Fatigue and Sleepiness in Multiple Sclerosis and Their Response to Short-Term Treatment With Modafinil
Abstract
Background: Patients with multiple sclerosis (pwMS) often experience significant fatigue. Simultaneously, previous studies show that pwMS frequently suffer from sleepiness and sleep problems. We aimed to contribute to the understanding of correlations between sleep and sleepiness/fatigue in pwMS to better identify responders to the wake-inducing drug, modafinil.
Methods: This open-label, two-centre study included 26 pwMS suffering from daytime tiredness. Sleepiness, fatigue, and sleep were evaluated by questionnaires (Epworth Sleepiness Scale [ESS], Modified Fatigue Impact Scale [MFIS]/Multidimensional Fatigue Inventory [MFI-20], and Pittsburgh), daily sleep diary, and a wrist-worn actigraph before and after 3-week modafinil treatment.
Results: One-third of the pwMS fulfilled the criteria of excessive daytime sleepiness (EDS, defined as ESS ≥ 10). Eighty-two percent (MFIS) of the patients were fatigued, and a high proportion (96%) of the pwMS were poor sleepers. Sleepiness (ESS) was inverse correlated to the subjective sleep quality. Actigraph data did not relate to subjective sleep, but total sleep time (TST) and higher sleep efficacy (SE) estimated by actigraph were positively correlated to the degree of fatigue. Modafinil had a positive effect on EDS.
Discussion: In the present study, we found that the tired multiple sclerosis (MS) patient may suffer from both EDS and fatigue. We found an inverse correlation between EDS and subjective sleep quality. On the contrary, fatigue was positively correlated with longer TST and higher SE, estimated by actigraph. Sleepiness and fatigue did not correlate, and thus, it is highly relevant to distinguish these two symptoms. Modafinil only showed an effect on fatigue when the patient also suffered from EDS, which could suggest that ESS is a possible screening tool for responders to modafinil treatment.
Conclusion: Sleepiness and fatigue do not seem to correlate in pwMS, and we recommend screening tools for sleepiness as well as sleep quality before initiating modafinil treatment to identify potential responders to the treatment.
1. Introduction
Multiple sclerosis (MS) is a chronical neurological disease characterized by immune-mediated inflammation, axonal damage, and demyelination resulting in deterioration of sensory and motor function, vision, bladder function, and cognition in addition to physical and mental fatigue [1]. Several theories about the aetiology of fatigue have been investigated, and it appears that damage to the central nervous system (CNS) has a significant impact [1]. White matter changes in areas related to motivation as well as impairments in cortico-subcortical interactions (thalamus and basal ganglia) seem to be affected. Moreover, the immune system has been proposed as a mechanism with activation of proinflammatory cytokines. Fatigue is among the commonest symptoms in patients suffering from MS and affects approximately 80% of the patients. As such, it is a major reason for unemployment, even for patients with otherwise minor disability. MS patients frequently report daytime tiredness, but it has proved difficult to distinguish between sclerosis-related fatigue and excessive daytime sleepiness (EDS)/tiredness caused by sleep disorders and/or metabolic conditions [2].
Fatigue and sleepiness are frequently used interchangeably in scientific literature and clinical practice, but each refers to distinct concepts. The International Classification of Sleep Disorders [3] has defined sleepiness as an inability to stay awake and alert during the day, leading to episodes of an irresistible need for sleep or unintended lapses into drowsiness or sleep. Fatigue can be defined as reversible physical and mental exhaustion with reduced motivation, and desire to rest [4]. Consequences of fatigue and sleepiness can appear similar and may include low energy levels, physical discomfort, lack of motivation, and reduced work ability. Accordingly, it can be difficult to clarify the causes of these symptoms resulting in treatment challenges [5]. However, there are still significant differences between sleepiness and fatigue in some circumstances, including driving.
Previous studies [2, 6] have shown that poor sleep is related to fatigue in patients with MS, and it has been suggested that management of sleep disorders could improve daily function. MS patients suffer from sleep disorders such as restless leg syndrome (RLS) and insomnia with higher frequency than healthy controls [4], and a study by Riccitelli et al. showed that MS patients who suffer from RLS had higher global fatigue scores compared to MS patients without a RLS diagnosis [7]. This underlines the importance of recognizing sleep disturbances in MS patients, and neglecting this can result in missed opportunities to treat patients, whom otherwise could have obtained reduction in their subjective tiredness.
Modafinil is a wakefulness-inducing compound used to treat EDS. It is generally well tolerated in most patients and has few adverse effects compared to other psychostimulants (although there is a risk of cardial arrhythmias). Several mechanisms of modafinil have been described, and it is accepted that it induces wakefulness by activating systems such as the dopaminergic and histaminergic systems. Modafinil has shown positive outcomes for treatment of sleepiness in shift workers, sleep apnoea, people with sleep disorders, and tiredness related to depression and is especially well studied in patients with narcolepsy [8–10]. It is also the most common treatment option to treat tiredness in MS patients; however, the treatment is still classified as off-label. Previous studies in MS have found various results. Some have shown a significant effect whereas others have found no significant effect on fatigue compared to placebo. A recent randomised, placebo-controlled, crossover, double-blind study by Nourbakhsh et al. found that modafinil was not superior to placebo in improving MS-related fatigue [11]. However, there still might be a clinically significant effect in patients who suffer from EDS.
If we could better understand the relationship between fatigue and sleepiness, as well as the influence of sleep on those symptoms in MS patients, it would help clinicians to introduce a more individually tailored treatment option.
The aim of the present study was to characterize sleep patterns, mental and physical fatigue, and EDS in a group of MS patients with self-reported daytime tiredness, as well as to evaluate possible correlations in these parameters.
Furthermore, we aimed to investigate how 3 weeks of modafinil treatment influences fatigue, sleepiness, sleep quality, and mood as well as grip strength in MS patients in an open-label study.
2. Methods
This study was designed as a two-centre, 4-week, open-label prospective trial. The participants were included from Aarhus University Hospital and Regional Hospital Central Jutland Viborg, Denmark. Clinical and demographic characteristic of participants can be found in Table 1. All participants were included, treated, and followed up at Aarhus University Hospital. The study was approved by the National Ethics Committee in 2019 (case number 72-205-19) and the Data Protection Agency (via the Central Denmark Region’s internal notification, no. 1-16-02-372-19). All participants signed an informed consent prior to inclusion. All data were kept electronically in the safe database REDCap (Aarhus University).
| Variable | |
|---|---|
| Age (median [min–max]) | 39.83 (20.51–78.27) |
| Gender (percentage) | |
| Female | 61.54% |
| Male | 38.46% |
| Civil status (percentage) | |
| Married | 50.00% |
| Partner | 34.62% |
| Single | 15.38% |
| EDSS (median [min–max]) | 2.5 (0–6.5) |
| Years since MS diagnosis (min–max) | 8 (0.5–26) |
| MS subtype | |
| Relapsing-remitting MS | 24 |
| CIS | 2 |
| BMI (median [min–max]) | 25.31 (19.25–45.91) |
| Grip test kg total (median [min–max]) | 20.33 (2.67–50.83) |
| Male | 29.17 (19.67–45.83) |
| Female | 16 (2.67–50.83) |
| Grip test kg dominant hand (median [min–max]) | 21.5 (4–60.33) |
| Male | 27.67 (16–48.67) |
| Female | 15.67 (4–60.33) |
| Disease-modifying medication (no.) | |
| Dimethyl fumarate | 5 |
| Teriflunomide | 3 |
| Ocrelizumab | 2 |
| Cladribine | 1 |
| Natalizumab | 1 |
| Fingolimod | 1 |
| Symptomatic medication (no.) | |
| Paracetamol | 4 |
| NSAID | 2 |
| Gabapentin | 3 |
| Muscle spasticity drugs | 2 |
| Overactive bladder drugs | 5 |
| Fampridine | 1 |
Study participation was voluntary, patients’ data was treated according to rules set by the General Data Protection Regulation and the Danish Data Protection Agency, and the participation did not affect patients’ general treatment. Withdrawal from study was possible at any time.
Inclusion criteria were MS or CIS (clinically isolated syndrome), subjective daytime tiredness, age between 18 and 80 years, and written consent. Exclusion criteria included ongoing treatment with modafinil or sleep medication, untreated heart disease, untreated hypertension, diagnosed depression, liver disease, pregnancy, and inability to follow study protocol.
At inclusion, a clinical examination of the patient was performed. The severity of MS disease was assessed by Kurtzke Expanded Disability Status Scale (EDSS) [12], a score that ranges from 0 to 10 with a higher score indicating greater disability. Blood samples (including vitamin D, vitamin B12, haemoglobin, thyroid function, creatinine, ferritin, and ALAT) and a routine ECG were done before treatment was initiated (Figure 1).
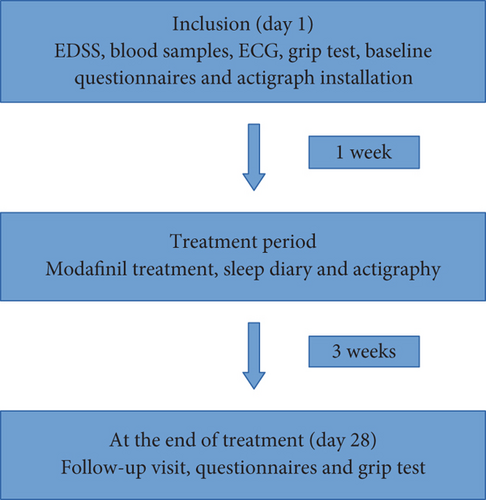
Sleep parameters were measured using actigraphy, as suggested by AASM guidelines [13]. We used ActiGraph wGT3X-BT [14, 15], which each participant wore at the wrist on the nondominant hand for the whole study period of 4 weeks (1-week baseline and 3-week treatment period). The sleep parameters given from ActiGraph data were estimated sleep efficacy (SE), total sleep time (TST), and wake time after sleep onset (WASO), and these were supplemented with a 4-week sleep diary to differentiate rest/sleep at night [14]. Additionally, participants filled out sleep-related questionnaires, including Pittsburgh Sleep Quality Index (PSQI) [16], insomnia scale (ISI) [17], and STOP-BANG Questionnaire [18]. We used a limit of 5 as cut-off in PSQI to distinguish between good and poor sleepers [16]. Participants also filled out Modified Fatigue Impact Scale (MFIS) [19] and Multidimensional Fatigue Inventory (MFI-20) [20] to assess fatigue, as well as Epworth Sleepiness Scale (ESS) [21] to evaluate sleepiness, and Major Depression Inventory (MDI) [22] to assess depression. For all the above-mentioned questionnaires, a higher score indicates greater disadvantage, referring to either worse sleep or a greater level of fatigue or sleepiness. In the same way, a higher score in MDI indicates a more severe form of depression [22]. MFIS consists of 21 items divided into 3 subscales: physical fatigue (9 items), cognitive fatigue (10 items), and psychosocial fatigue (2 items) [19]. A previous study has established a cut-off value of 38 for defining fatigue [23], which we used in our study. MFI-20 has 20 items divided equally with 4 items in each of the 5 subscales (general fatigue, physical fatigue, mental fatigue, reduced activity, and reduced motivation) [20], but previous studies have shown that the questionnaire works best with total scores instead of partial scores [24]. However, a previous study found a cut-off of 12 or more in the subscale “general fatigue” as pathological for fatigue [25]. Hand grip strength was established with a grip test using a hydraulic hand dynamometer from Jamar (serial number 5030J1) with three measurements on each side with the patient sitting in the upright position with the arm in a 90° angle [26]. An average of the three measurements on the dominant side was used for statistic calculations.
The questionnaires were answered electronically in Danish versions in REDCap (Aarhus University). Grip test and all the questionnaires were done both at baseline and again at follow-up 4 weeks after inclusion.
Modafinil treatment was initiated 1 week after baseline registration. The initial dose of modafinil was 100 mg as a single dose in the morning. After 3 days, this was increased to 100 mg bid (one dose in the morning and one at noon). Six days after the initiation of treatment, the dose was increased to 200 mg bid (two doses in the morning and two doses at noon). The maximum dose was 400 mg daily. This escalation plan was followed to find the optimal dose (effect and/or free of side effects). After the titration phase, the patients had 2 weeks on stable treatment before completing the questionnaires again.
Stata 15 software was used for all analyses conducted. Descriptive statistics were applied to summarise data on baseline characteristics of the included sample. Median and interquartile range (25–75 percentiles) were given for continuous variables and percentage for categorical variables. In data analysis, nonparametric tests were used. To measure the degree of association between two continuous baseline variables, Spearman’s rank correlation was used. Baseline values were compared with paired values after modafinil treatment for the following outcomes: MFIS, MFI-20, PSQI, ISI, ESS, and MDI. For actigraph data (TST, WASO, and SE), the median value from the baseline week was compared to the median value from the last week of active treatment. The predefined test was the Wilcoxon matched-pair signed-rank test.
3. Results
This study ran from September 1, 2019, to December 31, 2020. The main clinical and demographic data for the participants in this study are given in Table 1. A total of 26 MS/CIS patients were included, 62% of whom were women, with a median age of 39.8 years (between 20 and 78 years, one participant with age > 65 years) and a median disease duration of 8 years (from 0.5 to 26). EDSS median score was 2.5 (range 0–6.5). Twenty-four participants suffered from relapsing-remitting MS (RRMS), while two had a CIS diagnosis (Table 1). Two participants had well-treated hypertension, and no other significant comorbidities were found in the included sample based on medical history and prescriptions.
3.1. Baseline Measurements
At inclusion, six participants had low vitamin D level (less than 50 nmol/L), four had low ferritin levels, one had low TSH, one had low haemoglobin, and one had low vitamin B12. No interventions were performed. Median values for questionnaire scores at baseline are listed in Table 2.
| Questionnaire | Median (Q1–Q3) at baseline (26 participants) | Median (Q1–Q3) on treatment (22 participants) |
|---|---|---|
| MFISa | 45 (39–54) | 40 (19–50)* |
| Cognitive fatigue | 24 (19–28) | 20 (11–21)* |
| Physical fatigue | 19.5 (15–24) | 17 (6–22)* |
| Psychosocial fatigue | 3 (1–4) | 2 (0–3.5) |
| MFI-20 | 72 (61–78) | 67.5 (46–75)* |
| General fatigue | 18 (17–19) | 15.5 (11–18)* |
| Physical fatigue | 14 (12–17) | 13 (9–17) |
| Mental fatigue | 13.3 (12–14) | 12 (8–15) |
| Reduced activity | 15.50 (12–17) | 14 (9–16)* |
| Reduced motivation | 8 (7–9.5) | 8 (4–11.5) |
| PSQI (global score) | 8 (6–10) | 6 (5–8)* |
| ISI | 14 (12–18) | 10 (7–13)* |
| STOP-BANG | 2 (1–3) | 1 (1–3) |
| ESSb | 7.5 (5–10) | 5 (4–8)* |
| MDI | 16 (13–24) | 15 (7–22) |
| No depression (0–19) | 15 | 13 |
| Mild depression (20–24) | 4 | 6 |
| Moderate depression (25–29) | 2 | 3 |
| Severe depression (> 29) | 3 | 0 |
| Grip test kg total | 20.33 (15.33–29.17) | 24.42 (15.5–31.0)* |
| Grip test kg dominant hand | 21.5 (4–60.33) | 23 (7.33–56.67)* |
- Abbreviations: ESS = Epworth Sleepiness Scale, ISI = Insomnia Severity Index, MDI = Major Depression Inventory, MFI-20 = Multidimensional Fatigue Inventory, MFIS = Modified Fatigue Impact Scale, PSQI = Pittsburgh Sleep Quality Index.
- aFifty-five percent had a clinically significant decrease in MFIS of ≥ 10 points.
- bFifty percent had a clinically significant decrease in ESS of ≥ 2 points.
- *p < 0.05.
3.1.1. Fatigue
Median MFIS was 45 (ranged from 20 to 62) at baseline. With a cut-off value of 38 [23], 82% of the participants were defined as fatigued at inclusion.
MFI-20 had a median value of 72 (range 55–86) at baseline and a median value of 18 (range 11–20) in the “general fatigue” subscale. A total of 94.4% of the participants were fatigued at baseline based on a cut-off value of 12 or more in the “general fatigue” subscale [25].
At baseline, the mean value for hand grip strength was 22.11 ± 11.94 kg based on dynamometer with a general decrease from attempt 1 to 3. Median values (total and dominant hand) can be seen in Table 1.
3.1.2. Daytime Sleepiness
Median ESS was 7.5 (range 1–18). Defining EDS as an ESS score ≥ 10 [27, 28], 32% of the participants met this criterion at baseline.
3.1.3. Sleep
This study found a median value of global PSQI of 8 (range 3–16), and 95.5% of our sample had a PSQI score above 5 [13]. Median ISI score was 14 (range 0–24), and 10 (45.5%) of the participants had insomnia at baseline when using a cut-off score of 10 [17]. The median value of STOP-BANG was 2 (range 1–5), and 9% of the participants had a high risk of sleep apnoea with a value above 5 [29].
Data from actigraphs provided the values shown in Table 3.
| Actigraph parameter | Median (Q1–Q3) at baseline | Median (Q1–Q3) on treatment |
|---|---|---|
| Sleep efficacy (percent) | 87.51 (81.88–90.60) | 86.73 (83.74–90.17) |
| Latency (minutes) | 6.57 (3.29–11.57) | 6.62 (3.54–6.62) |
| Total time in bed (minutes) | 474.71 (441.29–502) | 475.55 (440–516.11) |
| Total sleep time (minutes) | 412.71 (387.71–439) | 419.90 (350.38–446.80) |
| Wake after sleep onset (minutes) | 48.29 (40.71–61.86) | 50.86 (37.67–67.53) |
| Number of awakenings (number) | 14.07 (12.14–18) | 13.58 (10–17.67) |
| Average awakening (minutes) | 3.42 (2.52–5.82) | 3.63 (2.86–4.77) |
| Movement index | 15.05 (12.06–17.89) | 16.10 (12.22–19.34) |
| Fragmentation index | 9.91 (7.71–12.09) | 11.54 (8.26–15.12) |
| Sleep fragmentation index | 24.27 (20.70–30.58) | 27.05 (19.61–34.78) |
3.1.4. Correlations (at Baseline)
In this study, we found no statistical significant relationship between total ESS score and MFIS (p = 0.3701), nor between ESS and MFI-20 (p = 0.6788).
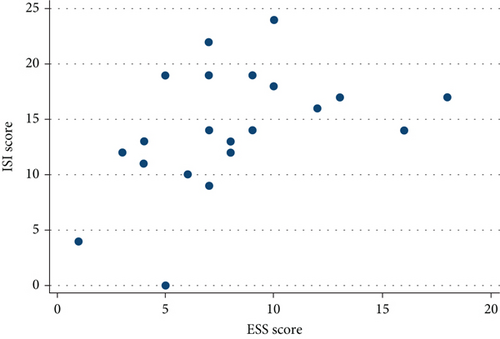
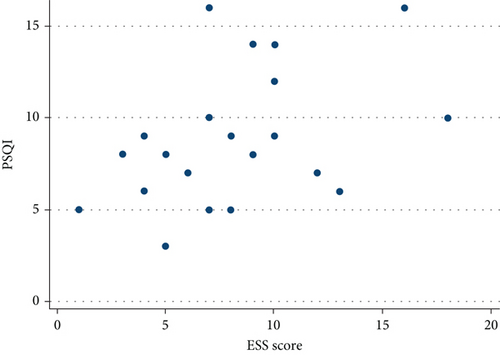
No correlation was found between ESS and SE, TST, WASO, or STOP-BANG, but a statistically significant inverse relationship was found between ESS and ISI (r = 0.51, p = 0.0152, Figure 2) as well as between ESS and PSQI (r = 0.44, p = 0.0415, Figure 3).
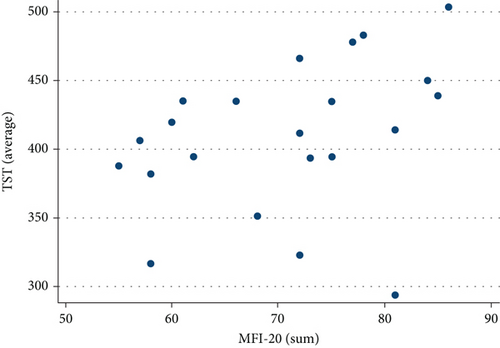
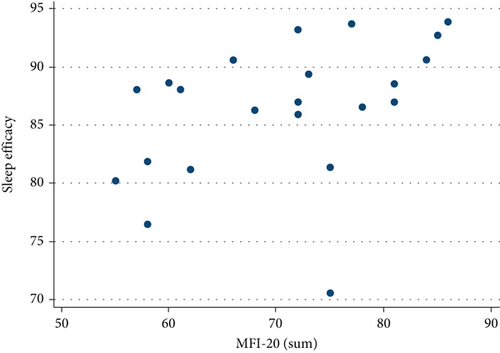
Neither MFIS nor MFI-20 correlated with WASO, ISI, PSQI, or global STOP-BANG score. MFI-20, however, showed a statistically significant positive relationship with TST (r = 0.4740, p = 0.0259, Figure 4) and SE (r = 0.4792, p = 0.0240, Figure 5).
Global PSQI score did not correlate with TST, SE, and WASO.
Based on grip test, no correlations between objective physical strength and MFIS, MFI-20, or ESS were found, and there was no correlation between grip test and the physical fatigue subscales on MFIS and MFI-20.
No significant correlation between subjective or sleep parameters by actigraph and disease severity was found.
3.1.5. Depression
Median MDI score was 16 (range 8–35, quartiles 13–24). Based on cut-off points for the MDI score, three participants suffered from undiagnosed mild depression, two suffered from undiagnosed moderate depression, and two suffered from undiagnosed severe depression. The MDI score correlated positively with MFIS (p = 0.0019), but not PSQI (p = 0.0836), ESS (p = 0.0639), or actigraph data.
3.1.6. Biochemistry
No significant correlations between TSH, vitamin D or B12, and fatigue or sleepiness were found.
3.2. Modafinil Treatment
Of the 26 included participants, 4 patients dropped out of the study before completing the final questionnaires. Three of them never started treatment due to personal reasons, and one declined to respond to the final survey invitation and was thus excluded from analysis. This resulted in a final participation sample of 22, of whom all received modafinil treatment. See Figure 6.
All participants followed the titration. The mean value for final daily dose of modafinil was 259.1 mg (range 100–400 mg).
Side effects were reported during the study, and the most frequent was headache with nine reported cases (40.9%). A lot of the reported side effects were temporary and thus only present the first days of treatment. An overview of reported side effects is shown in Table 4.
| Side effect | Frequency (n) | Percent |
|---|---|---|
| Headache | 9 | 40.9% |
| Gastrointestinal | 5 | 22.7% |
| Heart palpitations | 2 | 9.1% |
| Eyes | 2 | 9.1% |
| Mood related | 6 | 27.7% |
| Lowered alcohol tolerance | 2 | 9.1% |
| Dizziness | 1 | 4.5% |
| Oedema | 1 | 4.5% |
| Snoring | 1 | 4.5% |
| Allergic reaction | 1 | 4.5% |
| No reported side effects | 6 | 27.7% |
At follow-up visit, 69.6% of the participants reported a clear effect on daytime tiredness and no significant side effects. Thirteen percent reported to have a clear effect, but also significant side effects, and therefore only wanted to partially continue treatment with a reduced dose. Thirteen percent had significant side effects and wished to discontinue treatment. Out of all the participants, 4.4% felt no improvement on the medication. Overall, 82% of the participants wished to continue modafinil treatment to some extent, and 18% wished to discontinue treatment.
The median baseline ESS score was observed to be higher (ESS median 8 [6–10]) in participants who reported good treatment effect compared to those without perceived improvement (ESS median 5.5 [2.5–8]), but the difference did not prove statistically significant (p = 0.7646). The group with an improved subjective effect had a larger reduction in ESS after treatment (median ESS reduction from 8 to 5) compared to the group without subjective effect (median ESS reduction from 5.5 to 4.5) (p = 0.0143).
Median ESS scores improved from 7.5 (5–10, quartiles) to 5 (4–8, quartiles) after treatment (p = 0.0153). Previous studies have used a limit of 25% reduction or a 2-point reduction in ESS score as clinically significant. In the present study, 40% of the treated patients had more than a 25% reduction in their ESS score after treatment with modafinil, and 50% had a decrease of 2 points in ESS score [30, 31].
Defined by an improvement in MFIS score of 10 points or more [32], 54.6% of the participants had a clinically significant effect of modafinil on fatigue. Median MFIS scores decreased from 45 (39–54) at baseline to 40 (19–50) after treatment (p = 0.0048). Stratifying the subjects in two subgroups according to ESS at baseline (in subjects with ESS < 10 and subjects with ESS ≥ 10), a significant decrease in MFIS score was only seen in the group (n = 7) with ESS ≥ 10 (EDS). Both the cognitive fatigue and physical fatigue domains in MFIS were reduced after treatment (from median 24 [19–28] to 20 [11–21], p = 0.0028, and from 19.5 [15–24] to 17 [6–22], p = 0.019), but as with total MFIS score, this was only statistically significant when we included all participants, irrespective of the ESS value.
Based on MFI-20, there was a significant decrease in total score after treatment (p = 0.0294); however, as seen with MFIS score, the change in MFI-20 score was not significant when patients with EDS (ESS ≥ 10) were excluded from the analysis.
There was a statistically significant increase in objective physical strength estimated by grip test on the dominant hand (p = 0.0065) after modafinil treatment.
PSQI decreased significantly after modafinil treatment (p = 0.0006), but no statistically significant effect of treatment was seen on TST, WASO, and SE.
A statistically significant change in ISI score before and after treatment with modafinil (from median 14 [12–18] to 10 [7–13], p = 0.0086) was seen for the total sample, and the proportion of participants with defined insomnia decreased from 45.5% to 18.2%.
Modafinil had no statistical effect on the depression score (p = 0.0695).
4. Discussion
In a population of MS/CIS patients complaining of daytime tiredness, we examined the presence of fatigue as well as EDS, and the subjective and objective sleep quality were evaluated. In addition, we investigated the effect of 4-week off-label modafinil treatment on sleepiness, fatigue, and sleep in an open-label design.
In this sample of MS/CIS patients complaining of daytime tiredness, one-third fulfilled the criteria of daytime sleepiness (ESS > 10), and between 82% and 95% of the patients were fatigued depending on the inventory used (MFIS or MFI-20).
In accordance with previous studies [6], we found that MS/CIS patients suffer from poor sleep with a higher prevalence than the general population. In our sample, 95.5% had a PSQI score above 5, which is the limit used to distinguish between good and poor sleepers [16], compared to a previous study, which found 38.2% poor sleepers in the general population [33].
The higher incidence of poor sleep could not be confirmed objectively in the actigraph measurement, as only 9% of patients were under the limit for poor sleep, defined by Berger et al. as a SE below 80% [34]. However, TST in healthy mid-aged subjects is defined to be beneath average when under 420 min (7 h) [34, 35], and 59% of the participants in our study were below this limit. Actigraphy estimates wake and sleep times but does not assess sleep stages or arousals, and changes in microsleep might thus have been overlooked.
Keeping in mind these limitations of actigraphy, it can be argued that TST derived from actigraphy might better be referred to as “total rest time” than “sleep time.” However, previous studies and meta-analyses have found agreement between actigraphy and PSG with regard to sleep metrics TST and SL and have been suggested especially valuable in patients suffering from insomnia and/or EDS [13, 36, 37].
Poor sleep is one of the factors which may contribute to subjective daytime tiredness. A relevant proportion of the participants sleep/rest for less than 7 h per night indicating a level of sleep deprivation. This, as well as sleep disorders such as sleep apnoea (9%) and insomnia (46%), may contribute to the amount of participants with daytime tiredness. Another study found sleep-disordered breathing (SDB) in 41% of their population based on respirography, which suggests even more might suffer from sleep apnoea [38]. They also found a significant decrease in fatigue based on FSS score, but no change in ESS, when subjects with SDB were treated with CPAP. This underlies the importance of screening for sleep disorders in tired MS patients to help identify the best treatment option for their excessive daytime tiredness. We found a clear relationship between daytime sleepiness (ESS) and sleep quality (PSQI and ISI), indicating that poorer sleep quality is related to a higher daytime sleepiness. In contrast, MFI-20 (not MFIS) showed a positive correlation with objectively measured actigraph parameters SE and TST, suggesting that patients with a higher level of fatigue have a longer sleep duration at night. However, none of the fatigue scores were associated with subjectively reported sleep quality (PSQI or ISI).
Comorbidities like anomalies in baseline blood samples, depression, and age above 65 are also factors that can affect subjective daytime tiredness. We did not intervene during the study phase with antidepressants or vitamin supplements, which can be a possible limitation, and the sample size was too small to perform a regression analysis. We did however only have one participant above 65 years of age, and when statistical analysis was performed again with the exclusion of this participant, there were no significant differences in results. Comorbidities as known depression, hypertension, or cardiac disease were exclusion criteria, and no associations between blood samples or ESS/MFIS were found.
We found no correlation between fatigue (MFIS and MFI-20) and EDS (ESS), which supports the claim that it is highly relevant to distinguish the two symptoms, as their consequences can appear similar, but treatment strategy may differ.
4.1. Modafinil
Our study found the optimal dose of modafinil to be 250 mg based on the mean value where participants felt subjective effect while not suffering severe side effects. We increased modafinil to 400 mg daily within a week, and some participants reported minor passing side effects such as headaches. These might have been reduced if a slower titration had been used. There are, however, individual differences, and a slow titration schedule is recommended when starting treatment to find the lowest effective dose for each MS patient.
As hypothesised, patients suffering from EDS had a beneficial effect of modafinil treatment. A reduction in fatigue was found as well. However, when adjusting for ESS by only including those who did not suffer from EDS at baseline (ESS ≤ 10), modafinil had no effect on MFIS and MFI-20, indicating that excessive sleepiness might influence the effect seen on fatigue. This finding can potentially help better foresee which patients could benefit from modafinil treatment in the future.
Thus, using ESS as a screening tool might help to identify responders to modafinil treatment. However, larger RCT studies with focus on the effect of modafinil on sleepiness are warranted, as this study’s small size did not allow us to statistically adjust for confounders and limited our use of stratification on the data.
According to previous research, the physical fatigue MFIS subscale should be supplemented by objective measures [39]. Our population of MS patients had a lower mean value of physical strength estimated by grip test (22 kg) than the healthy population (33.5 kg) (right hand) [40], which is probably best explained by MS-related paresis. Surprisingly, physical strength improved on modafinil treatment both subjectively and objectively based on the MFIS physical component and grip test. It is unclear whether this is directly related to the treatment, mediated by decrease in daytime sleepiness or just due to fluctuations in MS motor symptoms. Thus, the mechanism behind this needs to be further investigated as no other studies have included objective measurement of physical fatigue as outcome measure in clinical trials with modafinil.
Participants who reported an effect of modafinil treatment had poorer objective and subjective sleep at baseline than those who felt no effect. Both ISI and PSQI improved on modafinil treatment, which is surprising as modafinil is a wake promoting drug, and insomnia is a reported side effect. A possible hypothesis for this result could be that the wake-inducing effect of modafinil benefits the patients during the day to a degree where they do not feel the need for daytime rest, which in turn might result in better nocturnal sleep/rest.
More participants reported an effect of modafinil than deducted from ESS, MFIS, and MFI-20 scores. Using an open-label design without a control group, the effect may be partially due to placebo response. This might explain the discrepancy between our results and the study by Nourbakhsh et al. [11], where modafinil was not found to be effective on MS-related fatigue compared to placebo. Nourbakhsh et al. did not include ESS as a primary outcome, but included it in a post hoc analysis, and found high ESS scores to possibly affect the measured MFIS improvement on modafinil treatment.
4.2. Limitations
The results of this study were significantly clearer in participants’ subjective reports of tiredness/sleepiness and sleep quality than the measurements by actigraph. It is possible that this difference would have been reduced if a more precise measurement for objective sleep (e.g., polysomnography [PSG]) was used. Actigraphy estimates quantitative sleep parameters out from movements. This might be one explanation for the discrepancy between subjective sleep and actigraph data. Moreover, actigraphy cannot assess sleep stage distribution and shifts, arousals, and short awakenings, in addition to periodic limb movement and sleep apnoea, which all probably contribute to the perception of unrefreshing or lacking sleep [41].
For grip test, we used three measurements on each side, which gives an information on hand strength. In order to have a measurement for fatigue, it would have been preferable to use at least 10 measurements on each side [42].
Our study had limitations regarding the open study design, multiple testing, the previously mentioned limitations for actigraphy, and the limited number of participants, which was partially due to a temporary shutdown during the COVID pandemic. Using self-reported questionnaires, one cannot rule out survey-related bias, including recall bias. Sampling bias is a risk when participants are asked to enter the study, as some of the patients with more severe symptoms might not have the energy to participate.
5. Conclusion
In the present study, we found that the tired MS patient may suffer from both excessive sleepiness and fatigue. We found an inverse correlation between EDS and subjective sleep quality. On the contrary, fatigue was positively correlated with longer TST and higher SE. Sleepiness and fatigue did not correlate, and thus, it is highly relevant to distinguish these two concepts. Our study underlines that screening of EDS and fatigue as well as insomnia and sleep apnoea should be included in the assessment of the tired MS patient. Future studies are recommended to focus on the association of sleep disorders and daytime fatigue/sleepiness in MS in the attempt to individualise and optimise the treatment.
Our small population, short-term study suggests that modafinil decreases fatigue in participants with coexisting sleepiness and that screening for sleepiness may predict benefit from treatment. Physical strength appeared to increase after treatment. The results need to be confirmed in a larger RCT study, including objective measurement of physical fatigue.
Conflicts of Interest
A.R.T. and M.O. have no conflicts of interest regarding the publication of this article. K.B.S. has served as a consultant for Takeda Pharma A/S and received travel grants from TEVA, Biogen, Merck, and Novartis. T.P. has received research grant support and travel support from Biogen Idec, Merck Serono, Novartis, Bayer Schering, Sanofi-Aventis, Roche, and Genzyme.
Funding
This study was funded by Neuroscience, the Lundbeck Foundation, in August 2019.
Open Research
Data Availability Statement
The study was registered at the national Ethics committee as well as the Central Danish Region. Data is contained within the article, but due to restrictions related to Danish law protecting private content, the full data set used in this study can only be made available through an application to Central Denmark Region by contacting the primary investigator.




