Electron Spin Resonance Spin Probe Technique for Investigating Non-TEMPO Radicals Dispersed in Nanospaces of a Crystalline Zn Complex
Abstract
An ESR spin probe technique with non-TEMPO radicals, such as nitronyl nitroxide (NN), benzonitronyl nitroxide (BzNN), and iminonitroxide (IN) radicals, was used for a porous metal-organic framework (MOF), [(ZnI2)3(TPT)2] (ZnTPT; TPT = tris(4-pyridyl)-1,3,5-triazine), at room temperature. The principal values of g and hyperfine coupling (A) tensors estimated from spectral reproduction were different from those for organic matrices for some of these radicals. These results indicate that host-guest interactions occur between the ZnTPT matrix and guest radicals. Thus, when using NN, BzNN, and IN radicals as spin probes for a porous MOF, the interaction between the metal atoms or organic ligands in host materials and guest radicals should be considered. The experimental ESR spectra for the derivatives of NN or BzNN radicals were reproduced only by the rigid-limit component in the ESR time scale. However, those for the derivatives of IN radicals were approximately reproduced only by rotational diffusion around the z-axis perpendicular to the plane in the IN group. Interestingly, this reproduction was not around the y-axis of the principal axes of the g tensors, parallel to the molecular long axis, as previously observed in a few organic matrices. The IN radicals dispersed in the ZnTPT matrix are expected to be accommodated in cylindrical or pseudocylindrical nanospaces sandwiched by the pyridyl or triazine rings of TPT in ZnTPT. These findings show that the ESR spin probe technique using non-TEMPO radicals can be used to investigate the chemical and biological structures of nanosized materials.
1. Introduction
Recent studies regarding various materials with tailored porous structures, sizes, and shapes, as well as interactions between host matrices and guest molecules, have grown remarkably. This has also accelerated the development of new functional materials using inclusion compounds (such as electric conductivity, [1] nonlinear optical property, [2] or magnetic property [3–5]), protection and storage of guest molecules (such as medical agents, [6] organic radicals, [7] or energetic materials [8]), and dynamics or mobility of guest molecules in a nanospace [9–12]. The detection or investigation of organic radicals confined in nanospaces or chemically decollated moieties by functional groups with unpaired electrons, such as N–O groups, requires the use of a powerful and unique technique, such as ESR spectroscopy. Using the ESR spin probe technique, the temperature dependence of the molecular dynamics of the organic radicals or moieties with unpaired electrons confined in the nanospace is studied to gain information on the structures, sizes, and shapes of the nanospaces present in organic [13–15] or inorganic porous materials [16], polymers [17, 18], peptide nanostructures [19], and biological membranes [20, 21].
The ESR spin probe technique is also used to investigate nanospaces formed in a variety of metal-organic frameworks (MOF) [11, 22, 23]. MOFs are expected to have applications such as adsorption or separation of small molecules on molecular sizes or host-guest interactions [11, 24] and catalysis for many chemical reactions [25]. Combinations of organic ligands and metals can lead to the development of various MOFs with unique pore sizes and shapes, as well as host-guest interactions. In many cases, derivatives of 2,2,6,6-tetramethyl-1-piperidinyloxyl (TEMPO) have been used as probe radicals. However, other radicals may be necessary in the future owing to the chemical diversity of the MOF pores in terms of the structures, sizes, shapes, and host-guest interactions between the MOF and guest radicals. For example, some TEMPO derivatives, such as 4-hydroxy-TEMPO (TEMPOL), introduced in MOF pores using the gas-phase sorption can be aggregated, resulting in the dipolar or exchange line-width modulation of ESR spectra [22]. In the impregnation method or encapsulation approach of probe radicals, the small spherical nitroxides, such as TEMPO, can be introduced into the narrow pore of the target materials, resulting in more complex ESR spectra due to the superposition of the spectra of the nitroxides in the narrow and large pores [18, 22].
The ESR spin probe technique has been used over the last decade to investigate non-TEMPO radicals such as 2-substituted nitronyl nitroxide (2-X-NN; 1–4; Figure 1(a)), 4-substituted phenyl benzonitronyl nitroxide (4-Y-PhBzNN; 5 and 6; Figure 1(b)), and 2-substituted-iminonitroxide (2-Z-IN; 7 and 8; Figure 1(c)), radicals for a nematic liquid crystalline phase [26]; organic 1D nanochannels such as 2,4,6-tris (4-chlorophenoxy)-1,3,5-triazine (CLPOT) or (o-phenylenedioxy) cyclotriphophazene crystals (TPP) [14, 27, 28]; or cylindrical cavities in polymethyl methacrylate (poly(methyl 2-methyl propenoate), PMMA) matrices [18]. The dipolar or exchange line-width modulation of ESR spectra for non-TEMPO radicals in nanospaces has not yet been reported [14, 18, 27, 28]. Moreover, the thin and long molecular shape of NN, PhBzNN, and IN radicals restricts their inclusion in excessively narrow pores. The principal values of g and hyperfine coupling (A) tensors of several NN or IN radicals had previously been studied, for example, in frozen organic matrices or cement [29, 30]. NN and IN radicals are important building blocks of porous films, vesicles, and nanoparticles [31], or ligands of magnetic metal complexes [32–34] as well as organic magnets [35]. In addition, NN and IN radicals are used as free radical scavengers to protect the peroxidation of cells [36] or as analgesic agents by selective binding with receptors [37]. Thus, NN or IN radicals have a potentially wide range of applications, particularly in the ESR spin probe technique. For instance, phenyl benzonitronyl nitroxide (PhBzNN) radicals [38, 39] can be used as spin probe radicals in two-dimensional (2D) sheet-type pores owing to their planar molecular shape [18].
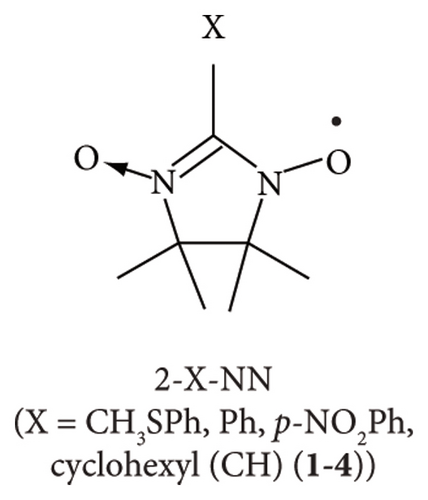
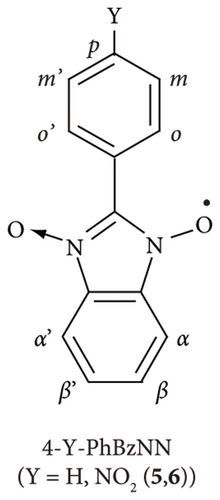
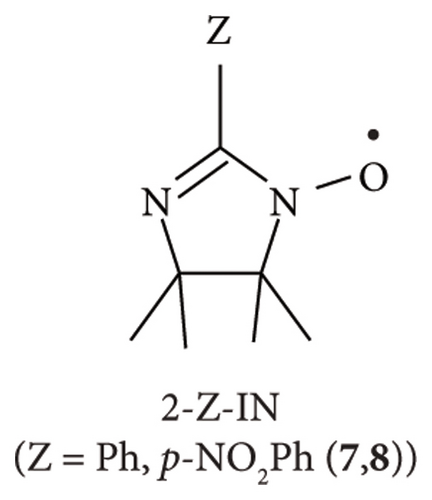
Herein, several 2-X-NN (X = Ph (2), p-NO2Ph (3), or cyclohexyl (CH; 4); Figure 1(a)), 4-Y-PhBzNN (Y = H (5) or NO2 (6); Figure 1(b)), and 2-Z-IN radicals (Z = Ph (7) or p-NO2Ph (8); Figure 1(c)) were dispersed in the three-dimensionally networked cage framework of a zinc complex, [(ZnI2)3(TPT)2] (ZnTPT; TPT = tris(4-pyridyl)-1, 3, 5-triazine) [24, 40–42]. The nanospace of ZnTPT shrinks or swells during the desorption or adsorption of guest molecules without destroying the crystal and is used to determine the structures of scarce target molecules, such as miyakosyne A (a marine natural product) or santonin (anthelmintic agent), using tiny crystals of [ZnTPT·(solvent molecule) (target molecule)] inclusion compounds (ICs) obtained by soaking ZnTPT in a solution of the target [24]. Electron-deficient panel-like TPT ligands in ZnTPT reduce the disorder of guest aromatics in the ZnTPT matrix. However, the preparation of single crystals of ZnTPT ICs is not always easy and it depends on the type of guest molecule. In addition, even if crystallization and determination of the crystal structure of ZnTPT ICs are successful, the pore structure of ZnTPT is dependent on the guest molecules, which are complex. In such cases, the ESR spin probe technique may be useful for determining the structures, sizes, shapes, and interactions between the pores of ZnTPT and the guest molecules. In addition, the MOF consisting of Zn(II) ion is suitable for the ESR measurement of adsorbed organic radicals because Zn(II) ion is nonmagnetic and gives no ESR signal. We conducted an ESR spin probe technique using non-TEMPO radicals with several NN, PhBzNN, and IN radicals in the nanospace of the ZnTPT. These studies have the potential to extend the reach of the application of the ESR spin probe technique and lead to the development of new magnetic materials formed by ordering magnetic molecules in nanospaces [4, 14, 43].
2. Experimental Details
2.1. Chemicals
ZnTPT [24, 40–42] and radicals 2–8 [44, 45] were synthesized following previously reported protocols. All the chemicals were used without further purification.
2.2. Sample Preparation
ZnTPT was synthesized as follows: 30 mg (9.6 × 10−2 mmol) of TPT was dissolved in 25 mL of nitrobenzene, and the residue was filtered (ca. 3.8 × 10−3 mol L−1; solution A). Concomitantly, 35 mg (0.11 mmol) of ZnI2 was dissolved in 5 mL of methanol (2.2 × 10−2 mol L−1; solution B). One milliliter of solution B was layered onto 5 mL of solution A in a test tube, and a colorless crystal precipitated after 3 days. The color of the ZnTPT crystal changed to yellow after drying in air; it was kept in a cyclohexane solution until just before use. Radicals 2–8 were dispersed in ZnTPT together with cyclohexane [41, 42] as follows: ca. 10 mg (6 × 10−3 mmol) of ZnTPT crystals and 0.2 mg of radicals 2–8 (from 6 × 10−4 mmol to 9 × 10−4 mmol, depending on the molecular weight of radicals) were added in 0.5 mL of cyclohexane and stored for 3 days. The crystals were filtered and washed well with dichloromethane, then dried just before any characterization measurement. [ZnTPT·(cyclohexane)x(guest radical)y] has a color similar to that of the original guest radical. For example, the color of [ZnTPT· ] was red because of the red color of 7.
2.3. Instrumentation
The ESR spectra were recorded at room temperature using an X-band spectrometer (JES-FA300, JEOL) equipped with a TE011 cylindrical cavity resonator. Colored crystals (2–3 mg) were packed in a commercial ESR tube (quartz glass; length: 270 mm; outer diameter: 5 mm). The X-band (e. g., 9.07 GHz) microwave power was set in the 0.001–1 mW range under nonsaturated conditions.
Single-crystal X-ray diffraction (XRD) analyses of [ZnTPT· and [ZnTPT· were conducted using a Rigaku Saturn diffractometer with graphite-monochromated Mo-Kα radiation (λ = 0.071075 nm) at 100 ± 1 K. For the determination of crystal orientation, plate-shaped crystals of the and (ca. 100 × 100 × 100 μm3) were placed at the bottom of a microtube and sealed with solvents. For the structure analysis, tiny crystals of [ZnTPT· and [ZnTPT· were mounted onto a single-crystal X-ray diffractometer while using a cryoloop, and diffraction data were collected. The crystal structures were solved and refined using SHELX97 software. The crystal structures of [ZnTPT· and gave rather poor factors because their crystal qualities were deteriorated after the adsorption of radical molecules; R = 0.1028 for [ZnTPT· and R = 0.2849 for [ZnTPT· , respectively.
Magnetic measurements for the [ZnTPT· (5.88 mg; 3.31 × 10−3 mmol) were performed with a Quantum Design MPMS-7 superconducting quantum interference device (SQUID) magnetometer. The sample was wrapped in a clean Al foil, and its magnetic susceptibilities were measured and subtracted separately. The magnetic curve for [ZnTPT· was measured in the magnetic field range of 0–50 kOe at 2 K. The static magnetic susceptibilities (χ) for [ZnTPT· were measured in the temperature range of 2.0–300 K at a magnetic flux density of 0.5 T. The diamagnetic contributions were calculated from Pascal’s constants (xdia = −8.0 × 10−4 emu mol−1).
Various core functions present in the EasySpin program package [46, 47] (version 5.2.35, ETH Zürich) such as garlic (for solution spectra), pepper (for solid-state spectra), and chili (for anisotropic slow-motion cw-ESR spectra) were used to simulate the spectra of radicals 2–8 dispersed in ZnTPT. Many parameters are required for spectral simulation. Therefore, high-speed calculations were performed in this study using parallel computing with a multicore computer. For example, computation with 12 cores was used to simulate the spectra using the step-by-step parameter variation technique, resulting in 12 reproductions within the time for a single calculation. Experiments and spectral simulations were performed with NN, PhBzNN, and IN radicals dispersed in ZnTPT. A personal computer (PC; RAM: <128 GB) was used to perform calculations to analyze the data using chili. The “Allocation” function of Opt was set as “Opt. Allocation = [4e7 2e5]” during the data analysis [46, 47].
2.4. Procedure to Obtain the Rigid-Limit or Rotational Diffusion ESR Spectra of the Radicals Dispersed in ZnTPT
The principal axes of the g, A (Ni), and A (Hj) tensors of the 2-X-NN, 4-Y-PhBzNN, and 2-Z-IN radicals were defined following previous reports [14, 18, 31]. The rigid-limit ESR spectra of the 2-X-NN, 4-Y-PhBzNN, and 2-Z-IN radicals have been previously reported [14, 18]. Note that the initial values of each component of the g and A tensors of the 4-Y-PhBzNN radicals in ZnTPT were obtained from those of PMMA [18]. The final principal components of the g and A tensors of the 2-X-NN, 4-Y-PhBzNN, and 2-Z-IN radicals were estimated using the step-by-step variation technique. Variations in the g and A tensor components, line-width parameters, and signal amplitudes were considered to minimize the sum of the squared differences between the data obtained from the experimental and simulated spectra.
Previously reported protocols used to determine the rotational axes of the radicals were followed to reproduce the spectra in cases where all or some of the radicals in the matrices underwent molecular motion (2-Z-IN radicals dispersed in ZnTPT in this study) [14, 18, 28]. The rotational axes of the radicals dispersed in ZnTPT were defined by the polar and azimuthal angles (θ and ϕ, respectively) relative to the principal axis of the g tensor of each radical [14, 48, 49]. The anisotropic rotational motion of each radical dispersed in ZnTPT can be described by a Markov process [49].
The simulation rate for the rigid-limit ESR spectra using the pepper (particularly for 4-Y-PhBzNN) increased manifold when the second-order perturbation theory (OPT method = “perturb2”) was used to carry out the calculations [47]. The line shape is expressed by a Voigt function [46, 48, 50].
3. Results and Discussion
3.1. Inclusion Amount of Guest Radicals in ZnTPT Matrix Estimated for [ZnTPT·(cyclohexane)x (non-TEMPO radical)y] ICs
The ESR spectra for [ZnTPT· (cyclohexane)x (non-TEMPO radical)y] were reproduced by a single rigid-limit component in the cases of 2–6 (Figures 3 and 4; Sections 3.2 and 3.3) or uniaxial rotational diffusion components in the cases of 7 and 8 (Figure 5; Section 3.4). These results indicate that dimerization of guest radicals, in addition to interspin dipolar or exchange interactions between guest radicals, was rare. Therefore, each radical in the ZnTPT matrix was expected to be isolated. Several methods have been used to estimate the inclusion amount of guest radicals in the ZnTPT matrix, as shown below. We evaluated all methods used previously.
Single-crystal XRD analyses were conducted for [ZnTPT· and [ZnTPT· . Precise estimation of the crystal structure of [ZnTPT· (cyclohexane)x (non-TEMPO radical)y] was difficult because of the low crystallizability of the single crystals and high R values (see Section 2.3). However, the host-guest ratio for the and could be estimated; the upper limits of the inclusion amounts of cyclohexane used as solvent and guest radicals per formula unit in the unit cell of ZnTPT crystals were 1 and 0.5, respectively, for the and 1 for the . For radicals 3, 4, 5, 6, and 8, two cyclohexane molecules were observed per unit formula in the unit cell of the ZnTPT crystal. Therefore, only trace amounts of guest radicals 3, 4, 5, 6, and 8 may be included in the ZnTPT matrix.
Magnetic measurements were performed to the It should be considered that the interspin distance between radical 2 dispersed in the ZnTPT matrix was adequately long, and that each radical was isolated based on the ESR results (see Section 3.2). Therefore, the is expected to exhibit paramagnetism with an electron spin quantum number S = 1/2.
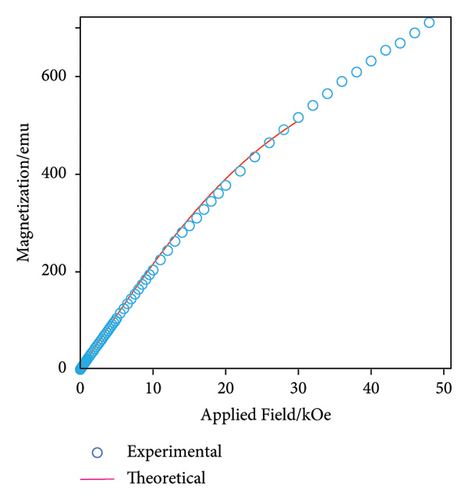
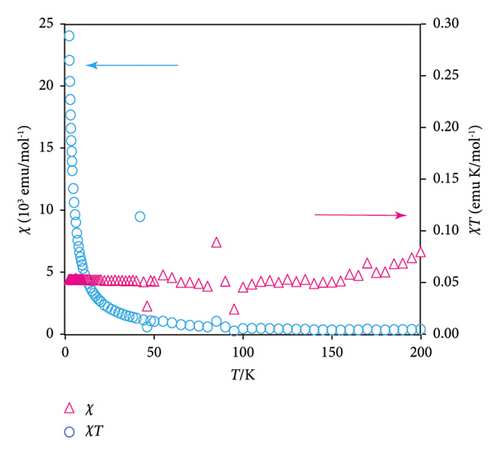
The sample mass, molecular weight, and Avogadro number are denoted by w, M, and NA, respectively, and N is equal to MNA/w. The occupancy of guest radicals per unit formula in the unit cell of the ZnTPT crystal is represented by p. The theoretical curve in the field range between 2 and 30 Oe, shown in Figure 2(a), demonstrates the case of p = 0.12, corresponding to y2 in .
Figure 2(b) shows the χ–T and χT-T plots of the from 2 to 200 K (magenta triangles and cyan circles, respectively). Using Curie’s law for the NA paramagnetic electron spins (S = 1/2), χT is estimated to be 0.375. In contrast, the experimental values of χT for were approximately constant at 0.05. The former corresponds to the case of x2 = 1 and y2 = 0.5 in the based on single-crystal XRD analyses, while the latter corresponds to x2 = 1 and y2 = 0.5p. p was estimated as 0.05/0.375 = 0.13. This value is consistent with the value of p estimated from the magnetization curve, as shown in Figure 2(a). In addition, the χ–T and χT-T plots of shown in Figure 2(b) were related to those of a typical paramagnet. These results demonstrate that the interspin interaction between the electron spins in the ZnTPT matrix is quite small, which is consistent with the ESR measurements (see below). Furthermore, similar or lower inclusion amounts of guest radicals are expected for radicals other than 2.
3.2. ESR Spectra of 2-X-NN Radicals Dispersed in ZnTPT Matrix
Figures 3(a)–3(d) show the ESR spectra recorded for 2-X-NN radicals (black) and the reproduced spectra (red); 2 (X = Ph; Figure 1(a)) dispersed in the PMMA matrix [16] and 2, 3 (X = p-NO2Ph; Figure 1(a)), or 4 (X = CH; Figure 1(a)) in the ZnTPT matrix at room temperature. Five major hyperfine interaction lines associated with the equivalent nitrogen atoms having unpaired electrons in the NN groups are shown in Figure 3(a)–3(d) [14, 18, 27, 28]. Therefore, Figures 3(b)–3(d) display the typical spectra of the dispersed NN radicals, which were well reproduced based on a rigid-limit powder pattern of an NN radical dispersed in the matrix, i.e., 10−6 s < τR, where τR denotes the rotational diffusion correlation time of the radicals. The NN radicals were adequately isolated in the ZnTPT matrix, and their molecular motion was terminated on the ESR time scale. This is consistent with the magnetic results described in Section 3.1. The molecular motion of the NN radicals was potentially hindered and restricted by the TPT ligands coordinately bonded to the Zn atoms of the ZnTPT matrix. This may have resulted in the confinement of a part of the NN radicals in the nanospace of the ZnTPT matrix with a diameter of approximately 1.0 nm from the molecular-size perspective at the smallest estimate.
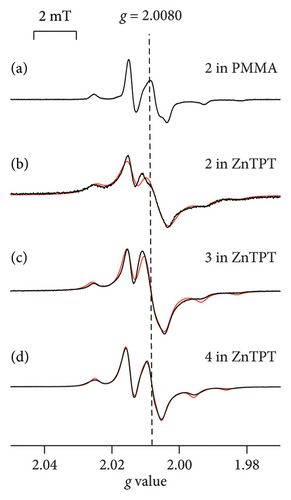
The spectra obtained for the 2-X-NN radical dispersed in the ZnTPT matrix (Figures 3(b)–3(d)) were simulated using the g and A tensor components listed in Table 1. The results of g and A tensor components for 2 agreed well with the results in which 2 was dispersed in various matrices [14, 18, 29, 30]. This indicates that few interactions between the ZnTPT matrix and dispersed radical 2 were observed. The g and A tensor components of 3 (or 4) in the Zn matrix are similar to those of 3 (or 4) in several other matrices (Table 1). However, the gzz components of 3 (or 4) in the ZnTPT matrix were shifted to higher g values than those of the organic 1D nanochannels of CLPOT [32] or for an acrylic resin such as PMMA [18]. Such findings indicate that the interaction between the ZnTPT matrix and 3 (or 4) was not negligible, at least for some of the NN radicals. The local electric field around the Zn atoms or the pyridyl or triazine rings of the TPT ligands in the ZnTPT matrix [41] may be attributed to the nitroxide moieties present in the NN group of 3 (or 4).
| Radicals | Matrices | gxx | gyy | gzz | Axx (N) | Ayy (N) | Azz (N) |
|---|---|---|---|---|---|---|---|
| /mT | |||||||
| 2 | Duco cement ∗1 | 2.0127 | 2.0068 | 2.0028 | 0.52 | 0.52 | 1.80 |
| Grassy toluene ∗2 | 2.0100 | 2.0065 | 2.0021 | — | — | 1.86 | |
| CLPOT ∗3 | 2.0118 | 2.0075 | 2.0031 | 0.34 | 0.12 | 1.74 | |
| TPP ∗4 | 2.0107 | 2.0086 | 2.0031 | 0.01 | 0.01 | 2.13 | |
| PMMA ∗5 | 2.0105 | 2.0072 | 2.0031 | 0.33 | 0.04 | 1.74 | |
| ZnTPT (this study) | 2.0104 | 2.0079 | 2.0030 | 0.58 | 0.01 | 1.70 | |
| 3 | CLPOT ∗3 | 2.0116 | 2.0076 | 2.0030 | 0.32 | 0.16 | 1.77 |
| PMMA ∗5 | 2.0111 | 2.0071 | 2.0031 | 0.23 | 0.19 | 1.74 | |
| ZnTPT (this study) | 2.0105 | 2.0082 | 2.0040 | 0.01 | 0.02 | 1.73 | |
| 4 | PMMA ∗5 | 2.0105 | 2.0070 | 2.0030 | 0.34 | 0.01 | 1.75 |
| ZnTPT (this study) | 2.0112 | 2.0083 | 2.0052 | 0.36 | 0.38 | 1.54 | |
- ∗1Ref. 29; ∗2Ref. 30; ∗3Ref. 27; ∗4Ref. 28; coinclusion with 7; ∗5Ref. 18. Spacer molecules in the case of asterisks 3 and 4 were abbreviated.
The principal axis xx and yy components of the hyperfine coupling tensor, Axx and Ayy, and (A(N)iso = (1/3)(Axx(N) + Ayy(N) + Azz(N)); also in other Aiso’s or giso’s) for 3 in the ZnTPT matrix were smaller than those for 3 in other organic matrices (see Table 1). The (A(N))iso for 4 in ZnTPT was almost the same as that for 4 in PMMA [18]. However, the principal axis zz component of the hyperfine coupling tensor, Azz, for 4 in the ZnTPT matrix was smaller than that for 4 in PMMA, whereas the Axx and Ayy for 4 were larger than those in PMMA [18]. These may be responsible for the host-guest interaction between the planar electron-deficient pyridyl or triazine rings of TPT ligands in the ZnTPT and the NO moieties present in the NN group of 3 (or 4) (see Section 3.3) [41].
3.3. ESR Spectra of 4-Y-PhBzNN Radicals Dispersed in the ZnTPT Matrix
Figures 4(a)–4(c) show the ESR spectra recorded for 4-Y-PhBzNN (black) and the reproduced spectra (red); 5 dispersed in PMMA (Y = H; Figure 1(b)), [18] and 5 and 6 (Y = p-NO2; Figure 1(b)) dispersed in ZnTPT. The ESR spectra for 4-Y-PhBzNN radicals are more complex than those for 2-X-NN radicals due to the superhyperfine coupling between the unpaired electron and the protons in the annulated aromatic and aryl moieties in PhBzNN (see Figure 1(b) and Section 2.4). The superhyperfine coupling between the unpaired electron and protons in 4-Y-PhBzNN perturbed the characteristic rigid-limit powder pattern of the 2-X-NN radicals (see Section 2.4), resulting in five major hyperfine interaction lines associated with the equivalent nitrogen atoms having unpaired electrons in the BzNN groups (Figure 4(a)–4(c)), which are broader than the ESR spectra of the 2-X-NN radicals (Figures 3(a)–3(d)) [18]. The ESR spectra for 4-Y-PhBzNN in ZnTPT were similar to those for PMMA and could be reproduced using a rigid-limit powder pattern of the PhBzNN radical dispersed in a matrix, i.e., 10−6 s < τR [18]. The isolation of 4-Y-PhBzNN radicals in the ZnTPT matrix was confirmed, and the molecular motion of the PhBzNN radicals in the ZnTPT matrix was terminated on the ESR time scale. The molecular motion of the PhBzNN radicals was potentially hindered and restricted by the TPT ligands of the ZnTPT matrix. This may lead to the confinement of the BzNN radicals in the nanospace of the ZnTPT matrix, which has a diameter of approximately 1.2 nm from the molecular-size perspective at the smallest estimate.
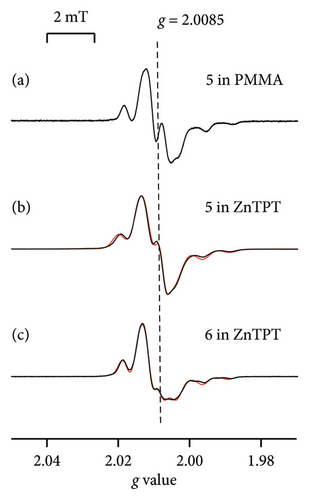
The spectrum recorded with 5 (or 6) dispersed in the ZnTPT matrix was simulated using the principal axes’ components of the g and A tensors, as shown in Table 2. They were relatively similar to those for 5 (or 6) in PMMA [18]. However, the principal axis components of the g tensor and giso estimated from the principal axes’ components of the g tensors of 5 (or 6) in the Zn matrix were totally shifted to a higher g value side than those for PMMA (Tables 2 and 3) [18]. This indicates that the interaction between the ZnTPT matrix and 5 (or 6) was not negligible. Furthermore, the local electric field around the Zn atoms or the electron-deficient pyridyl or triazine rings of the TPT ligands in ZnTPT [41] may be attributed to the nitroxide moieties present in the BzNN group of 5 (or 6), similar to the case of the 2-X-NN radicals in ZnTPT. The planar molecular configuration of 5 (or 6) may be influenced by the electron-deficient pyridyl or triazine ring of the TPT ligands in the ZnTPT.
| Tensor components | States | ||||
|---|---|---|---|---|---|
| 5 in PMMA ∗1 | 5 in ZnTPT | 6 in PMMA ∗1 | 6 in ZnTPT | ||
| gxx | 2.0121 | 2.0127 | 2.0118 | 2.0129 | |
| gyy | 2.0071 | 2.0078 | 2.0068 | 2.0084 | |
| gzz | 2.0032 | 2.0039 | 2.0028 | 2.0037 | |
| Axx (N) | /mT | 0.11 | 0.17 | 0.07 | 0.07 |
| Ayy (N) | 0.03 | 0.00 | 0.02 | 0.00 | |
| Azz (N) | 1.17 | 1.23 | 1.20 | 1.16 | |
| Axx (Hα) | 0.10 | 0.08 | 0.09 | 0.00 | |
| Ayy (Hα) | 0.00 | 0.00 | 0.07 | 0.01 | |
| Azz (Hα) | 0.14 | 0.20 | 0.09 | 0.11 | |
| Axx (Hβ) | 0.11 | 0.27 | 0.09 | 0.00 | |
| Ayy (Hβ) | 0.00 | 0.00 | 0.02 | 0.06 | |
| Azz (Hβ) | 0.09 | 0.00 | 0.09 | 0.05 | |
| Axx (Ho) | 0.09 | 0.00 | 0.06 | 0.00 | |
| Ayy (Ho) | 0.00 | 0.00 | 0.05 | 0.00 | |
| Azz (Ho) | 0.06 | 0.17 | 0.06 | 0.09 | |
| Axx (Hm) | 0.05 | 0.00 | 0.01 | 0.00 | |
| Ayy (Hm) | 0.00 | 0.00 | 0.02 | 0.02 | |
| Azz (Hm) | 0.03 | 0.14 | 0.01 | 0.05 | |
| Axx (Hp) | 0.07 | 0.00 | — | — | |
| Ayy (Hp) | 0.00 | 0.00 | — | — | |
| Azz (Hp) | 0.05 | 0.12 | — | — | |
- ∗1Ref. 18.
| States | giso | (A(N))iso | (A(Hα))iso | (A(Hβ))iso | (A(Ho))iso | (A(Hm))iso | (A(Hp))iso | |
|---|---|---|---|---|---|---|---|---|
| /mT | ||||||||
| 5 | In benzene solution ∗1 | 2.007 | 0.437 | 0.093 | 0.065 | 0.049 | 0.022 | 0.041 |
| The average of the principal axes’ components in PMMA ∗2 | 2.0075 | 0.438 | 0.081 | 0.066 | 0.052 | 0.029 | 0.041 | |
| The average of the principal axes’ components shown in Table 2 (this study) | 2.0081 | 0.467 | 0.092 | 0.090 | 0.058 | 0.047 | 0.040 | |
| 6 | In benzene solution ∗2 | 2.0068 | 0.432 | 0.078 | 0.064 | 0.057 | 0.010 | — |
| The average of the principal axes’ components in PMMA ∗2 | 2.0072 | 0.434 | 0.083 | 0.067 | 0.058 | 0.012 | — | |
| The average of the principal axes’ components shown in Table 2 (this study) | 2.0083 | 0.409 | 0.042 | 0.039 | 0.030 | 0.024 | — | |
| p-pyridinylBzNN ∗1 | 2.0072 | 0.426 | 0.091 | 0.073 | 0.041 | 0.020 | — | |
- ∗1Ref. 38; ∗2Ref. 18.
The estimation of (A(N))iso and (A(Hj))iso (j = α, β, o, m, and p) from the principal axis components of the A tensor components in ZnTPT differed from the isotropic values previously studied for 5 (or 6) in the solution-state or in PMMA (Table 3) [18, 38]. Those for 5 were relatively larger than the estimation based on the solution-state ESR spectra or the results in PMMA, whereas those for 6 were smaller. These could explain the difference in the resonant conditions between the solution- and solid-states. The other reason may be the plainer molecular shape of 4-Y-PhBzNN radicals (Figure 1(b)); the pyridyl or triazine rings of TPT ligands in the Zn matrix can potentially influence the unpaired electron density on the BzNN group or aryl moiety. The low (A(N))iso and (A(Hj))iso values for 6 may be associated with the electron-withdrawing NO2 group on the nitrophenyl ring of 6 as in the case of 3 (see Section 3.2).
3.4. ESR Spectra Recorded with 2-Z-IN Radicals Dispersed in the ZnTPT Matrix
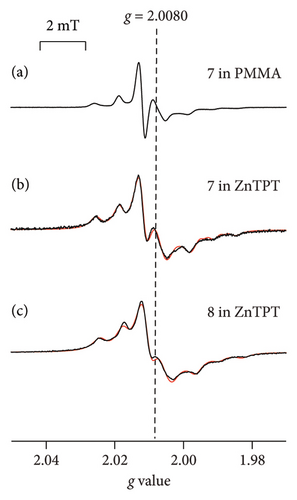
Figures 5(a)–5(c) show the ESR spectra recorded at room temperature for 2-Z-IN radicals (black), and the reproduced spectra (red); 7 (X = Ph; Figure 1(c)) dispersed in the PMMA matrix, [18] and 7 and 8 (X = p-NO2Ph; Figure 1(c)) in the ZnTPT matrix. Seven major hyperfine interaction lines associated with the inequivalent nitrogen atoms having unpaired electrons in the IN groups are shown in Figures 5(a)–5(c) [14, 18, 28]. We attempted to reproduce the spectra of 7 (or 8) using a rigid-limit or anisotropic rotational diffusion component. The sum of the squared differences between the data obtained from the experimental and simulated spectra for the reproduction of anisotropic rotational diffusion component in 7 (or 8) was estimated to be as quarter of that of the rigid-limit component. The spectra could be more accurately reproduced using the rotational diffusion component of the dispersed IN radical than with rigid-limit components. The results revealed that 7 (or 8) in the ZnTPT matrix underwent anisotropic rotational diffusion in the cylindrical or pseudocylindrical nanospaces of the ZnTPT matrix. The spectra recorded with 7 (or 8) in the ZnTPT matrix (Figures 5(b) and 5(c), respectively) were simulated using the g and A tensor components, as shown in Table 4. The value of gxx for 7 (or 8) in the ZnTPT matrix (also gzz for 7) was larger than those for 7 (or 8) in other organic matrices, whereas those for gyy were smaller. These may be responsible for the host-guest interaction between the Zn atoms or pyridyl or triazine rings of TPT ligands in the ZnTPT and the NO moieties present in the IN group of 7 (or 8).
| Tensor components | States | |||||
|---|---|---|---|---|---|---|
| 7 | 8 | |||||
| In glassy toluene ∗1 | In TPP ∗2 | In PMMA ∗3 | In ZnTPT (this study) | In PMMA ∗3 | In ZnTPT (this study) | |
| gxx | 2.0093 | 2.0103 | 2.0098 | 2.0134 | 2.0098 | 2.0121 |
| gyy | 2.0060 | 2.0050 | 2.0067 | 2.0048 | 2.0063 | 2.0045 |
| gzz | 2.0022 | 2.0028 | 2.0023 | 2.0046 | 2.0022 | 2.0030 |
| Axx (N1) | — | 0.02 | 0.33 | 0.53 | 0.29 | 0.49 |
| Ayy (N1) | — | 0.01 | 0.00 | 0.00 | 0.01 | 0.09 |
| Azz (N1) | 2.32 | 2.36 | 2.43 | 2.18 | 2.45 | 2.24 |
| Axx (N2) | — | 0.01 | 0.08 | 0.19 | 0.06 | 0.28 |
| Ayy (N2) | — | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 |
| Azz (N2) | 1.28 | 1.16 | 1.18 | 1.11 | 1.15 | 1.12 |
- ∗1Ref. 30; ∗2Ref. 28; ∗3Ref. 18.
The ESR spectra for 7 and 8 (Figures 5(b) and 5(c)) were simulated using a model that represents the anisotropic rotational diffusion of dispersed IN radicals (a slow-motion regime relative to the ESR time scale, i.e., 10−6 s > τR > 10−9 s). Rotational diffusion was assumed to occur around a tilted axis ((θ, ϕ) = (3°, 87°) for 7 and (θ, ϕ) = (12°, 87°) for 8) relative to the principal system of the g tensor with appropriate line-width components. The principal axes of the 2-Z-IN radicals are defined as follows: the z-axis is perpendicular to the molecular plane of the IN group, the y-axis is parallel to the bond between the 2-position carbon atom of the IN group and the phenyl ring, and the x-axis is perpendicular to the yz plane [28]. According to this definition, the rotational diffusion of 7 (or 8) dispersed in ZnTPT is expected to be approximately around the z-axis of the principal axes, that is, perpendicular to the IN plane. The θ and ϕ values for 7 (or 8) in the ZnTPT matrix were completely different from those for 7 (or 8) in organic 1D nanochannels of TPP [28] (caution: TPP is not to be confused with TPT) or in an acrylic resin, such as PMMA (Figure 5(a)) [18]. Such examples suggested rotational diffusion around the y-axis of the principal axis, which is parallel to the molecular long axis of 2-Z-IN radicals [14, 18, 28]. These results indicate the rarity of 2-Z-IN radicals exhibiting rotational diffusion around the principal z-axis. 2-Z-IN radicals could be used as spin probes if rotational diffusion can be performed not only around the y-axis of the principal axes of the g tensor (for example, as a spin probe for a nanochannel or nanosized cylindrical space from the viewpoint of the radical shape of 7 (or 8)) but also around the z-axis (for example, as a spin probe for a nanosized flat cylinder or 2D sheet-type pores), depending on the sizes and shapes of the nanospaces of host materials and the host-guest interactions between host materials and guest radicals. From the molecular-size perspective for 7 (or 8), the size of the nanospace in which 7 (or 8) is included is a flat cylinder or cylinder with a diameter of 1.0 nm and a height of 0.6 nm at the smallest estimate. In ZnTPT, 7 (or 8) is expected to be accommodated in flattened-cylindrical or pseudocylindrical nanospaces sandwiched between the pyridinyl or triazine rings of the TPT ligands of ZnTPT. A few examples have been reported for the case in which cyclohexane was used as a solvent and coincluded with other molecules in the nanospace of the ZnTPT matrix (the inclusion amount of cyclohexane per formula unit of ZnTPT complex was 1.0–2.5) [24, 41, 42]. In a previous report, the included cyclohexane molecules were accommodated in relatively large nanospaces in ZnTPT crystals, whereas coincluded plainer guest molecules, such as perylene, were stacked on the TPT ligands [42]. The nanospace in the ZnTPT matrix studied in this study by 7 (or 8) may be consistent with the space over the TPT ligands in previous studies rather than the large space in which cyclohexane was included.
The τR values corresponding to 7 and 8 dispersed in ZnTPT were estimated to be 1.1 × 10−7 s for 7 and 1.2 × 10−7 s for 8. The molecular dynamics of 7 and 8 in ZnTPT were similar and were barely influenced by the substituent at the 4-position of the phenyl group present in the radicals. These τR values were comparable to those recorded for 7 or 8 in PMMA [18] and were 102 times higher than those for TPP organic 1D nanochannels at room temperature [28]. These findings can be attributed to the strong host-guest interaction between ZnTPT and guest radicals.
The ESR spectra of 7 (or 8) dispersed in ZnTPT indicate that the nanospaces in the ZnTPT matrix can be studied using 7 (or 8) as the ESR spin probe. Thus, 2-Z-IN radicals can be used as spin probe molecules for ZnTPT complexes as well as polymers such as PMMA [18], and probably for other MOFs with nanosized pores. In particular, the ESR spin probe technique can be used when the preparation of single crystals of MOFs is difficult or when the pore structure of MOFs is complex. Apparently, the precision for the estimation of pore size in the ESR spin probe technique is lower than the single-crystal XRD analyses due to the indirect measurement method. Therefore, the ESR spin probe technique for MOFs should be used as a complementary approach to the single-crystal XRD. Suitable organic radicals, such as 2-X-NN and 4-Y-PhBzNN, can be selected as probes depending on the pore structure, size, and the host-guest interaction of the MOF if the molecular dynamics of guest radicals are performed with increasing temperature without destroying the pores of the host MOF. However, the g, A, and superhyperfine tensor components for the 2-X-NN, 4-Y-PhBzNN, and 2-Z-IN radicals often differ from those of the organic matrices. When non-TEMPO radicals are used as ESR spin probes for the pores of MOF, the host-guest interactions between the host MOF and guest radicals should always be considered.
4. Conclusion
The nanospaces in the ZnTPT matrix were examined by ESR spectroscopy. Some of the g, A, and superhyperfine tensor components for the 2-X-NN, 4-Y-PhBzNN, and 2-Z-IN radicals in the ZnTPT matrix were shifted when compared with the estimations for organic matrices. When using non-TEMPO radicals as ESR spin probes for porous MOF, the interactions between the host materials and guest radicals should be considered. The spectra corresponding to 2-Z-IN were reproduced using uniaxial rotational diffusion components around the principal gzz axis. The ESR spectra and simulation results for the 2-Z-IN radicals indicate that ZnTPT had flattened-cylindrical or pseudocylindrical nanospaces. These results demonstrate that 2-Z-IN radicals could potentially be used as spin probes to study the characteristics of the nanospace of the ZnTPT matrix. The ESR spin probe technique using 2-Z-IN radicals can be used while considering the host-guest interactions between the host MOF and guest radicals in cases when the preparation of single crystals of MOFs is difficult or when the pore structure of the MOF is complex even if they consist of metal ions other than Zn(II). However, it is to be noted that the magnetic properties of the metal ions other than Zn(II) may affect the ESR signal. The signal of the molecular motion in 2-X-NN and 4-Y-PhBzNN (dispersed in ZnTPT) could not be determined using ESR spectroscopy. However, they can be used as spin probes for other MOFs if the structures, sizes, and shapes of the nanospaces in the MOF matrices are appropriate.
These findings show that the ESR spin probe technique can be used to investigate the chemical and biological structures of nanosized materials using samples containing non-TEMPO derivatives. Nanosized spaces containing imino or nitronyl nitroxide groups have only been reported in recent years. In particular, paramagnetic vesicles, nanoparticles, and porous films with NN radicals can be used in molecule-based magnetism, magnetic resonance imaging (MRI) contrast agents, biosensing, and drug delivery [31]. In many cases, the preparation of single crystals of these materials is difficult. Although the precision of the estimation of the pore size is always discussed, the results of this study can be used to develop novel systems. Our research group is currently conducting additional investigations on this topic, with the goal of expanding this field even further.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This work was partially supported by the Strategic Research Base Development Program for Private Universities of the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT), 2009–2013. We would like to thank Prof. S. Stoll, working at the University of Washington, for helping with the EasySpin calculations. We would like to thank Editage (https://www.editage.com/) for the English language editing.
Open Research
Data Availability
No underlying data were used to support the results of this study.




