Remodeling on Lateral Side of Posterior Mitral Leaflet in Recurrent Mitral Regurgitation after Mitral Annuloplasty for Patients with Atrial Functional Mitral Regurgitation
Abstract
Objective. The high recurrence rate of mitral regurgitation (MR) in patients with atrial functional mitral regurgitation (AFMR) who underwent mitral annuloplasty (MAP) is reported. However, the mechanism of recurrence is not fully understood and appropriate surgical intervention remains unknown. Herein, we reviewed patients with AFMR who underwent MAP at our institution and investigated the preoperative geometric characteristics of the mitral valve in terms of MR recurrence after surgery. Methods. We retrospectively evaluated 20 patients with AFMR who underwent MAP between 2010 and 2022. The mean follow-up period was 3.2 ± 2.3 years. Preoperative three-dimensional transesophageal echocardiography (3D TEE) was available for all patients, and geometric analysis of the mitral valve was performed using the Philips Q-Lab software. Results. MR recurred in six patients. The rates of freedom from MR recurrence were 79% and 57% at one and three years, respectively. The lateral portion of the posterior mitral leaflet (PML) in patients with recurrent MR was longer and thicker than that in patients without recurrent MR (length of P1; 10 ± 3 vs. 15 ± 5 mm, p < 0.01, length of P2; 11 ± 4 vs. 14 ± 4 mm, p = 0.23, length of P3; 8 ± 3 vs. 10 ± 3 mm, p = 0.13). Conclusions. Patients with remodeling of the lateral portion of PML tended to have recurrent MR after MAP. This factor could indicate progressive remodeling, and MAP alone may not be a sufficient intervention for these patients.
1. Introduction
Long-standing atrial fibrillation causes left atrial enlargement and mitral annulus dilatation, leading to functional mitral regurgitation (MR). In recent studies, its pathology is defined as atrial functional MR (AFMR) [1–3]. Previous studies reported that the recurrence rate of MR in patients who underwent MAP (mitral annuloplasty) for AFMR was between 13 and 20% [4–6]. However, the underlying mechanism for the recurrence of MR after MAP for AFMR remains unclear [7]. The quality of three-dimensional transesophageal echocardiography (3D TEE) has greatly improved in the past decade, and its quality allows 3D TEE to be used to diagnose mitral valve morphology and guide surgical intervention [6–10].
We reviewed patients with AFMR who underwent MAP at our institution and investigated the preoperative geometric characters of the mitral valve with 3D TEE in terms of MR recurrence after MAP for AFMR.
2. Methods
2.1. Patients
MAP for AFMR was performed on 25 patients at Osaka University Hospital between 2010 and 2022. This study reviewed 20 patients whose TEE data were available. The mean patient age was 75 ± 7 years, 14 (60%) were female, and the mean follow-up period was 3.2 ± 2.3 years. All patients had long-standing AF that persisted for more than 1 year. Patients with organic abnormalities of the mitral valve leaflets or subvalvular structures, including degenerative mitral valve disease, low left ventricle ejection fraction (LVEF) (less than 50%), abnormal wall motion of the left ventricle (LV), history of acute myocardial infarction, infectious endocarditis, or rheumatic fever, were excluded from the study.
2.2. Echocardiography
Preoperative and postoperative transthoracic echocardiography (TTE) was performed in all patients, and the LV end-diastolic dimension (LVEDD), LV end-systolic dimension (LVESD), LVEF, and left atrium (LA) dimension were measured. The MR and tricuspid regurgitation (TR) grades on Doppler echocardiography were classified as follows: none, 0; trivial, 1; mild, 2; moderate, 3; severe, 4. Tethering of PML was defined as the immobility of PML in preoperative transthoracic echocardiography. Preoperative 3D TEE was available for all patients, and geometric analysis of the mitral valve was performed using the Philips Q-Lab software (Philips Medical Systems, Andover, MA, USA). The area, length, and thickness of the mitral annulus and leaflet were measured during end-systole. The anterior mitral leaflet (AML) and posterior mitral leaflet (PML) angles were defined as the angles between the annular line and the line joining the anterior or posterior annulus and the coaptation point, respectively. The AML and PML were divided into three parts: lateral A1 and P1, middle A2 and P2, and medial A3 and P3, and the length or thickness of each part was measured. Coaptation height after surgery was measured using postoperative TTE and defined as the length of contact between the AML and PML in the end-systole. Postoperative recurrence on MR was defined as moderate or severe MR on postoperative or follow-up echocardiography.
2.3. Surgical Procedures
Surgery was performed through median sternotomy or right mini-thoracotomy. The mitral valve was approached using the left-sided atrial approach. All patients underwent MAP. After sizing the intercommissural distance and AML size, the size of the annuloplasty ring was selected between the just size and two sizes down size. The ring (Memo-3D, Sorin Biomedica Cardio S.r.I., Saluggia, Italy, or Physio II, Edwards Lifestyle, Irvine, CA, USA) was implanted with 2 − 0 Ethibond sutures. If there was a large gap between the AML and PML, anterior mitral leaflet chordal reconstruction, posterior mitral leaflet patch augmentation, edge-to-edge, or cleft closure reconstruction was performed at the discretion of the attending surgeons. All patients underwent concomitant left atrial appendage closure, and those with moderate or severe tricuspid valve regurgitation underwent tricuspid annuloplasty.
2.4. Statistical Analysis
Continuous variables are presented as medians and 95% confidence intervals. Categorical variables were reported as frequencies. All statistical analyses were performed using JMP 16.0 (SAS Inc., Cary, NC, USA). Categorical variables were summarized as frequencies and percentages and compared among groups using chi-square or Fisher’s exact tests. Continuous variables were summarized as the mean ± SD or median (interquartile range). All p values for statistical analyses are two-tailed, and statistical significance was set at p < 0.05. The Kaplan–Meier analysis was used to calculate the freedom rate from MR recurrence after surgery and the overall survival rate.
2.5. IRB Information
This study was approved by the Osaka University Graduate School of Medicine (reference number: 16105).
3. Results
MR recurrence occurred in six patients; the causes were recurrent functional MR in four patients and detachment of the MAP ring in two. The recurrent functional MR occurred at the lateral portion in three patients and at the medial portion in one patient. In the two patients with detachment of the MAP ring, the MAP ring was detached at the medial portion of AML (Table 1). The freedom rates from MR recurrence were 79%, 71%, and 57% at 1, 2, and 3 years after surgery, respectively. The survival rates 1, 3, and 5 years after surgery were 100%, 100%, and 83%, respectively (Figure 1).
| Case | Details of recurrent MR after MAP |
|---|---|
| 1 | Functional MR on the lateral side |
| 2 | Functional MR on the lateral side |
| 3 | MAP ring detachment at the medial side of AML |
| 4 | MAP ring detachment at the medial side of AML |
| 5 | Functional MR on the medial side |
| 6 | Functional MR on the lateral side |
- MR, mitral regurgitation; AML, anterior mitral leaflet; MAP, mitral annuloplasty.
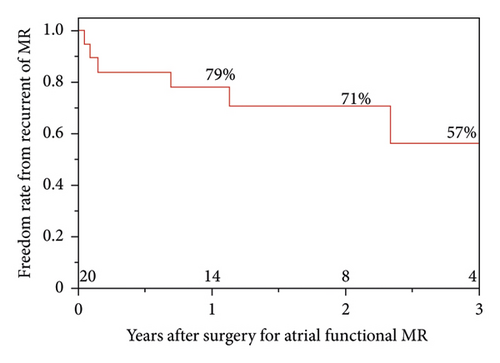
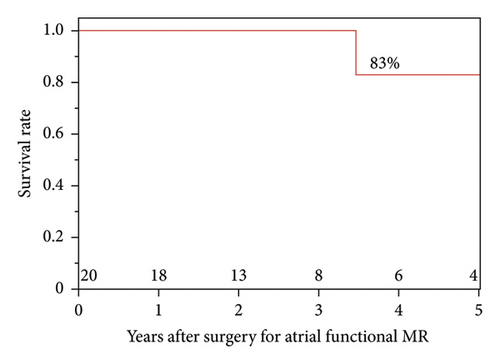
3.1. Characteristics of Patients and Preoperative TTE
Table 2 summarizes the patient characteristics and their preoperative TTE data. No significant differences were observed in sex, body surface area, heart failure symptoms, or comorbidities between patients with and without recurrent MR. LVEDD, LVESD, LVEF, LA dimension, MR grade, TR grade, and tethering of PML were not significantly different between the patients with and without recurrent MR.
| Recurrent MR (−) (n = 14) | Recurrent MR (+) (n = 6) | p value | |
|---|---|---|---|
| Female sex | 11 (79%) | 3 (50%) | 0.201 |
| Age, y | 76 ± 7 | 74 ± 7 | 0.532 |
| Body surface area, m2 | 1.49 ± 0.2 | 1.55 ± 0.1 | 0.447 |
| New York heart association class | |||
| II, n (%) | 12 (86%) | 4 (67%) | 0.344 |
| III/IV, n (%) | 2 (14%) | 2 (33%) | 0.344 |
| Comorbidities | |||
| Hypertension | 7 (50%) | 2 (33%) | 0.317 |
| Hyperlipidemia | 3 (21%) | 1 (17%) | 0.689 |
| Diabetes | 1 (7%) | 1 (17%) | 0.596 |
| Chronic kidney disease | 5 (36%) | 1 (17%) | 0.289 |
| Cerebrovascular event | 1 (7%) | 1 (17%) | 0.596 |
| Chronic respiratory disorder | 2 (14%) | 1 (17%) | 1.000 |
| Preoperative TTE | |||
| LVEDD, mm | 54 ± 9 | 55 ± 4 | 0.681 |
| LVESD, mm | 35 ± 6 | 34 ± 2 | 0.553 |
| LVEF, % | 63 ± 6 | 68 ± 3 | 0.078 |
| LA dimension, mm | 60 ± 17 | 67 ± 12 | 0.358 |
| MR grade | 3.5 ± 0.7 | 3.8 ± 0.4 | 0.221 |
| TR grade | 3.0 ± 1.2 | 2.3 ± 1.9 | 0.296 |
| Tethering of PML | 5 (36%) | 3 (50%) | 0.552 |
- MR, mitral regurgitation; TTE, transthoracic echocardiography; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; LVEF, left ventricular ejection fraction; LA, left atrium; TR, tricuspid regurgitation; PML, posterior mitral leaflet.
3.2. Surgical Outcome
Surgical outcomes are summarized in Table 3. There were no significant differences in the repair technique, type and size of the mitral annuloplasty ring, or concomitant surgery between the patients with and without recurrent MR.
| Recurrent MR (−) (n = 14) | Recurrent MR (+) (n = 6) | p value | |
|---|---|---|---|
| Repair technique | |||
| Resection and suture | 0 (0%) | 0 (0%) | |
| AML chordal reconstruction | 2 (14%) | 0 (0%) | 0.218 |
| PML patch augmentation | 1 (7%) | 0 (0%) | 0.391 |
| Others ∗ | 8 (57%) | 3 (50%) | 0.769 |
| MAP only | 4 (29%) | 3 (50%) | 0.363 |
| Left atrial plication | 1 (7%) | 1 (17%) | 0.515 |
| Mitral annuloplasty ring | |||
| MEMO 3D | 13 (93%) | 5 (83%) | 0.515 |
| Physio II | 1 (7%) | 1 (17%) | 0.515 |
| Mitral annuloplasty ring size | 29 ± 3 | 29 ± 3 | 0.702 |
| Concomitant surgery | |||
| Maze procedure | 1 (7%) | 1 (17%) | 0.515 |
| PFO closure | 2 (14%) | 0 (0%) | 0.218 |
- Others ∗ included edge to edge and cleft closure. MR, mitral regurgitation; AML, anterior mitral leaflet; PML, posterior mitral leaflet; MAP, mitral annuloplasty; PFO, patent foramen ovale.
3.3. Preoperative TEE
Table 4 summarizes the geometric analysis of the mitral valve using preoperative 3D TEE and Figure 2 shows the comparison in the geometric analysis of the mitral valve between the typical case with and without recurrent MR. Patients with recurrent MR had a longer circumference of the mitral annulus and a larger area of PML than patients without recurrent MR (circumference of the mitral annulus: 137 ± 11 vs. 124 ± 12 mm, p = 0.02, PML area: 797 ± 231 vs. 552 ± 144 mm2p = 0.01). Particularly, the lateral portion of PML in patients with recurrent MR was longer and thicker than those in patients without recurrent MR (length of P1; 10 ± 3 vs. 15 ± 5 mm, p < 0.01, length of P2; 11 ± 4 vs. 14 ± 4 mm, p = 0.23, length of P3; 8 ± 3 vs. 10 ± 3 mm, p = 0.13, thickness of P1; 2.1 ± 0.5 vs. 3.5 ± 1.0 mm, p < 0.01, thickness of P2; 1.9 ± 0.5 vs. 2.7 ± 0.7 mm, p < 0.01, thickness of P3; 2.0 ± 0.5 vs. 2.3 ± 0.6 mm, p = 0.20). Regarding the AML and PML angles, there was no significant difference between patients with and without recurrent MR.
| Recurrent MR (−) (n = 14) | Recurrent MR (+) (n = 6) | p value | |
|---|---|---|---|
| Mitral annulus | |||
| Anteroposterior diameter, mm | 36 ± 5 | 40 ± 5 | 0.086 |
| Anterolateral-posteromedial diameter, mm | 35 ± 5 | 41.2 ± 6.5 | 0.021※ |
| Circumference, mm | 124 ± 12 | 137 ± 11 | 0.020※ |
| Mitral leaflets | |||
| AML angle, ° | 13 ± 6 | 16 ± 6 | 0.180 |
| PML angle, ° | 34 ± 12 | 37 ± 16 | 0.671 |
| AML area, mm2 | 848 ± 168 | 932 ± 144 | 0.263 |
| PML area, mm2 | 552 ± 144 | 797 ± 231 | 0.008※ |
| Length of MV A1, mm | 19 ± 5 | 19 ± 4 | 0.902 |
| Length of MV A2, mm | 27 ± 4 | 28 ± 4 | 0.364 |
| Length of MV A3, mm | 21 ± 5 | 24 ± 3 | 0.282 |
| Length of MV P1, mm | 10 ± 3 | 15 ± 5 | 0.002※ |
| Length of MV P2, mm | 11 ± 4 | 14 ± 4 | 0.225 |
| Length of MV P3, mm | 8 ± 3 | 10 ± 3 | 0.131 |
| Thickness of MV A1, mm | 2.0 ± 0.6 | 2.3 ± 0.5 | 0.170 |
| Thickness of MV A2, mm | 1.9 ± 0.4 | 2.1 ± 0.4 | 0.440 |
| Thickness of MV A3, mm | 1.9 ± 0.6 | 2.1 ± 0.4 | 0.659 |
| Thickness of MV P1, mm | 2.1 ± 0.5 | 3.5 ± 1.0 | 0.001※ |
| Thickness of MV P2, mm | 1.9 ± 0.5 | 2.7 ± 0.7 | 0.005※ |
| Thickness of MV P3, mm | 2.0 ± 0.5 | 2.3 ± 0.6 | 0.204 |
- MR; mitral regurgitation, AML; anterior mitral leaflet, PML; posterior mitral leaflet, MV; mitral valve.
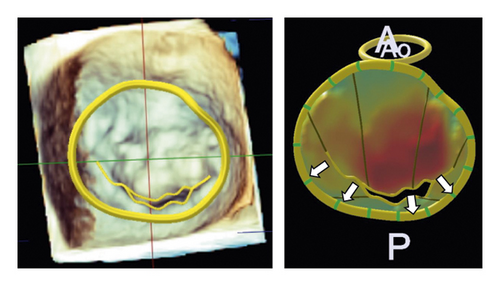
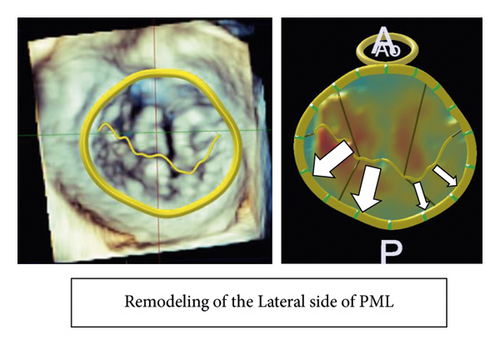
3.4. Postoperative Coaptation Height
Patients with recurrent MR had a shorter postoperative coaptation height than patients without recurrent MR (10.0 ± 2.0 vs. 5.1 ± 1.5 mm, p < 0.01) (Figures 3 and 4).

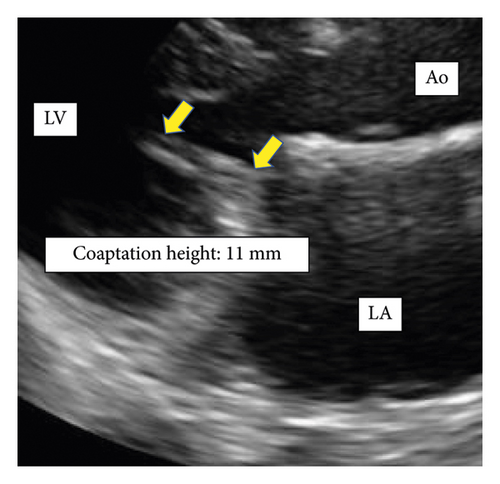
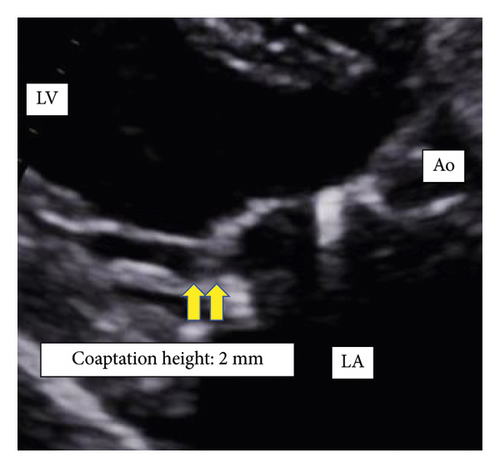
4. Discussion
This study showed that (1) patients with recurrent MR after MAP for AFMR had a larger leaflet area on the lateral side of PML than those without recurrent MR after MAP for AFMR. (2) Patients with recurrent MR after MAP for AFMR had a shorter postoperative coaptation height than those without recurrent MR after MAP for AFMR.
Previous studies demonstrated preoperative risk factors for recurrent MR after MAP for AFMR, such as larger LVEDD [4], LVESD [5, 6], and a greater degree of leaflet tethering [4] in patients with recurrent MR compared to patients without recurrent MR. However, the risk factors shown in one research were not consistent with those in other studies, and there is still no certain underlying cause for recurrent MR after MAP for AFMR. This could be not only because the AFMRs reported to date have been small studies with few patients, but also because the pathogenesis of AFMR involves multiple factors, including left atrial enlargement due to long-term atrial fibrillation, valve leaflet remodeling and tethering [11, 12]. In addition, this could also be because additional surgical procedures with MAP varied among those studies. In the current study, remodeling of the lateral portion of PML, meaning further remodeling of the lateral portion in PML as a preoperative risk factor, and short coaptation height after MAP as a postoperative one were found. It has previously been shown that postoperative coaptation height after mitral valve repair has a negative correlation with residual MR grade [13]. It would be a unique finding that remodeling of the lateral portion of PML correlated with short coaptation height after MAP for AFMR and led to MR recurrence.
It is an interesting finding that ring detachment was included as a recurrent mode after MAP for AFMR in previous studies [5, 6] and in the present study. It has been reported that mitral annular dilatation and PML area expansion were geometric changes due to AFMR [11, 12, 14] and that functional MR caused cellular changes in the mitral valve leaflet and increased the thickness of the mitral valve [15, 16]. Moreover, it also demonstrated that LA dilatation is caused by the fact that the LA is internal to the posterior mitral annulus and the crest of the LV is external to the posterior mitral annulus in AFMR [17]. In AFMR, pathohistological changes in the mitral valve are found in both the mitral valve leaflet and annulus, and they could cause recurrent functional MR or detachment of the MAP ring in patients with AFMR who underwent MAP. In this study, the recurrent functional MR after MAP tended to occur at the lateral portion, where PML progressed remodeling. The remodeling at the lateral portion of PML might cause less-effective MAP with insufficient coaptation, resulting in recurrent functional MR. It is still unclear if the remodeling of the lateral portion of PML found in the current study would be associated with an advanced stage of pathohistological disorder in the mitral valve with AFMR. Further pathohistological studies are needed to clarify the mechanism and indicate appropriate surgical procedures to improve outcomes in patients with AFMR.
4.1. Limitations
This study has some limitations. This was a retrospective single-center study with a small number of patients in each group. However, we analyzed the high-quality image data in 3D TEE, and this study is informative for cardiovascular surgeons who perform surgery on patients with AFMR.
5. Conclusion
Patients with AFMR with remodeling of the lateral portion of PML tended to have recurrent MR after MAP. This factor could indicate progressive remodeling and an advanced disease stage of AFMR. Thus, MAP alone might not be sufficient for these patients.
Disclosure
This paper was read at the Mitral Conclave of The American Association for Thoracic Surgery in May 5, 2023 [18].
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Open Research
Data Availability
The deidentified participant data will not be shared.




