Efficacy and Safety of Topical Dapsone for Acne Vulgaris: A Systemic Review with Meta-Analysis
Abstract
Topical dapsone is an alternative medication for acne treatment. However, which topical dapsone formulation is superior remains uncertain. Furthermore, data on the efficacy of dapsone compared with other topical acne treatments are lacking. We aimed to review the efficacy and safety of topical dapsone at different concentrations and to compare it with other topical acne treatments. We systematically reviewed literature related to the use of topical dapsone in treating acne published from January 2005 to September 2022. We searched databases from selected research studies for inclusion criteria and performed a network meta-analysis to compare the efficacy of using dapsone at different concentrations. Nine eligible studies were identified; among these, two studies with 7,350 patients were analyzed using network meta-analysis. At 12 weeks, the percentage of achieving a Global Acne Assessment Score and the mean percentage reduction in inflammatory acne were higher with 7.5% than with 5% dapsone, but the difference was not statistically significant. However, the mean percentage reduction in noninflammatory acne and total acne lesion-count at 12 weeks was lower with 7.5% than with 5% dapsone, but the difference was not statistically significant. Both concentrations of dapsone were more effective in treating inflammatory than comedonal acne and particularly effective in female patients and those aged ≥18 years. The side effects of dapsone were mild. Thus, topical dapsone is an effective alternative treatment for acne. Both concentrations of topical dapsone are effective in treating acne with no significant difference in efficacy and minimal side effects.
1. Introduction
Acne is a common dermatological condition, affecting 9.4% of the global population, and is found in all age groups, particularly in adolescents and young adults. Although acne is not life-threatening, it significantly affects patients’ physical and mental health [1, 2]. The current standard first-line treatments for acne include topical benzoyl peroxide, retinoids, and antibiotics, which can be limiting for some patients owing to their side effects such as skin irritation at the application site. Moreover, long-term use of topical antibiotics raises concerns about bacterial resistance. Therefore, alternative topical medications are used in patients who cannot tolerate standard acne treatments [2–4].
Dapsone has anti-inflammatory and antibacterial effects [5, 6]. In the past, oral dapsone was used to treat severe nodulocystic acne and was considered effective. However, oral dapsone has side effects owing to its systemic absorption, such as hemolytic anemia and methemoglobinemia [5, 7]. Thus, topical dapsone was developed to reduce these systemic side effects. Currently, topical dapsone is an alternative treatment for acne and is available at two concentrations: 5% topical dapsone applied twice daily and 7.5% topical dapsone applied once daily. The latter was more recently developed for increased convenience. Both concentrations were approved by the US Food and Drug Administration for acne treatment in 2005 [6, 8] and 2016 [6, 9], respectively.
However, there remain no clear statistical conclusions regarding the efficacy of different concentrations of topical dapsone. Therefore, we aimed to conduct a systematic review that was divided into two parts. The first part involved a network meta-analysis comparing the efficacy of different concentrations of dapsone for acne treatment. The second part was a systematic review compiling important data about topical dapsone regarding long-term use, onset of action, side-effects, and safety, based on current research findings.
2. Materials and Methods
2.1. Search Strategy and Study Selection
We systematically searched for related research using computers from databases, including PubMed, Embase, and the Cochrane Library, from January 2005 to September 2022. The keywords and Medical Subject Headings terms used for the search were: ([acne] OR [acne vulgaris] OR [inflammatory papule] OR [comedone]) AND ([dapsone] OR [topical dapsone] OR [aczone] OR [4,4-diaminodiphenylsulfone]). Moreover, the researchers searched and reviewed related research from cited references that were additionally discovered.
2.2. Eligibility Criteria
Research studies were included if they met the following inclusion criteria: (1) population: patients with acne aged ≥12 years; (2) intervention: topical dapsone 5% or 7.5%; (3) comparison: vehicle, other standard acne treatments, or comparison before and after treatment; (4) outcome: mean percent reduction from baseline in acne lesion count, acne lesion count, acne score, and adverse effects; and (5) type of design: randomized and nonrandomized controlled trials. Unpublished studies, conference abstracts, trial protocols, and preclinical studies were excluded.
2.3. Data Extraction and Quality Assessment
Two reviewers independently searched for relevant studies based on the inclusion and exclusion criteria. They independently recorded information from the collected studies using a data extraction form and assessed the risk of bias using the Cochrane risk-of-bias tool [10] for randomized controlled trials and the Risk of Bias Assessment Tool for Nonrandomized Studies [11] for nonrandomized controlled trials. If there was a discrepancy in the recorded data or the assessment of the risk of bias, the researchers and assistants consulted a senior dermatologist (a third reviewer) to reach a consensus.
2.4. Statistical Synthesis and Analyses
2.4.1. Direct Meta-Analysis
A direct meta-analysis was performed to compare dapsone in both concentrations and vehicle using risk ratios (RRs), and 95% confidence intervals (CIs) were estimated for dichotomous outcome, which was the percentage of achieving a Global Acne Assessment Score (GAAS). Alternatively, we used mean differences and 95% CIs for continuous outcomes, which was the mean percentage reduction in acne lesion count. Subsequently, they were pooled across studies using the inverse variance method if the heterogeneity was low (I²<25% and Q test P > 0.1); otherwise, the DerSimonian and Laird method was used.
2.4.2. Network Meta-Analysis
A two-stage network meta-analysis was performed to estimate the RR and mean difference, as mentioned previously, by comparing 5% and 7.5% topical dapsone with a common comparator (vehicle) as the reference treatment. A two-stage network meta-analysis using a multivariate meta-analysis with a consistency model and common between-study variance was performed to assess the relative treatment effects across the network. Treatments were compared and tested accordingly. Transitivity was explored by comparing the patient characteristics between the treatment and comparison groups. All the analyses were performed using Stata version 17 (StataCorp, College Station, TX, USA). Statistical significance was set at P < 0.05.
3. Results and Discussion
3.1. Search Strategy
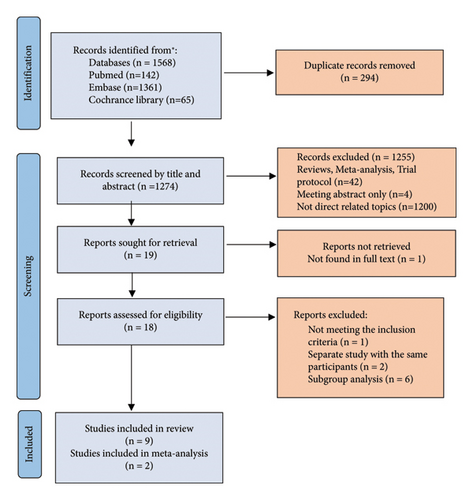
The systematic search results from the databases are shown in Figure 1. In total, 1,568 studies were identified, which were reduced to 1,274 after removing duplicates. After further exclusion of irrelevant studies, unpublished studies, and studies without full-text articles, 18 full-text articles [12–29] were considered for review. Upon evaluation, one study [21] did not meet the inclusion criteria, two substudies [22, 23] were included as multicenter studies, and six subgroup analyses [24–29] were excluded. Ultimately, nine studies [12–20] met the inclusion criteria.
3.2. Study Characteristics
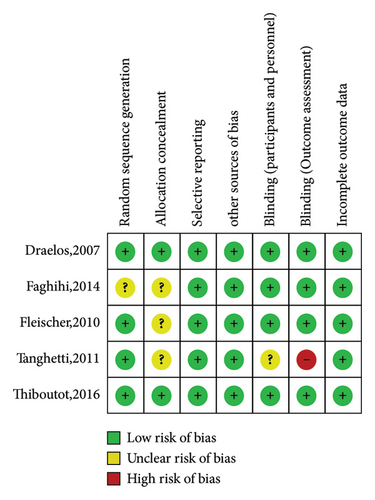
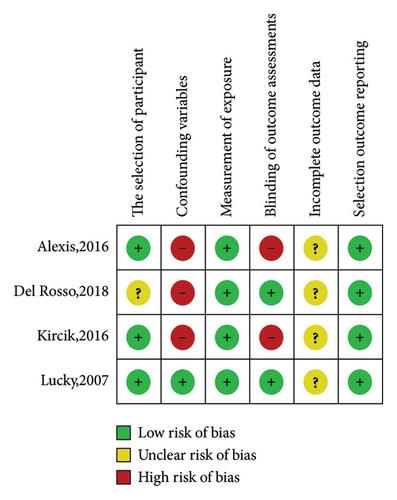
The characteristics of the nine eligible studies are summarized in Supplementary Table 1. These included five randomized controlled trials (four double-blind and one single-blind) comparing the effects of topical dapsone against vehicle in two studies and against other standard acne treatments in three studies. There were also four nonrandomized controlled trials (all open-label, comparing pre- and posttreatment results in the same volunteers). These studies were published between 2007 and 2018. Seven studies investigated 5% topical dapsone, and two investigated 7.5% topical dapsone, primarily examining the efficacy of topical dapsone on facial acne, with only one study focusing on truncal acne. The duration of these studies ranged from 8 weeks to 1 year, with most studies having a 12-week duration. Only one study had a 1-year duration, which aimed to assess the long-term efficacy and safety of topical dapsone. The risk-of-bias assessment results for these studies are presented in Figures 2 and 3.
3.3. Meta-Analysis of Efficacy of 5% vs. 7.5% Topical Dapsone
This study performed a network meta-analysis to compare the efficacy of different concentrations of topical dapsone. Only two studies [12, 20] with 7,350 patients were suitable for network meta-analysis to compare the efficacy of 5% and 7.5% topical dapsone against vehicle . These studies were similar in terms of study design, volunteer baseline characteristics, and other factors affecting treatment outcomes, such as sex, age, number and severity of acne lesions at baseline, and ethnicity.
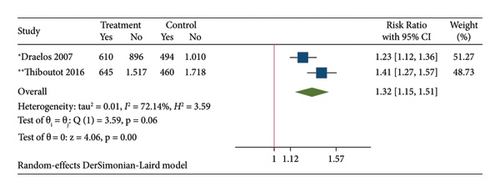
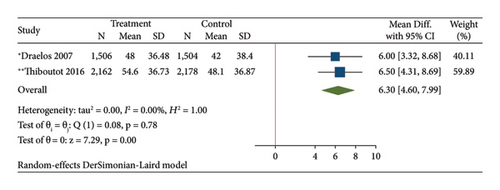
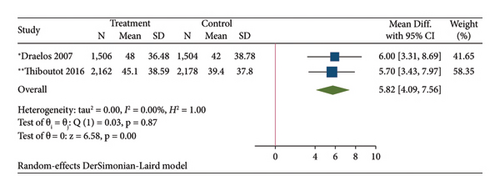
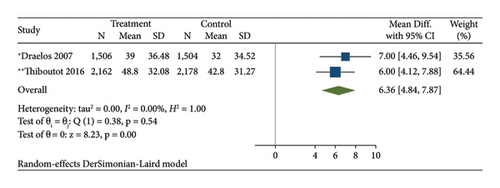
The results of the direct meta-analysis comparing the efficacy of topical dapsone against vehicle at 12 weeks posttreatment showed that topical dapsone had a significantly higher percentage of achieving GAAS than the vehicle, with a pooled RR of 1.32 (95% CI, 1.15–1.51) and high heterogeneity (I2, 72.14%) (Figure 4(a)). Topical dapsone also showed a significantly higher mean percentage reduction in inflammatory, noninflammatory, and total acne lesion count than the vehicle, with pooled mean differences (95% CI) of 6.30 (4.60–7.99), 5.82 (4.09–7.56), and 6.36 (4.84–7.87), respectively (Figures 4(b), 4(c), and 4(d)), with no heterogeneity (I2, 0%).
The results of the network meta-analysis comparing the efficacy of different concentrations of topical dapsone at 12 weeks posttreatment revealed that 7.5% topical dapsone had a higher percentage of achieving GAAS and a higher mean percentage reduction in inflammatory acne than 5% topical dapsone, but the differences were not statistically significant. The pooled RR and pooled mean difference were 1.15 (95% CI, 0.19–6.95) and 0.5 (95% CI, −1.94 to 2.94), respectively. At 12 weeks posttreatment, 7.5% topical dapsone showed a lower mean percentage reduction in noninflammatory and total acne lesions compared with 5% topical dapsone. However, these differences were not statistically significant, with pooled mean differences of −0.3 (95% CI, −3.74 to 3.14) and −1 (95% CI, −7.2 to 5.2), respectively.
3.4. Efficacy of Topical Dapsone in Long-Term Treatment
Long-term use of 5% topical dapsone applied twice daily over 12 months showed a significant statistical reduction in all types of acne compared with baseline (58%, 20%, and 49% reductions in inflammatory, noninflammatory, and total acne lesions, respectively; P = 0.002 for all acne types) [13]. The most significant reduction was observed in inflammatory acne, starting as early as 1 month, whereas noninflammatory acne started reducing from the 3rd month. Both types of acne gradually decreased and stabilized at 6–12 months.
This aligns with the findings of Kircik et al. on the long-term (6 months) effectiveness of 5% topical dapsone as a maintenance therapy after initial treatment with oral doxycycline [18]. As oral antibiotics are not recommended for long-term acne treatment owing to side effects and drug resistance, the continuous use of only 5% topical dapsone in that study resulted in a consistent reduction in all acne types.
3.5. Duration to Reduce Acne Lesions by 25%
Several studies have shown that 5% dapsone applied twice daily can reduce inflammatory, noninflammatory, and total acne lesions by 25% within approximately 2–4, 8–12, and 4–12 weeks, respectively [12–14]. For 7.5% dapsone applied once daily, the reduction times were approximately 2, 2–4, and 2–4 weeks, respectively (Table 1) [20].
| Drug | Time for 25% reduction in inflammatory acne (weeks) | Time for 25% reduction in noninflammatory acne (weeks) |
|---|---|---|
| 5% dapsone applied twice daily | 2–4 | 8–12 |
| 7.5% dapsone applied once daily | 2 | 2–4 |
| 2.5% benzoyl peroxide applied twice daily | 1.6–2.8 | — |
| 5% benzoyl peroxide applied twice daily | 1.5–6.5 | 4.4–>10 |
| 5% benzoyl peroxide + 1% clindamycin applied twice daily | 0.7–2.6 | 3.7–4.9 |
| 2.5% benzoyl peroxide + 0.1% adapalene applied once daily | 1.7 | — |
| 0.1% adapalene applied once daily | 0.6–8.4 | 0.9–10.5 |
| 0.025% tretinoin applied once daily | 1.3–5.1 | 1–3.9 |
| 0.05% tretinoin applied once daily | 1.1–7.6 | 1.5–3 |
In clinical practice, the onset of action of a medication is another important factor in advising patients with acne and in the physicians’ choice of acne treatment. Therefore, we compared the onset of action of topical dapsone with other standard acne treatments. According to Jacobs et al., who compiled the onset durations of other standard acne treatments [30] (Table 1), the time taken for a 25% reduction in inflammatory acne lesions was similar for both concentrations of topical dapsone and other standard treatments that primarily reduced inflammation and treatment of Cutibacterium acnes bacteria, such as 2.5% and 5% benzoyl peroxide and 5% benzoyl peroxide with 1% clindamycin, with a timeframe of approximately 2–4 weeks. However, these standard treatments often result in more side effects related to irritation at the application site [2–4].
Moreover, when comparing the time for a 25% reduction in noninflammatory acne lesions between dapsone and standard treatments from the topical retinoid group, such as 0.1% adapalene and tretinoin at concentrations of 0.025% and 0.05%, respectively, 7.5% topical dapsone had a similar onset of action, reducing the number of comedonal acne lesions by 25% in approximately 2–4 weeks. However, 5% topical dapsone had a relatively slower onset of action compared with these treatments. Therefore, other treatments with comedolytic effects may need to be considered in combination with 5% topical dapsone to help reduce the number of comedonal acne lesions.
3.6. Efficacy of Topical Dapsone in Different Populations
Studies indicated that both concentrations of topical dapsone were statistically significantly more effective than vehicle in treating acne across all sexes, age groups (12–17 years, ≥18 years), ethnicities, and skin types. However, subgroup analysis revealed higher efficacy in female than male patients [12, 25] and in the ≥18-year age group compared with the 12–17-year group [12, 26]. The efficacy was similar across ethnicities (Caucasian and non-Caucasian) and skin types (Fitzpatrick skin types I–III and IV–VI) [20, 28, 29] (Table 2).
| Study (author/year) | Original study | Subgroup analysis in dapsone treatment group | Conclusion | |
|---|---|---|---|---|
| 5% topical dapsone | Pickert and raimer, 2008 [24] | Draelos et al., 2007 [12] | Age 12–15 years (N = 578) |
|
| Lucky et al., 2007 [13] | Age 12–15 years (N = 181) | (i) At 12 months, volunteers aged 12–15 years using topical dapsone had a greater reduction in inflammatory and total acne compared with baseline (IP reduced by 44%, total by 30%, P < 0.001), but without significant difference in noninflammatory acne (NP reduced by 6%, P < 0.454) | ||
| Tanghetti et al., 2012 [25] | Draelos et al., 2007 [12] |
|
|
|
| Del Rosso et al., 2015 [26] | Draelos et al., 2007 [12] |
|
|
|
| 7.5% topical dapsone | Tanghetti et al., 2018 [27] | Thiboutot et al., 2016 [20] |
|
(i) At 12 weeks, female volunteers using topical dapsone showed a significantly greater reduction in all types of acne compared with male volunteers in all severity levels of acne |
| Taylor et al., 2018 [28] | Thiboutot et al., 2016 [20] |
|
|
|
| Draelos et al., 2017 [29] | Thiboutot et al., 2016 [20] |
|
(i) At 12 weeks, volunteers aged ≥18 years using topical dapsone had a significantly higher percentage of achieving GAAS score than those aged 12–17 years (37% vs. 22.5%, OR = 1.81, 95% CI = 1.54–2.08) | |
|
(i) At 12 weeks, volunteers using topical dapsone showed no significant difference in the percentage of achieving GAAS score between Caucasians and non-Caucasians (28.5% vs. 31.7%) | |||
|
(i) At 12 weeks, female volunteers using topical dapsone had a significantly higher percentage of achieving GAAS score than male volunteers (33.9% vs. 24.7%, OR = 0.8, 95% CI = 0.68–0.93) | |||
3.7. Efficacy of Topical Dapsone When Compared/Combined with Other Topical Acne Treatments
Dapsone 5% enhanced the effects of topical retinoids in acne treatment. It was more effective in reducing noninflammatory acne when used with 0.1% adapalene compared with 5% dapsone alone [14]. Similarly, the combination of 5% dapsone with 0.1% tazarotene was more effective than tazarotene alone in treating noninflammatory acne [15]. However, combining 5% dapsone with 4% benzoyl peroxide showed no significant difference compared with dapsone alone [14]. Another study showed enhanced efficacy when 5% dapsone was combined with oral isotretinoin [16]. Moreover, dapsone 5% was used as long-term maintenance therapy following initial treatment with oral doxycycline, showing a continuous reduction in all acne types [18]. There are no comparative studies on the efficacy of 7.5% topical dapsone with other acne treatments or when used in combination.
3.8. Safety during Pregnancy, Lactation, and When Used with Oral Contraceptive Pills
Topical dapsone is classified under FDA pregnancy risk category C, indicating evidence of embryotoxicity in animal studies where the animals received doses more than 500 times the typical human topical dose. However, no teratogenic effects have been reported in humans from oral or topical dapsone [31]. This is consistent with the report by Tuffanelli DL et al. in 1982, which successfully and safely used oral dapsone to treat dermatitis herpetiformis during pregnancy [32]. Therefore, topical dapsone is likely safe for use in pregnant women, but it should be discontinued before the last month of pregnancy to reduce the risk of neonatal hyperbilirubinemia [33]. Nonetheless, direct human studies are still lacking.
Regarding lactation, topical dapsone is categorized under Hale lactation risk category L4 (evidence of risk to the infant; potential benefit may warrant use). It has been found that oral dapsone can be excreted in breast milk [33], and there are reports of hemolytic anemia in infants breastfed by mothers taking oral dapsone [34]. Therefore, the use of topical dapsone is not recommended for treating acne in breastfeeding women.
For women using oral contraceptives, oral dapsone has minimal effect on contraceptive efficacy and is likely safe to use in conjunction with oral contraceptives [35]. Additionally, no drug interactions have been reported between topical dapsone and oral contraceptives [36], thus allowing their concurrent use.
3.9. Adverse Effects
3.9.1. Cutaneous and Systemic Adverse Effects
The common adverse effects at the application site included dryness (reported to vary from 0 to 20%), redness (reported to vary from 1.6 to 30%), oily skin (reported to vary from 6 to 15%), skin peeling (reported to vary from 0 to 11%), itching (reported to vary from 1 to 5%), and burning (reported to vary from 1 to 15%). These symptoms are generally mild to moderate and reversible upon discontinuation of the medication. [12–14, 17, 19, 20] (Summarized in Supplementary Table 2) However, using topical dapsone in combination with benzoyl peroxide can lead to temporary brown discoloration of the skin and clothing, which is difficult to wash off. When necessary, patients should be advised to apply both medications at different times or to wash off the benzoyl peroxide before applying topical dapsone [14, 37]. Common systemic adverse effects reported are headaches, colds, and sore throats [12–14, 19, 20]. In a long-term study (1-year duration) conducted by Lucky et al., headache and nasopharyngitis were common systemic adverse effects observed [13]. However, in comparative studies between topical dapsone and a vehicle, the adverse effects at both application and non-application sites were similar. This finding was observed in studies involving 5% and 7.5% topical dapsone [12, 20].
3.9.2. Systemic Absorption
Some studies have reported the plasma concentrations of 5% dapsone, with mean plasma dapsone concentrations of 4.75–11 ng/mL and mean N-acetyl dapsone concentrations of 2.34–4.5 ng/mL [13, 14]. In studies that examined hemoglobin levels to assess for hemolysis after topical dapsone use, no significant changes in hemoglobin levels were observed before and after drug use [12, 13, 16]. Additionally, no significant systemic adverse events have been reported in participants with glucose-6-phosphate dehydrogenase (G6PD) deficiency [12, 13].
This aligns with the findings of a study by Thiboutot et al. in 2007 [38], which investigated the systemic absorption of 5% dapsone gel after both short- and long-term usage. After 14 days of using 5% dapsone, systemic absorption was 126 times less than that of a single dose of oral dapsone (100 mg). After 12 months of use, the systemic absorption was 100 times less than that of a single dose of oral dapsone (100 mg). Throughout the 12-month study period, plasma dapsone levels remained relatively constant and did not increase with prolonged use. Piette et al. [39] studied the safety of 5% dapsone in volunteers with G6PD deficiency, who were at a higher risk of hemolytic anemia, and found that plasma levels reached a steady state within 2 weeks of use and rapidly decreased upon cessation. This study found that the systemic absorption was 100 times lower than that of a single dose of oral dapsone (100 mg). None of the studies reported systemic adverse effects. Therefore, 5% topical dapsone is safe for both normal volunteers and patients with G6PD deficiency. Regarding 7.5% topical dapsone, Jarratt et al. [40] reported that the systemic absorption of 7.5% topical dapsone applied once daily was 25–40% less than that of 5% topical dapsone applied twice daily. Furthermore, this study also reported that adverse effects at the application site in the group using 5% topical dapsone did not show any clinically significant difference from those in the group using 7.5% topical dapsone. No adverse systemic effects were observed.
3.9.3. Drug Interactions
Dapsone is classified as a sulfone drug and not as a sulfonamide, although it has a similar chemical structure. No cross-reactions were found; thus, those allergic to sulfonamides are not contraindicated for the use of topical dapsone [41]. However, oral dapsone with oral trimethoprim/sulfamethoxazole (TMP-SMX) can increase the blood concentration of both drugs by 1.5 times. Thiboutot et al. [38] found that the systemic absorption levels of topical dapsone increased when used with oral TMP-SMX but were still 100 times less than a single dose of oral dapsone. TMP-SMX levels in the blood did not increase when topical dapsone was used.
3.9.4. Postmarketing Surveillance
Postmarketing surveillance revealed three cases of methemoglobinemia in dapsone users: two with a 5% concentration [42, 43] and one with a 7.5% concentration [44]. One case was suspected to be due to the concurrent use of oral citalopram, an inhibitor of the cytochrome P450 enzyme system that affects the metabolism of drugs, such as dapsone, resulting in higher blood levels [42]. Another case was a 19-month-old child who accidently received a large amount of 5% dapsone [43]. The third patient was suspected of using an excessive amount of the drug [44]. All three patients with methemoglobin levels of 20.3%, 20.6%, and 34.1%, respectively, recovered from methemoglobinemia after treatment with methylene blue. None of these three patients had hemolytic anemia or any complications. Recently, the fourth case of methemoglobinemia was reported in a 15-year-old girl who developed persistent methemoglobinemia with subsequent autoimmune hemolytic anemia after 2 days of topical dapsone use [45]. Topical dapsone was used for acne treatment; however, the concentration, amount, or body surface area that the patient applied was not mentioned. The patient was treated with ascorbic acid, methylene blue, and cimetidine, and the fluctuation in methemoglobin levels resolved within 10 days.
4. Discussion
This study aimed to compare the effectiveness of 5% topical dapsone applied twice daily with 7.5% dapsone applied once daily using a network meta-analysis. At 12 weeks posttreatment, there was a slight difference in the reduction of all types of acne and an increased percentage of achieving GAAS between the two concentrations of topical dapsone. However, this difference was not statistically significant. Both concentrations were more effective in reducing inflammatory acne than noninflammatory acne. However, 7.5% topical dapsone reduced both inflammatory and noninflammatory acne faster than 5% topical dapsone. This might be because the higher concentration enhanced the ability to reduce inflammation and the amount of C. acnes bacteria. The anti-inflammatory mechanism of dapsone involves a dose-dependent reduction in the production of reactive oxygen species and the inhibition of neutrophil chemotaxis [5, 6].
Topical dapsone can be an alternative treatment option to standard acne treatments. It has similar effectiveness in reducing inflammatory acne with a comparable onset time of action. Topical dapsone can also be an alternative treatment for adult female acne, as it is more effective in women than in men and in adult acne than in adolescent acne [25, 26], since adult female patients with acne often have sensitive skin and may not tolerate standard acne treatments well. Additionally, dapsone enhances the effects of topical retinoids in reducing comedonal acne. Therefore, topical dapsone can be an effective option for acne patients who are irritated by other topical medications, particularly benzoyl peroxide, which can be administered as monotherapy or in combination with retinoids.
Long-term use of dapsone for acne treatment is feasible, and treatment results tend to stabilize from the sixth month onward [13]. In cases of poor response, a change in treatment or combination therapy with other acne medications may be considered. If the response is favorable, topical dapsone can be continued as long-term maintenance therapy [18].
Topical dapsone generally causes mild to moderate side effects that are reversible upon drug discontinuation. In a previous study, no significant systemic side effects were observed at either concentration of topical dapsone [12, 13, 16]. Although postmarketing surveillance found four cases of methemoglobinemia, these are considered rare when compared with the widespread use of 5% and 7.5% topical dapsone since 2005. Currently, 5% and 7% topical dapsone approved in the United States and Europe do not require blood tests for methemoglobin levels during treatment.
Despite our efforts to conduct a thorough systematic review, this study had some limitations. First, only a few original studies were included, and only two were eligible for meta-analysis. Second, a possible publication bias is that studies with positive results tend to be accepted for publication. Although the literature search strategy was comprehensive, we did not acquire unpublished trials or in-house studies from the manufacturers. Therefore, a precise comparison of the efficacy of topical dapsone at different concentrations or even with other acne treatments requires direct clinical research for further confirmation.
5. Conclusions
Both 5% and 7.5% topical dapsone are effective in treating acne with minimal irritation and high safety. They can be used in both the general population and patients with G6PD deficiency. Dapsone is particularly effective in female patients with acne and in those aged >18 years. It is more effective in treating inflammatory compared to comedonal acne and reducing inflammatory acne by 25% within approximately 2–4 weeks. Treatment outcomes with topical dapsone should be assessed approximately 6 months posttreatment, and it can be used continuously as a long-term maintenance therapy for acne. Additionally, topical dapsone enhances the efficacy of topical retinoids in reducing comedonal acne. Therefore, topical dapsone is a viable alternative treatment for patients with acne who cannot use standard topical acne medications.
Disclosure
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors would like to thank Dr. Kunlawat Thadanipon for his valuable comments.
Open Research
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.