The Use of Perioperative Liposomal Bupivacaine during Spine Surgeries Does Not Produce Improved Postoperative Pain Control in Adults: A Systematic Review
Abstract
Recovery following spinal surgery can be challenging, particularly concerning postoperative pain management. The first-line treatment for postoperative pain is opioid analgesia, but opioid overuse can lead to adverse effects. Liposomal bupivacaine (LB), an extended-release local anesthetic, has shown promise as a method of enhancing postoperative pain control. This systematic review aims to provide a comprehensive evaluation of the effectiveness of LB in improving postoperative outcomes after spinal surgery. Following the PRISMA guidelines, 18 studies met inclusion criteria and were evaluated for pain, opioid consumption, length of stay (LOS), and adverse events. Six studies showed no significant difference between treatment groups in all three major outcomes. Only two studies showed statistically improved pain control, opioid use, and LOS with LB. Similarly, another study also demonstrated improved pain control in the LB group; however, there was no significant change in opioid use or LOS. Two other studies also showed significantly improved postoperative pain control after the administration of LB, but the effect lasted only 24 hours after surgery. Three other reports saw a quicker transition off intravenous opioids with LB use, but no difference in total opioid use. Three of the four studies that focused on pediatric populations found improvement in all major outcomes with LB use; the fourth saw no significant difference between treatment groups. Currently, evidence is poor to suggest that LB significantly improves postoperative pain, opioid use, or LOS in adults. However, several patterns were identified that may influence study results: reports performed at military bases have different, more stringent protocols for recovery, and differences in spinal surgeries, some more invasive than others, can lead to different pain levels. The results of this systematic review demonstrate that the addition of LB does not provide improved postoperative pain control after spinal surgery in adults as demonstrated by both randomized controlled trials (RCTs) and the majority of retrospective cohort studies (RCSs). However, the use of LB for spine surgeries may be justified for the pediatric population, but further research is warranted given the limited reports available.
1. Introduction
Pain is often expected after surgery due to the inherent tissue damage that occurs during the procedure; however, there is growing evidence that this pain is inadequately managed in many patients [1]. Many spinal procedures are often associated with intense pain in the immediate and early postoperative period due to extensive dissection and muscle retraction [2, 3]. Poorly controlled pain often causes a reduction in patient mobility, which can lead to complications such as deep vein thrombosis, pulmonary embolus, wound issues, and pneumonia. Opioid analgesics are a first-line agent in the management of postoperative pain; however, overuse can be associated with significant adverse side effects. Among surgical specialties, orthopedic and neurosurgery procedures tend to have the highest rates of opioid use [4]. Effective pain control is a crucial aspect of patient comfort and a pivotal determinant of overall long-term outcomes.
Liposomal bupivacaine (LB; Exparel), an extended-release local anesthetic, has garnered increasing attention for its potential to enhance postoperative pain control and reduce opioid utilization in various surgical settings [5]. In the context of spinal surgery, where pain management is of particular importance, the utilization of liposomal bupivacaine presents a promising opportunity to optimize postoperative outcomes. Understanding the effectiveness of liposomal bupivacaine in this specific surgical context is critical for improving patient care and refining anesthesia protocols.
We reviewed the effectiveness of liposomal bupivacaine in improving postoperative outcomes in spinal surgery. Our primary objectives encompass a multifaceted exploration of the impact of LB during spinal surgery, focusing on postoperative pain control, postoperative opioid consumption, length of stay (LOS), and the incidence of complications. We aim to provide a nuanced understanding of how perioperative use of liposomal bupivacaine fares in comparison to traditional anesthesia modalities for patients undergoing spinal surgery, using a meticulous analysis of randomized controlled trials (RCTs) and retrospective cohort studies (RCSs). By accomplishing this, we aim to inform clinical practice and decision-making, offering guidance to healthcare providers, as they strive to optimize patient care and enhance surgical outcomes.
2. Methods
2.1. Search Strategy
We conducted a systematic review based on the Preferred Reporting items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The protocol for the systematic review was not registered. PubMed, Scopus, and clinicaltrials.gov were the databases evaluated. The search strategy was focused on “liposomal bupivacaine” for “spine” surgeries. Multiple search phrases and keywords were used to limit bias and capture missed studies that may not have shown up using a single search. The snowball method was used to collect references from other systematic reviews for potentially relevant articles that were missed with the initial search. The final search date was on December 18th, 2023. At the start, all abstracts were read in their entirety for initial screening. The full text of studies with potential for final inclusion was evaluated for eligibility based on inclusion and exclusion criteria.
2.2. Inclusion and Exclusion Criteria
Inclusion criteria required studies to be RCT or RCS in the English language that evaluated the impact of perioperative liposomal bupivacaine during spinal surgery on postoperative pain and analgesia. The studies must involve the use of LB for any type of spine-based surgery and report more than one standardized outcome measure. Studies that were not RCTs or RCSs of human patients, did not report any outcome data, and involved surgeries not focused on the spine were excluded.
2.3. Data Extraction and Quality Assessment
We collected data regarding the age range, total number of participants, type of surgery, treatment characteristics, and type of anesthesia mixture. Outcome data included intraoperative and postoperative opioid consumption, postoperative pain, LOS, and adverse events. Continuous variable data were reported as a mean ± standard deviations or a median (interquartile range). Categorical variable data were reported as frequency with percentages. Associations were reported with statistical significance at a p value <0.5. Studies were grouped based on the type of population (adults vs pediatrics). The critical appraisal of included studies was evaluated using the Jonna Briggs Institute (JBI) assessment tool for risk of bias for RCTs and RCSs [6]. This tool includes 11 items for RCTs and RCSs, each of which aims to assess a variety of biases such as selection, performance, and measurement. Each question can receive an answer of yes, no, unclear, or not applicable. Studies with a higher number of answers to be “yes” have a low risk of bias and those with a higher number of “no” answers have a high-risk of bias.
3. Results
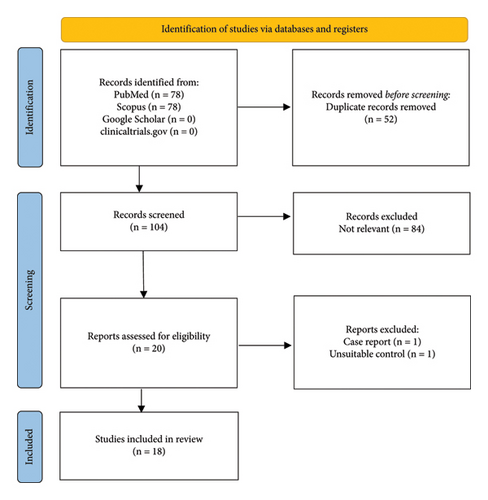
A total of 18 studies (2 RCTs and 16 RCSs) were included in this study (Figure 1). Four studies included only the pediatric population (18 years old or less). The risk of bias was moderate given there were several different areas where it was questionable if the criteria were met for each study (Tables 1(a) and 1(b)).
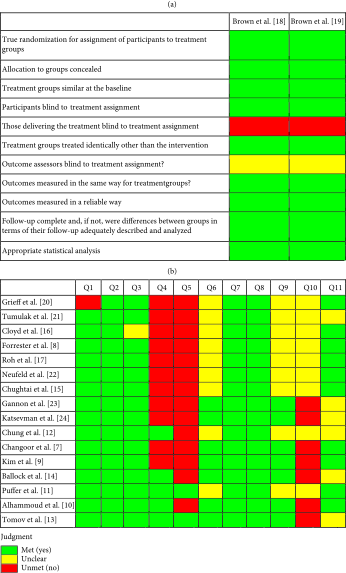 |
- (1) Two groups similar and recruited from the same population. (2) The exposures measured similarly to both exposed and unexposed groups. (3) Exposure measured in a valid and reliable way. (4) Confounding factors identified. (5) Strategies to deal with confounding factors stated. (6) Groups/participants free of the outcome at the start of the study. (7) Outcomes measured in a valid and reliable way. (8) Follow-up time reported and sufficient to be long enough for outcomes to occur. (9) Follow-up complete, and if not, were the reasons to loss of follow-up described and explored. (10) Strategies to address incomplete follow-up utilized. (11) Appropriate statistical analysis used.
The surgeries performed, most of which were at the lumbar level, include spinal fusions and/or decompressions and microdiscectomies. After induction with general anesthesia (GA) and before wound closure, LB was injected into paraspinal muscles and/or subcutaneous tissues surrounding the incision site for all studies except three. Changoor et al. [7] injected LB into the erector spinae muscles as an erector spinae block. Cloyd et al. [16] and Ballock et al. [14] did not provide the location of the anesthetic injection. The use of LB was often compared against the use of bupivacaine (n = 5), normal saline (n = 2), or no additional anesthesia (n = 11). Liposomal bupivacaine was often diluted with normal saline or mixed with the nonliposomal form of bupivacaine and then injected bilaterally along the incision site (Table 2).
| Author (year) | Study type | Age (years) | Surgery type | Treatment groups | Pain (VAS) | Opioid use | Length of stay (days) | Adverse events |
|---|---|---|---|---|---|---|---|---|
| Forrester et al. [8] (2022) | RCS | Elderly | Cervical or lumbar decompression and/or fusion |
|
|
Total: 54 ± 38 vs 44 ± 45 MME | 2.7 ± 2.4 vs 1.8 ± 2.0 | None |
| Cloyd et al. [16] (2018) | RCS | 8–18 | Posterior spinal fusion |
|
1 (0–3) vs 1 (0–3) | Total: 2.02 ± 0.98 vs 1.76 ± 0.82 mg/kg | 5 (4–5) vs 5 (4–5) | |
| Brown et al. [18] (2018) | RCT | >17 | Posterior lumbar decompression and fusion |
|
4.8 ± 2.8 vs 5.0 ± 2.7 | Total: 13.4 ± 8.9 vs 11.6 ± 9.3 MME | 3.7 ± 1.5 vs 3.6 ± 1.4 | |
| Roh et al. [17] (2020) | RCS | >17 | Posterior lumbar spinal fusion |
|
3.5 ± 1.4 vs 3.8 ± 1.2 |
|
|
|
| Chung et al. [12] (2021) | RCS | >17 | Spinal fusion |
|
|
|
4.8 vs 4.7b | Similar rates between groups |
| Brown et al. [19] (2019) | RCT | >17 | Posterior lumbar decompression and fusion |
|
3.9 ± 1.46 vs 3.9 ± 1.62 | Similar rates between groups for nausea, vomiting, and constipation | ||
| Grieff et al. [20] (2016) | RCS | 18–80 | Posterior cervical and lumbar decompression and fusion |
|
|
|
Rate (%): 8.8 vs 16.6 (cervical) and 18.4 vs 26.5 (lumbar) | |
| Chughtai et al. [15] (2020) | RCS | <19 | Spinal deformity surgery |
|
|
Total: 129 vs 78.2 ∗b MME | 4 (4–6) vs 3 (3–4) ∗ | Similar rates between groups for nausea, vomiting, and constipation |
| Tumulak et al. [21] (2017) | RCS | No age range given | Lumbar spinal fusion |
|
2.9 ± 0.931 vs 3.0 ± 0.962 | Total: 283.63 ± 244.38 vs 269.14 ± 195.16 mg | 83.63 vs 88.53b h | No significant difference between groups |
| Puffer et al. [11] (2016) | RCS/PCS | >17 | Single-level lumbar microdiscectomy |
|
|
3.5 ± 0.7 vs 2.5 ± 0.2 | No significant difference between groups | |
| Neufeld et al. [22] (2022) | RCS | No age range given | Anterior cervical discectomy and fusion (ACDF) |
|
Total: 987.4 ± 1,299.2 vs 918.7 ± 1,470.4 MME | 3.2 ± 2.8 vs 3.3 ± 1.6 | ||
| Gannon et al. [23] (2023) | RCS | >18 | One and two level posterior lumbar fusion |
|
No significant difference in VAS pain scores | Total: 233.1 vs 153.5 ∗b OME | 3.17 vs 3.02b | |
| Ballock et al. [14] (2021) | RCS | 1–17 | Primary spinal surgery |
|
2,437 vs 1,288 ∗b MME | 4 vs 3.5 ∗b | ||
| Changoor et al. [7] (2023) | RCS | <19 years | Posterior spinal fusion |
|
|
Total: 70.2 ± 30.3 vs 44.5 ± 29.0 ∗ OME | 2.74 ± 0.58 vs 2.43 ± 0.61 ∗ | Nausea and vomiting (%): 31.8 vs 37.7 |
| Katsevman et al. [24] (2020) | RCS | >18 | Elective PLIF or TLIF |
|
|
No significant difference in total MME from 0 to 72 h | 3.08 vs 1.94c ∗ | |
| Kim et al. [9] (2016) | RCS | No age range given | TLIF |
|
|
|
4.3 ± 1.3 vs 3.1 ± 0.9 ∗ | Rate (%): 8.1 vs 8.1 |
| Tomov et al. [13] (2018) | RCS | >23 | Single-level TLIF |
|
|
|
5.1 vs 4.8 | |
| Alhammoud et al. [10] (2022) | RCS | 67.8 ± 10.5 | Multilevel lumbar decompression |
|
3.1 ± 1.9 vs 1.6 ± 1.6 ∗ |
|
Rate (%): 7.5 vs 13.3 | |
- When 2 or more sets of results are reported with “vs,” the scores are reported as control vs treatment; if multiple patients were reported, treatments results were listed in patient numerical order. Data were reported as a mean ± standard deviations or a median (interquartile range). BPVC = bupivacaine, ESPB = erector spinae plane block, LB = liposomal bupivacaine, HCl = hydrochloride, h = hours, IV = intravenous, kg = kilogram, mg = milligram, ml = milliliter, MME = morphine milligram equivalent, NS = normal saline, PCA = patient-controlled analgesia, PLIF = posterior lumbar interbody fusion, POD = postoperative day, TLIF = transforaminal lumbar interbody fusion, and VAS = visual analog scale. ∗Statistical significance between groups. aReported as geometric mean (95% confidence interval) in the original study. bReported as mean only, and no standard deviation provided in the original study. cReported as median only, and no IQR provided in the original study.
The three main outcomes that were analyzed in this review were postoperative pain, postoperative opioid consumption, and length of stay in the hospital. Pain intensity was reported using the visual analog scale (VAS), which provides a scale of 1–10. Narcotic consumption most often included the amount of opioid use and occasionally the total time spent on analgesia. LOS was reported as the number of days hospitalized unless a different measure of time was mentioned. The most reported complications were nausea and vomiting, pruritus, respiratory depression, and sleepiness. The incidence of complications was low and insignificant between treatment groups.
Overall, the addition of LB during spinal surgery in adults does not appear to make a significant impact on postoperative pain. Six studies did not show a significant difference in treatment groups in postoperative pain, postoperative opioid use, and LOS [18–22, 24]. Two studies observed a significant decrease in narcotic use with LB administration; however, this did not translate to a statistically significant decrease in pain [17, 23]. On the other hand, Forrester et al. [8] demonstrated a statistically significant decrease in pain over multiple postoperative days, but not a difference in opioid consumption or length of hospitalization. Kim et al. [9] and Alhammoud et al. [10] were the only two studies that provided evidence of significantly improved postoperative pain control with LB. However, the significant difference does not appear to be long-lasting, only providing improved pain control up to 24 hours postsurgery as seen in the study by Kim et al. [9]. Interestingly, Puffer et al. [11], Chung et al. [12], and Tomov et al. [13] observed a quicker transition off IV opioids in the group receiving LB, but total narcotic use was insignificant between treatment groups.
While LB did not provide a significant impact on pain control after surgery for adults, LB administration in pediatrics does seem to make a difference. Changoor et al. [7], Ballock et al. [14], and Chughtai et al. [15] all saw decreased pain, opioid use, and LOS when children 18 years or less were given LB. However, one study by Cloyd et al. [16] saw no difference in postoperative outcomes with injection of LB.
While total hospital costs are reported in only three studies [9, 14, 17], all three show a decrease in costs for patients given LB. However, Ballock et al. [14] was the only study to show a significant difference. Possible reasons behind decreased hospital costs could be due to the impact on room and board and time in the operating room. Specifically in the study conducted by Bullock et al. [14], cost savings with LB versus non-LB analgesia were mostly attributable to hospital stay cost by room and board and central supply cost.
4. Discussion
Conventional spinal surgeries often involve extensive dissection of subcutaneous tissues, bones, and ligaments, resulting in a high degree of postoperative pain [2, 4]. The current mainstay of pain control after surgery includes narcotics, with a heavy reliance on opioids [25]. Long-term consequences of postoperative opioid analgesia surrounding dependence and addiction are well documented and a feared sequela of physicians prescribing these medications. Thus, effective pain control is an important aspect of postoperative care, supporting the clinical value of our study. LB has the potential to be part of a multimodal pain regimen to provide better management of pain after spine surgery. Thus, a comprehensive understanding of the impact of LB is necessary.
The placement of local anesthetics at the surgical site has become a popular strategy to manage postoperative pain [26]. The anesthetic administered is often a mixture of local anesthetics like bupivacaine or lidocaine, diluted with nonlocal anesthetic adjuvants such as epinephrine. Liposomal bupivacaine is a multivesicular formulation of 1.3% bupivacaine, developed to improve the analgesic duration of bupivacaine, prolonging pain relief and decreasing the need for narcotics.
To date, there are only two RCTs, both of which look at the addition of LB for posterior lumbar decompression and fusion (PLDF) in adults. There were no statistically significant differences in postoperative opioid consumption, postoperative pain, LOS, and complication rates between the intraoperative LB field block group and the placebo saline group. However, a subanalysis of narcotic tolerant (NT) and narcotic naive (NN) patients found decreased opioid use in NT patients when given LB versus saline injections compared to NN patients. A RCS by Grieff et al. [20] found a similar trend toward increased opioid requirements in the NT subpopulation that received nonliposomal bupivacaine versus LB. Thus, LB may provide an increased benefit for certain populations, such as NT patients.
Across most retrospective cohort studies, the addition of LB did not provide a significant improvement in pain control following spine surgery. However, several authors mentioned important patterns. First, in studies where LB significantly reduced opioid use but not pain [17, 23], it is possible that enough narcotics were given to reduce pain to a level typical for adult patients undergoing lumbar spinal fusion, rather than a level that was significantly less. For Tomov et al. [13] and Puffer et al. [11], even though total opioid use was not significantly reduced with LB, there was a significant decrease in IV narcotics and pain consults, indicating a faster recovery. The need for IV-dosed opioids can often be a barrier to discharge. While LOS should be expected to decrease, the lack of significance may be in part due to the setting of the studies taking place outside of the United States at a military practice where LOS is more protocol-based. Both Kim et al. [9] and Chung et al. [12] did not conduct studies at military bases and saw a decline in IV narcotic use following LB injection. However, only Kim et al. [9] had a significantly decreased LOS. Although Tumulak et al. [21] showed no superiority of LB injection over continuous plain bupivacaine infusion, there are inherent drawbacks that exist with a continuous infusion delivery system such as catheter setup, increased training, and cost of pumps that make an LB injection preferred. Lastly, there are concerns regarding the cost to patients given the cost of a single injection of LB is around $300 compared to only $3 for nonliposomal bupivacaine [27]. However, all three studies that provided a cost analysis showed decreased total hospital costs with the administration of LB. The use of LB for spine surgery may be justified when its cost benefits are taken together with the potential postoperative benefits.
The type of spinal surgery is another factor that plays a role in the success of LB. Not all spinal procedures require the same amount of tissue dissection. Some procedures are more intensive than others, resulting in different intensities of pain after surgery. Compared to decompression procedures, fusion procedures necessitate wider exposure, increased muscle dissection, and a longer period of retraction [8]. Thus, postoperative pain with fusion procedures is often much higher. A subgroup analysis by Forrester et al. [8] saw significantly increased opioid requirements in the fusion subgroup compared to the decompression subgroup, despite LB administration. However, it is unclear whether this difference accounts for the amount of preoperative medications used. Furthermore, Neufeld et al. [22] conducted a linear regression analysis that found that the number of cervical vertebrae involved in the procedure significantly predicted postoperative opioid use. Schoenfeld et al. [28] showed that less intensive discectomy and decompression procedures had a greater likelihood of patient opioid discontinuation. Thus, specific surgical characteristics are crucial to keep in mind when considering postoperative opioid use.
It is also possible that the current dose of 20 mL (266 mg) of LB may not be sufficient to produce an analgesic benefit for more invasive spinal surgeries, leading to a lack of significant results across studies. A RCT conducted a dose-response analysis of LB for total knee arthroplasty and found that only 532 mg liposomal bupivacaine, twice the concentration currently approved by the FDA, was superior to standard bupivacaine [29].
When isolating the pediatric population, spinal fusion surgery is commonly used to treat spinal deformities [30]. Deformity correction causes significant tissue trauma that can result in debilitating pain. Thus, IV patient-controlled analgesia with opioids has been used to manage pain in these patients [31]. However, early opioid misuse is associated with later opioid use disorder and high-risk behavior persisting into adulthood [32]. The use of LB in the pediatric population was recently approved in 2021. Cloyd et al. [16] saw similar use of narcotics between LB and the bupivacaine groups; however, the study suffered many limitations such as no data on LB dosage, lack of standard pain management protocol, and no assessment for readmission for pain after 72 hours postsurgery. Chughtai et al. [15] attempted to avoid such limitations and saw significantly decreased narcotic consumption, pain up to 48 hours, and LOS in pediatric patients who had undergone local infiltration with LB. Ballock et al. [14], Changoor et al. [7], and a quality improvement study by McIntosh and McLeod [33] all saw similar outcomes. LB has the potential to be part of a multimodal analgesia regimen in pediatric patients; however, future studies are needed to verify LB’s efficacy and safety in the pediatric population.
Our review includes a total of 18 studies and provides updates to the previous systematic review of only 10 studies by Nguyen et al. [34]. In general, our results are consistent with the previous study, with some differences. Like the previous review, there exists low-quality evidence that LB can provide improved postoperative pain control in patients undergoing spinal surgery. However, our review includes additional studies focused on the pediatric population, which is potentially a promising use for this medication.
4.1. Limitations
While we believe this systematic review provides valuable insights into the use of liposomal bupivacaine in spinal surgery, we recognize there are limitations to this study. The quality of studies included should be considered and could create an opportunity for publication bias as positive outcomes are more likely to be published by researchers. This bias could influence the overall perception of the effectiveness of liposomal bupivacaine in spinal surgery. Additionally, administration of liposomal bupivacaine and comparison controls were not standardized across all studies included in this review. Variations in injection sites, dilution methods, and comparisons against different anesthesia modalities allow for potential variability in observed outcomes making it difficult to draw definitive conclusions about the effectiveness of liposomal bupivacaine in spinal surgery [35]. Notably, there was heterogeneity among the spinal procedures in analyzed studies. This difference could contribute to variations in postoperative pain experiences and outcomes. Different surgical techniques, levels of invasiveness, and patient populations across studies could introduce confounding factors that may limit the generalizability of our findings. Finally, it should be noted that the duration of follow-up varied across studies, with some assessing outcomes up to 24 hours postsurgery, while others extended the observation period. The variability in follow-up duration may impact the detection of long-term effects of liposomal bupivacaine on postoperative pain and opioid consumption warranting subsequent studies standardizing follow-up duration.
5. Conclusion
Opioids for postoperative pain management following spinal surgery have been a longstanding practice. However, there is a push to encourage multimodal pain regimens to limit opioid use and decrease opioid dependence. The results of this systematic review demonstrate through low-quality RCSs that the addition of LB may decrease postoperative pain, opioid use, and length of stay without a significant impact on adverse events in adults; however, the positive impact on postoperative pain management does not appear long-lasting or clinically significant. In the pediatric population, the effectiveness of LB appears to be stronger; however, the number of available studies is limited. Higher-quality RCTs do not show a benefit with LB over standard pain regimens, but there were only two published. More high-quality studies like prospective RCTs are needed to assess the efficacy of LB for spine surgeries and to justify the use of LB to manage pain. Currently, the available research does not demonstrate strong evidence for improved pain management with the use of LB during spinal surgery.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
Open Access funding was enabled and organized by BTAA 2023.
Open Research
Data Availability
All data generated or analyzed during this study are included within this article.




