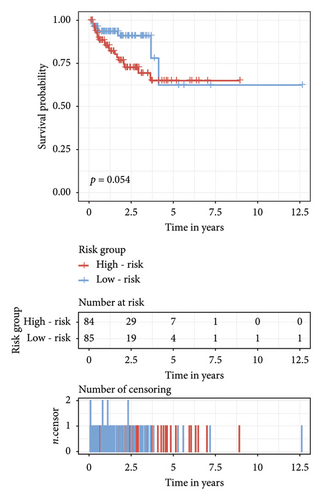
3.3. Identification of Independent Prognostic lncRNAs
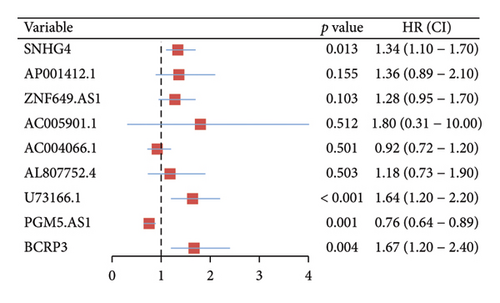
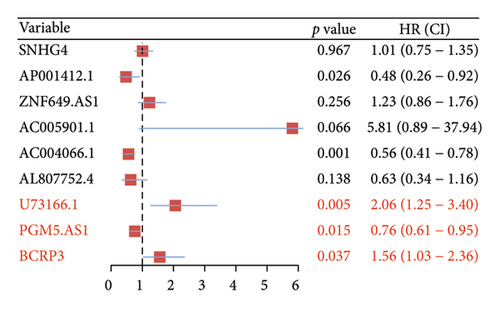
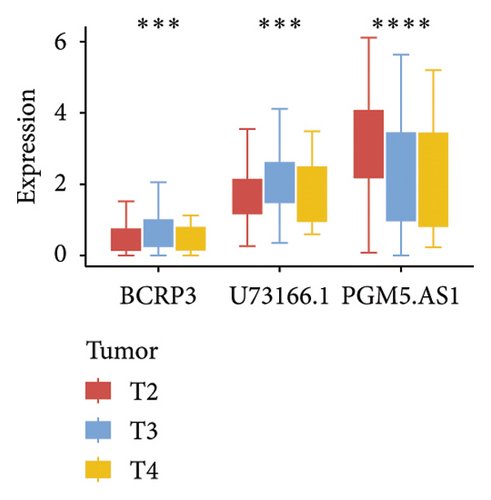
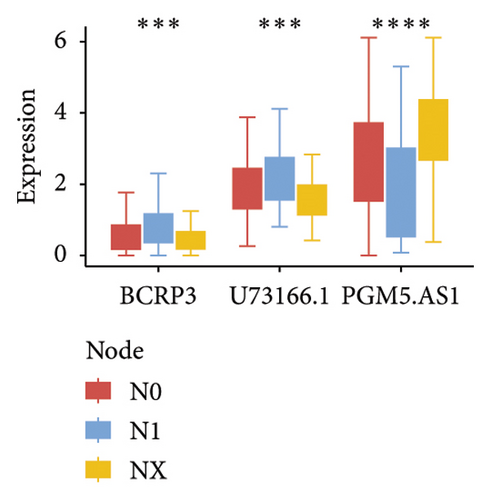
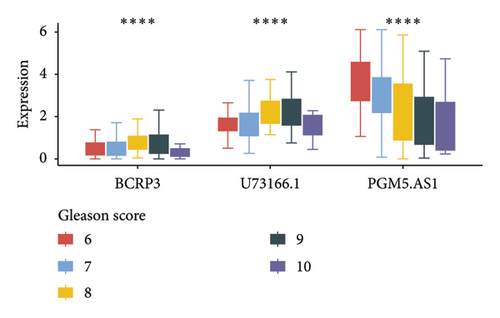
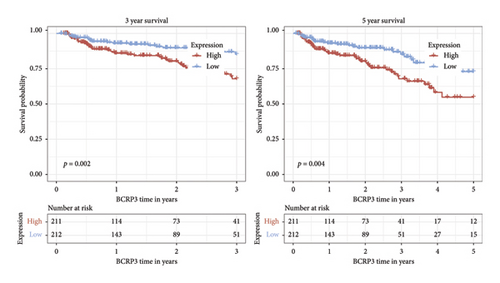
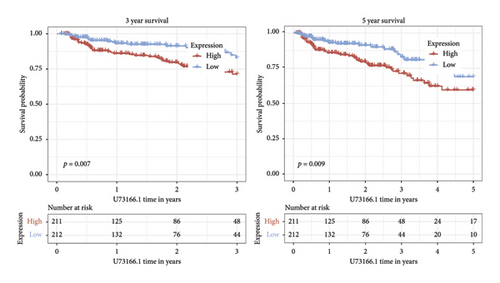
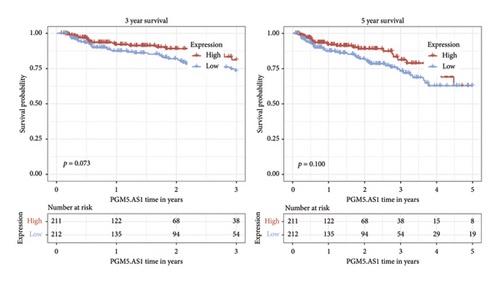
Independent prognostic lncRNAs were further selected according to the cox regression analysis. It was shown that lncRNAs of U73166.1 (p < 0.001 and p = 0.005), PGM5.AS1 (p = 0.001 and p = 0.0015), and BCRP3 (p = 0.004 and p = 0.037) were significantly related with the biochemical recurrence in PCa patients with the p value < 0.05 both in the univariate and multivariate Cox regression (Figures 4(a) and 4(b)). Additionally, the relationships between expression of lncRNAs and clinical characteristics were also presented in Figure 4. It was noted that all the three lncRNAs were significantly co-related with the T-stage (Figure 4(c)), N-stage (Figure 4(d)), and Gleason score (Figure 4(e)). Subsequently, we also analyzed the survival curves of the three lncRNAs (Figure 5). The results revealed that the 3- and 5-years PSA-free survival curves of BCRP3 contained the highest p value (p = 0.002 and p = 0.004; Figure 5(a)), which is higher than U73166.1 (Figure 5(b)) and PGM5.AS1 (Figure 5(c)). Finally, the lncRNA BCRP3 was selected as the targeted lncRNA for detecting the promotion of PCa.
3.4. Downregulation of BCRP3 Inhibits Proliferation, Migration, and Invasion of PC3
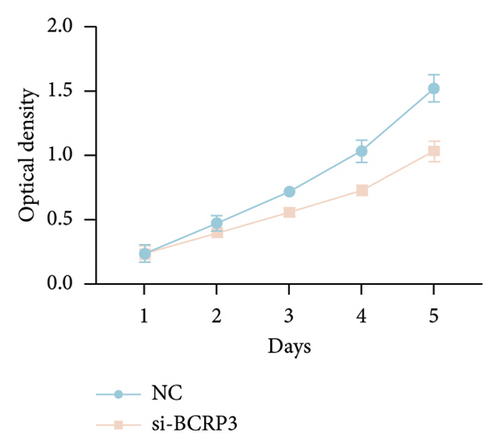
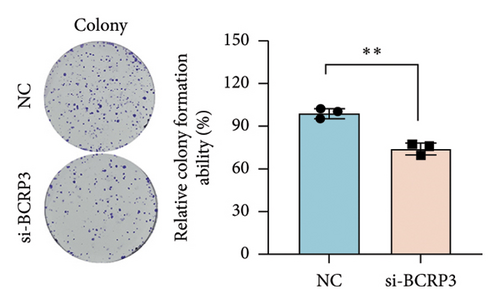
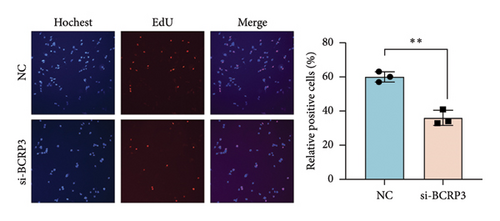
The expression of BCRP3 was measured in different cell lines. It was found that PC3 processed the highest BCRP3 expression compared with the other two cell lines and RWPE-1 cells (normal prostate epithelial cells) (Figure S2). Therefore, the cell line of PC3 was selected as the model for in vitro assays. To measure the effects of BCRP3 on proliferation, migration, and invasion, we establish a BCRP3 downexpression system. PC3 cells were transfected with si-BCRP3 which was determined by the results of real-time PCR (Figure 6(a)). Based on the downregulation of BCRP3, we observed that inhibiting BCRP3 expression effectively reduced cell proliferation by CCK-8 and colony experiments (Figures 6(b), 6(c)), which was further proved by the results of EdU experiments (Figure 6(d)). It was confirmed that BCRP3 knockdown in cells could impact the proliferation of PC3, indicating the tumor promoting function of BCRP3 in prostate cancer. In addition, wound-healing and Transwell assays both proved that BCRP3 downregulation assisted in the inhibition of cell migration and invasion in vitro (Figures 6(e), 6(f), and 6(g)). The related results of experiments conducted to measure the cell migration and invasion proved that BCRP3 knockdown in cells could inhibit the tumor progression of PC3, assisting in the PCa therapy in the future. In summary, the above results revealed that downregulating BCRP3 would inhibit cell proliferation, migration, and invasion in PCa, which contributing to expending the PSA-free survival. All the experiments conducted in this study confirmed that BCRP3 could function as the tumor promoting lncRNA participated in the PCa progression, indicating that BCRP3 knockdown could be considered as a potential therapy in PCa treatments.

3.5. BCRP3-miRNA Co-Regulatory Network
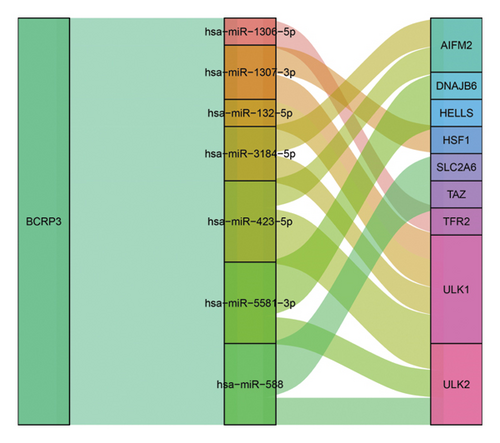
To explore the potential mechanism of BCRP3 tumor promoting function, the BCRP3-miRNA co-regulatory network was determined through the data from Starbase database. The downstream of BCRP3 was predicted through R software. It was shown that 7 miRNAs were significantly related with BCRP3 in PCa, which would affect the 9 ferroptosis-related mRNA expression for changing the tumor behaviors (Figure 7). Those interactions would account for the tumor promoting performances of BCRP3 and further indicate the underlying mechanism for ferroptosis regulation.
4. Discussion
In decades, kinds of therapies toward cancers have been broadly investigated, contributing to the social health [17–19]. The novel therapy of cancers will assist in the improvements of clinical treatments. PCa as the second most common cancer among men world-widely, deserves more attention and studies from the society [1]. Even the numbers of researches have demonstrated the progression of advanced clinical therapies toward PCa, there are various difficulties should be handled to expend survival of patients [20]. Biochemical recurrence of PCa refers to the higher PSA level than 0.2 ng/mL among two consecutive occasions after treatments, and represents adverse prognosis of patients with PCa [21]. It is noteworthy that timely identification of biochemical recurrence will significantly improve the clinical outcomes among the PCa patients. Meanwhile, ferroptosis, the novel kind of programmed cell death (PCD), is considered as an essential tumor suppressor in kinds of cancers, including PCa [22], renal cancer [23], et al. [24]. Establishment of a predictive model toward biochemical recurrence of PCa based on ferroptosis related lncRNAs will promote the progression of advanced clinical therapy, which is also the aim of this work to improve the individualized treatment of PCa.
PCD incessantly happens in the body, and the ferroptosis, as one kind of PCD, is characterized by iron-dependent LPO during the cell death [25]. The detailed processes contained in ferroptosis consist of iron metabolism, oxidative stress and et al., which involving kinds of altered metabolites such as LPO, nicotinamide adenine dinucleotide phosphate (NADPH) and glutathione (GSH) [26]. Based on the complex mechanism of ferroptosis, an increased number of studies have presented that ferroptosis participated in the tumor progression and would function as a suppresser in cancers [27, 28]. It is reported that ferroptosis also inhibit the progression in prostate cancer, which will accelerate the improvements of clinical therapy [29, 30]. Inspired by the exciting research results, advanced attempts should also be conducted to timely predict and avoid biochemical recurrence of PCa based on the ferroptosis. In this study, we establish a risk model based on the ferroptosis related lncRNAs according to the in silico assays, assisting in the survival prediction for patients with PCa.
Noncoding RNA has been confirmed as a functional RNA in tumor behaviors through numerous studies [31, 32]. LncRNAs also play an important role in regulating tumor progression in various cancers, such as PCa [33], renal caner [18], et al. [34]. Meanwhile, the lncRNAs are also proved as a regulator of ferroptosis in kinds of diseases [9, 35, 36]. The detailed molecular mechanism can be demonstrated that the lncRNAs will regulate miRNA as a ceRNA, which finally affecting functional protein level [11, 12]. There are various studies proved that lncRNAs participated in the tumor progression through regulating the ferroptosis [37, 38]. And this study of ferroptosis related lncRNAs in PCa will enrich the research toward malignant tumors therapy. In fact, our study has also proved that downregulation of BCRP3, a ferroptosis related lncRNAs, inhibited the proliferation, migration, and invasion of PCa in vitro, which assisting in the selection of novel target toward PCa treatments. Additionally, we further predict the potential molecular mechanism of the tumor inhibition after downregulating BCRP3. It is assumed that BCRP3 will function as a competing endogenous RNA (ceRNA) in regulating 7 miRNAs, and finally affects the expression of ferroptosis related proteins. The detailed mechanism involved ceRNA had been widely confirmed as the regulating method in special RNA expression change. LncRNA could competitively bind to corresponding miRNAs, affecting the regulation of miRNAs on target mRNA, thereby achieving regulation of target genes, finally affecting the tumor progression [39, 40]. In this study, we predicted the related miRNAs which could be regulated by BRCP3 through ceRNA mechanism. It was found that miR-1306-5p, miR-1307-3p, miR-132-5p, miR-3184-5p, miR-423-5p, miR-5581-3p, and miR-558 could potentially be regulated by BRCP3. Subsequently, we further analyzed the downstream of those regulated miRNAs. The ferroptosis related genes, such as AIFM2, SLC2A6, TAZ, et al. [41–43], were considered as the targets of the above miRNAs, providing the evidences that BRCP3 could function as the ferroptosis related lncRNAs regulating the PCa progression.
In conclusion, our study establishes a novel risk model in predicting biochemical recurrence of PCa patients, which will provide a clinical tool in determining the individualized treatment. Besides, we also confirm that the lncRNA BCRP3 function as an oncogene in PCa can be considered as the target in clinical therapy. This comprehensive risk model will promote the improvement of PCa therapy based on the novel molecular mechanism. Nevertheless, there were some limitations needed to be handled in the next steps. First, the in vitro experiments were conducted in one cell line, which should be further explored in other cell lines. Secondly, the influence of BCRP3 on ferroptosis needs further valuation to comprehensive reveal the BCRP3 tumor promoting function. Besides, the co-regulatory network between BCRP3 and miRNAs should be confirmed to prove the underlying mechanism for ferroptosis regulation. Additionally in vivo experiments should be subsequently conducted to prove the function of those ferroptosis related lncRNAs, and this risk model should be applied in the prospective study for the assessment of predictive performances.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Conception and design: P.Z., Z.X., and X.J. Administrative support: X.J. Provision of study materials or patients: W.Z., H.W., and Y.G. Collection and assembly of data: P.Z., W.Z., and Y.G. Data analysis and interpretation: H.W. and P.Z. Manuscript writing: all authors. Final approval of manuscript: all authors. P.Z. and Y.G. contributed equally to this work.
Funding
This work was supported by the Research Foundation of Taizhou People’s Hospital (ZL202004). The authors are grateful for the invaluable support and useful discussion with other members of the Urological Department.
Acknowledgments
The authors are grateful for the invaluable support and useful discussion with other members of the Urological Department.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
Raw data were generated at the laboratory of Taizhou People’s Hospital. Derived data supporting the findings of this study are available from the corresponding author on request.




