Macromolecular Poly(N-isopropylacrylamide) (PNIPAM) in Cancer Treatment and Beyond
Applications in Drug Delivery, Photothermal Therapy, Gene Delivery and Biomedical Imaging
Abstract
Poly(N-isopropylacrylamide) (PNIPAM) is a versatile polymer known for its phase transition properties, exhibiting a lower critical solution temperature (LCST) of approximately 32°C. Below this temperature, PNIPAM is hydrophilic, while above it, the polymer becomes hydrophobic, making it ideal for thermosensitive drug delivery systems (DDSs). In tissue engineering, PNIPAM provides a biocompatible, nontoxic and stimuli-responsive surface for cell culture. Its nontoxic nature ensures safety in medical applications. PNIPAM enhances biosensing diagnostics through its affinity for biomolecules, improving accuracy. Widely used in hydrogels, smart textiles, soft robotics and various medical applications, PNIPAM adapts to environmental changes. Its straightforward synthesis allows for the creation of diverse copolymers and composites, applicable in selective reactions and conjugations with fluorescent tags or chemical modifications. PNIPAM’s versatility extends to pH-responsive alternatives, broadening its application spectrum. Practical examples include phase separation in water treatment and cleaning processes. This discussion explores PNIPAM’s biomedical and drug delivery applications, particularly in cancer treatment, photothermal therapy (PTT) and photodynamic therapy (PDT), gene delivery and medical imaging. Additionally, it highlights PNIPAM’s noncancerous applications, such as small interfering RNA (siRNA) targeting of oncogenes and detailed imaging of deep and tumour tissues.
Summary
- •
Poly(N-isopropylacrylamide) (PNIPAM) exhibits phase transition properties ideal for drug delivery.
- •
PNIPAM’s biocompatibility ensures safety in medical applications.
- •
PNIPAM has versatile applications in hydrogels, textiles and medical fields.
- •
PNIPAM enhances biosensing diagnostics with improved accuracy.
- •
PNIPAM has potential in photothermal therapy (PTT), gene delivery and imaging.
1. Introduction
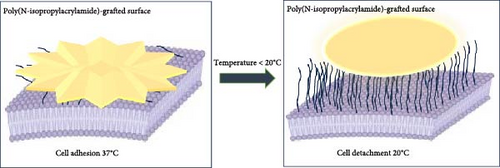
Poly(N-isopropylacrylamide) (PNIPAM) is a polymer known for its unique properties, particularly its temperature-sensitive behaviour [1, 2]. This amphiphilic nonionic surfactant has a low critical solution temperature (LCST) of around 32°C, just above room temperature. Below this temperature, PNIPAM is hydrophilic and dissolves in water. However, when the temperature rises above LCST, it becomes hydrophobic and water-insoluble, causing the polymer to collapse and precipitate out of solution. This property makes PNIPAM ideal for designing thermosensitive drug delivery systems (DDSs), where the polymer can release drugs at specific sites within the body in response to temperature changes [3–5]. PNIPAM is also valuable in tissue engineering due to its temperature-responsive properties, making it an ideal substrate for cell culture. As the temperature decreases, PNIPAM becomes more hydrophilic, allowing cells to easily adhere and grow on its surface. This temperature sensitivity allows for controlled cell detachment by simply lowering the temperature, offering a gentle and efficient method for harvesting cells without the need for enzymes or chemicals [6, 7]. Biocompatibility is a key feature of PNIPAM, making it suitable for a wide range of applications in medical and biological fields. Its nontoxic nature ensures that it does not cause any harmful effects when used in biomedical products for the human body. This lack of toxicity, combined with its other beneficial properties, makes PNIPAM an ideal material for use in areas like drug delivery, tissue engineering and other medical applications where safety is crucial [8, 9]. PNIPAM is highly useful in biosensors due to its ability to recognize and bind biomolecules with specificity, leading to accurate and reliable diagnostic and monitoring results. Its temperature-sensitive nature allows for controlled interaction with target molecules, which enhances the precision of biosensing applications. This property, coupled with its biocompatibility, makes PNIPAM an excellent material for creating biosensors that can provide secure and dependable results in medical diagnostics and monitoring [10]. Some of the uses of PNIPAM include its blending with other components in fabrication of hydrogel coatings [11] and smart textiles [12]. Smart hydrogels based on PNIPAM are used for various purposes, including in soft robotics [13] and as wound dressings because of its ability to alter the response to environmental changes [14, 15]. Moreover, PNIPAM is synthesized conveniently and is versatile; hence, tailored copolymers and composites can be developed depending on a particular application. Still contemplating the fact that copolymerization of PNIPAM with other monomers is allowed, the researchers are likely to extend its uses and improve its performance [16]. Covalent modification of PNIPAM by introducing various chemical groups can enhance its functionality for specific applications, such as conjugation with fluorescent markers or specific binding agents makes PNIPAM more versatile and effective in specialized roles. Additionally, while PNIPAM is well-known for its temperature responsiveness, it can also react to other stimuli like pH changes, further broadening its range of applications in areas such as drug delivery, biosensing and targeted therapies. These modifications allow PNIPAM to be fine-tuned for particular tasks, making it a highly adaptable material in biomedical and technological fields [17]. The current applications of PNIPAM in various fields demonstrate its potential and practicality for use in complex environments. Its ability to respond to multiple stimuli, such as temperature and pH, combined with its biocompatibility and adaptability through chemical modifications, makes PNIPAM a versatile material. These qualities suggest that PNIPAM can be effectively employed in intricate settings, such as in advanced biomedical applications, environmental sensing and smart material design, where precise and responsive behaviour is crucial [18–20]. Another notable application of PNIPAM is in everyday tasks, such as water treatment and washing. PNIPAM can be used for temperature-controlled phase separation, effectively removing dirt and other unwanted substances from water. When the temperature is adjusted, PNIPAM undergoes a phase change that can help in capturing and separating contaminants, making it a valuable tool for cleaning and purification processes in domestic and industrial settings. This temperature-sensitive behaviour adds a practical dimension to PNIPAM’s use in routine activities, enhancing the efficiency of cleaning and water treatment operations [21, 22].
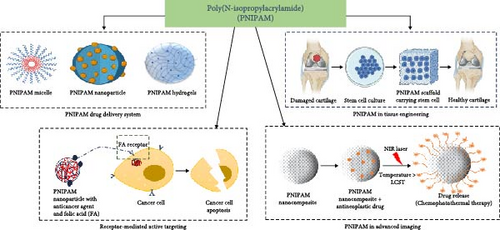
This review aims to provide a comprehensive overview of the various applications of PNIPAM, making it a valuable resource for scientists, medical practitioners and engineers. By examining the latest advancements and challenges, the review evaluates the potential of PNIPAM and its future applications based on its effectiveness and limitations. Additionally, it identifies gaps in the current research and suggests areas for further investigation, especially in the development of personalized treatment plans. The review emphasizes how PNIPAM-based technologies could enhance targeted drug delivery, diagnostics, cancer research and tissue engineering, ultimately improving patient outcomes through more precise and efficient therapies (Figure 1).
2. PNIPAM: A Versatile Smart Material
2.1. Chemical Structure and Properties
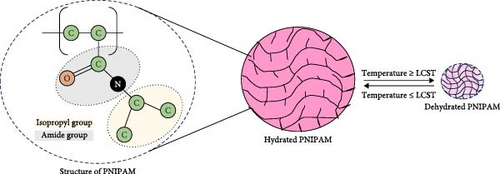
PNIPAM is a thermoresponsive polymer distinguished as an intelligent material with a chemical formula (C6H11NO)n. It exhibits negative thermosensitive properties and can form sol–gel transformation above LCST. PNIPAM shows amphiphilic properties having both hydrophilic and hydrophobic groups: amide (–CONH–) and isopropyl (–CH(CH3)2) [23].
Below the lower consolute temperature, the system demonstrates complete miscibility, while partial liquid miscibility occurs above it. The degree of polymerization, branching in polymer solution, may alter the LCST. PNIPAM exhibits LCST close to human body temperature which is about 32°C in aqueous media. Below this temperature, it will again be soluble [24]. The amide groups of PNIPAM polymer chains have a hydrophilic nature that allows them to interact with water molecules favourably, resulting in their dissolution in water [25]. PNIPAM is soluble in aqueous solutions as below its LCST, it is hydrophilic and has strong hydrogen bond between the amide groups and the water molecules. Near the LCST, these bonds are much weakened [26], while hydrophobic interactions between chains of PNIPAM predominate which results in the exclusion of water and its decreased solubility. Above the LCST, PNIPAM becomes highly hydrophobic in its nature derived from its molecular configuration [27]. This interaction balances out with the hydrophobic interaction from isopropyl groups, leading to a negative Gibbs free energy (ΔGmix < 0). The negative enthalpy change of mixing (ΔHmix < 0) mainly contributes to this energy. The amide groups are surrounded by water molecules, which lowers the entropy (ΔSmix < 0) [28]. Hydrophobic contacts strengthen when the temperature rises beyond LCST, weakening the entropy term. This phase separation is indicated by a positive Gibbs free energy (ΔGmix > 0) when the enthalpy change magnitude is less than T times the entropy change. During this phase, PNIPAM chains dehydrate and congregate into densely packed globular conformations [29]. Studies have demonstrated that PNIPAM polymers, specifically the 15-mer chain length, are effective for drug delivery due to their stability and temperature responsiveness [30]. The reversible temperature-dependent behaviour of PNIPAM and its use in cell culture are clearly shown in Figure 2. Below its LCST, PNIPAM expands and becomes hydrophilic; above it, it becomes hydrophobic and contracts. This characteristic makes it possible to adjust the temperature at which cells adhere to and separate from PNIPAM-grafted surfaces, opening the door to noninvasive, straightforward cell-harvesting methods.
2.2. Chemical Composition Identification
PNIPAM-containing materials are intricate and necessitate advanced techniques for their analysis. Fourier transform infrared spectroscopy (FTIR) holds significance in identifying the functional groups that are present in PNIPAM by detecting characteristic absorption peaks at specific wavenumbers, such as 1650 and 1550 cm−1 for amide I and II. On the other hand, nuclear magnetic resonance (NMR) provides detailed information about the molecular structure of PNIPAM-based compounds, including chemical shifts, peak shapes, integral areas and coupling constants. The strong signal detected at approximately 1.15 ppm in the NMR spectrum provides evidence of the presence of isopropyl methyl groups (–CH₃) in the PNIPAM chains [31].
2.3. Effect of Surfactant on PNIPAM
Surfactants can alter the responsive properties of PNIPAM. Anionic surfactants containing sulphate heads like sodium dodecyl sulphate (SDS) can raise the LCST and suppress the thermoresponse of PNIPAM by interacting with its isopropyl group. The strength of the surfactant–polymer connection depends on the charge of the surfactant’s head group. Micelle tails can replace the water environment surrounding the surfactant’s hydrophobic moieties and suppress the thermoresponse of PNIPAM [32].
2.4. Effect of Molecular Weight
PNIPAM with molecular weight over 50 kDa, the effect of molecular weight and polydispersity on LCST is minimal. Increasing the PNIPAM content in water up to 40 wt% only slightly raises the LCST, with variations typically around 3–3.5°C, ranging between 28.5 and 32°C. Copolymerization with other comonomers is necessary to further adjust the LCST [1]. Higher molecular weights of PNIPAM result in a relatively stable LCST, while lower molecular weights cause more significant changes in solubility with temperature fluctuations [33]. Higher molecular weights also lead to different relaxation dynamics and stronger interactions at surfaces, impacting the polymer’s behaviour in solutions and interfaces [34]. In contrast, at lower molecular weights, PNIPAM shows a greater tendency for interchain aggregation, especially at higher temperatures, which can affect solubility and stability, particularly in biological applications [35].
2.5. Effect of Water/Organic Solvent Mixtures
In mixtures of water and organic solvents, LCST is affected by type and volume fraction of the cosolvent present [36]. The decrease in LCST is observed as the amount of the organic solvent is increased but there is a specific volume at which the LCST increases instead. At lower volume ratios, the hydration of PNIPAM is reduced due to the competition between PNIPAM chains and cosolvent molecules for water molecules. This results in the emergence of a particular phenomenon. Furthermore, cosolvent polarity is vital, as less polar solvents result in a lower volume fraction at which the LCST starts to increase [1].
2.6. Effects of Cross-Linking Density
Cross-linked hydrogels composed of PNIPAM do not dissolve in solvents but can still absorb a considerable amount of solvent. On the other hand, non-cross-linked PNIPAM can dissolve in water, while cross-linked PNIPAM cannot be dissolved, although it still retains the ability to absorb water [37].
2.7. Effect of Salt
The performance of PNIPAM hydrogels that are cross-linked can be affected by various solvents and aqueous solutions. In aqueous solutions, the LCST of PNIPAM can be influenced by anions and cations, with kosmotropic anions showing a strong kosmotropic effect and encouraging dehydration of PNIPAM) [38], following the Hofmeister series: CO32− > SO42− > S2O32− > H2PO4− > F− > Cl− > Br− ≈ NO3− > I− > ClO4− > SCN−, arranged in decreasing order of kosmotropicity. The way anions affect the separation of single-walled carbon nanotubes (SWCNTs) by PNIPAM depends on their kosmotropic properties. The sorting behaviour of SWCNTs is impacted by the micellar environment surrounding them, which is influenced by the concentration of surfactant. Finally, it has been observed that non-cross-linked PNIPAM has the ability to dissolve in water, while cross-linked PNIPAM is unable to dissolve but can still absorb water [39]. Table 1 shows the overview of various synthesis methods of PNIPAM including free radical polymerization, radical precipitation, copolymerization and reversible addition–fragmentation chain transfer (RAFT) polymerization.
| Synthesis method | Description | Key features | Reference |
|---|---|---|---|
| Free radical polymerization | Involves radical initiators like AIBN and potassium peroxydisulphate |
|
[10] |
| Radical precipitation polymerization | Polymerization takes place in a nonsolvent environment in which microgel formation can be achieved through homogeneous nucleation | Polymerization process is normally found to take place at a temperature range of 60–70°C | [40] |
| Two-step freezing polymerization | A two-cycle procedure that involves subjecting the polymerization to freezing at 20°C followed by thawing at 4°C to increase synthesis rate | Can synthesize PNIPAM hydrogels in as little as 2 h, thus reducing production time. The possibility of creating high-performance hydrogels with enhanced biomorphic compatibility and mechanical properties enables both chemical and physical cross-linking | [41] |
| Copolymerization | The synthesis method includes copolymerization with other monomers which makes it possible to change the properties and functions thereof |
|
[42] |
| Reversible addition–fragmentation chain transfer (RAFT) polymerization |
|
Predictable molecular weight, high end-group integrity | [43] |
| Microwave-assisted polymerization | Use microwave irradiation to accelerate polymerization. It reduces reaction time and generate high production yield | May result to faster response time and possibly higher returns. Retains a strong control over characteristics of polymer. Minimize generation of unwanted by-product | [44] |
- Abbreviations: AIBN, azobisisobutyronitrile; LCST, lower critical solution temperature; PNIPAM, poly(N-isopropylacrylamide).
3. PNIPAM-Based DDSs
3.1. PNIPAM Nanoparticles (NPs)
PNIPAM has been exploited to prepare thermo-sensitive NPs for applications. They are prepared using various methods like emulsion polymerization [45], photoemulsion polymerization [46], atom transfer radical polymerization (ATRP) [47], free radical polymerization [47] and in situ chemical cross-linking [48]. Paclitaxel (PTX) and other low molecular weight (LMW) drugs can be encapsulated and liberate from these NPs with ease. The release characteristics are governed by temperature, especially in the area of the LCST. Sizes and shapes of the hydrophobic monomers determine assembly of NPs and drug release where variable compositions from the hydrophobic monomers alter swelling profiles that impact on PTX release [49]. In addition, it is suggested to adjust the NPs with polyethylene glycol (PEG) to enhance their stability as well as for utilizing in the cellular study as hydrophilic shell of PEG acts as a steric barrier and preventing aggregation [49]. Tyrosine, alanine, arginine and alanine (YARA) peptide and piplartine are encapsulated in PNIPAM, a temperature-responsive nanocarrier, as the medications are directly administered to the site of infection. Consequently, the peptide SILY that has an affinity to collagen that is abundant in breast tumour is linked to these NPs with an aim of targeting them towards the tumour site. NPs can be internalized into cells easily, and they are small to plug the ducts showing high effectiveness ex vivo for cancer cells and even more when combined with both drugs. Further, it was established that peptidyl PTX NPs, decorated with the peptide SILY and encapsulated in nanostructured lipid carriers (NLC), present a prospect for selectivity of killing malignant breast cancer cells without significant toxicity on normal cells [50, 51]. The work under consideration concerns the preparation of ultra-small iron oxide (Fe3O4) NPs with PNIPAM coating through surface-initiated ATRP. These NPs possess extremely small size, a high drug-to-mass ratio and thermally sensitive drug release mechanism proportional to the solution pH. Because of their superparamagnetic properties, they possess the ability to be used as contrast agents in magnetic resonance imaging (MRI) because they have the possibility of both therapeutic and diagnostic uses [52]. PNIPAM-coated Fe3O4 NPs with the assistance of chitosan (CS) for drug delivery purposes have been considered. These stimuli-responsive NPs when loaded with vincristine sulphate, an anticancer drug which comes under chemotherapy, show controlled release characteristic based on the LCST under collapsed state. Notably, the release mechanisms are different at different temperatures, suggesting that there may be temperature-triggered drug release [53]. Design of temperature-sensitive PNIPAM-grafted–poly(glycidyl methacrylate) (PGMA)-coated magnetite core/shell NPs (PNIPAM–PGMA NPs) solves the problem of colloidal instability detected in the case of PNIPAM-coated NPs. These NPs remain as colloids which are stable and make it possible to directly assess the change in magnetic resonance properties due to phase transition of PNIPAM without the confounding effect of NP aggregation. This innovation enables probe for stimuli responsiveness of drug delivery and therapy imaging in biomedical research and therapy [54]. Also, studies have shown that magnetic NPs coated with span a PNIPAM polymer have advised to release chemotherapy drugs. Thus, upon application of an alternating magnetic field, these NPs can be heated and cause release of the carried drug. This case of eradicating the tumour through the help of NPs which is combined with chemotherapy and hyperthermia has been proven to reduce the count of the tumour cells to half within a space of a day [55]. Magnetic microspheres were obtained by immobilizing the core–shell NPs on the surface of Fe3O4@SiO2 magnetic microspheres using cross-linked PNIPAM-co-acrylic acid (PNIPAM-co-AA) NPs. The obtained microspheres are superparamagnetic and sensitive to the changes of temperature and pH. They hold some promises for use in drug shipment, biosorting and catalysis. The study found a way to improve their polymerization conditions, sensitivity to the environment and magnetic properties [56]. PNIPAM NPs containing acetaminophen were produced via photopolymerization, a method known for its environmental benefits. These NPs were later integrated into gelatine hydrogels using response surface methodology. The hydrogels displayed reduced swelling at temperatures past the phase transition, exhibited antibacterial effects against both gram-positive and gram-negative bacteria and demonstrated controlled drug release [8]. Researchers developed tiny NPs coated with PNIPAM, loaded with the chemotherapy drug etoposide and designed to target and treat metastatic prostate cancer. The NPs were small (57 nm) and released the drug slowly over time in cancerous environments. In lab experiments, the NP-loaded etoposide was significantly better at killing metastatic prostate cancer cells than free etoposide. The NPs induced apoptotic activities more especially in the most refractorily of the cancer cells; this was well evidenced by the increase in apoptosis in the overly aggressive PC-3 cells [57]. The release of the drug erlotinib (ERL) from the superparamagnetic iron oxide NPs (SPIONs) occurs when exposed to pH values that are observed within the cancer cells as they have a lower pH than the normal tissues. PNIPAM is a pH-sensitive core within the NPs which helps in retaining the drug within the NPs under physiological pH and release the drug at the lower pH under tumour condition. This mechanism of action improves drug specificity for cancer cells, improves internalization within the cells and enhances the induction of cancer cell death when activated [58].
3.2. PNIPAM Hydrogels
Hydrogels possess enhanced swelling characteristics when exposed to a number of influences. The hydrogels that are prepared through physical cross-linking are capable of reversible gelation as well as used in drug delivery and scaffolding of tissues. Chemical cross-linking also involves cross-linking of polymer chains by covalent bonds to provide enhanced stability to the hydrogel structure [59]. Some of the common responses are radical polymerization in which one can use a photoinitiator as well as chemical initiator [60]. The ability to initiate the polymerization process of the hydrogel at specific locations is known as photoinitiated polymerization and is used often in the fabrication of hydrogels with intricate structures or shapes, for example, in tissue engineering. Apart from that, for applications needing high mechanical strength and stability like use in regenerative medicine scaffold, bulk polymerization method can be used to synthesize hydrogels [61]. Microgels are cross-linked polymer structures of colloidal dimensions which typically have dimension of size between 100 nm and several metres and micrometres. As a subtype of microgels, nanogels are generally softer in nature and possess three-dimensional (3D) cross-linked polymeric structures which are usually in the nanometre range. For template-assisted methods, it is also possible to produce nanogel with desired size and shape by using templates for its formations. This approach has a significant effect of ensuring some form of standardization as well as replicability in the fabrication process [60]. A PNIPAM hydrogel is experiencing steady progression in numerous fields but especially in the DDS field [62]. These hydrogels are 3D, water-loving, cross-linked with the capacity of accommodating much water and, with controlled biocompatibility, biodegradability and acute environmental sensitivity [63]. The synthesis of PNIPAM-based microgels is revealing a promising tendency while using the method of the surfactant-free precipitation polymerization in the rotating packed bed (RPB) reactor, which works at high gravity and enhance mass transfer as well as accelerate chemical reactions. This method offers controlled size modulation by adjusting cross-linker and initiator quantities, yielding higher outputs within a shorter reaction time compared to conventional stirred tank reactors. In vitro studies demonstrate PNIPAM-based microgels efficacy in sustained drug release, particularly in doxorubicin (DOX)-loaded formulations [64]. In another innovative approach, PNIPAM was attached to cellulose nanofibers (CNFs) through silver-catalysed decarboxylative radical polymerization, resulting in PNIPAM-graft-CNFs (PNIPAM-g-CNFs). These hydrogels respond to temperature changes and have diverse applications, particularly in designing hydrogels suitable for biomedical and functional applications [65]. Lactoferrin (LF)-PNIPAM-co-AA is designed for targeted breast cancer therapy. These nanohydrogels can respond to changes in pH and temperature, making them highly effective in delivering the anticancer drug Honokiol (HK) to tumour sites. They boast a high drug loading capacity and have demonstrated potent anticancer effects in both laboratory and animal tests [66]. Thermoresponsive hydrogels with controlled hydrophilicity and conductivity can be created by adding carboxyl-functionalized multiwalled CNTs (MWCNTs-COOH) to a PNIPAM matrix. This incorporation method has proven to be effective and efficient [67]. However, PNIPAM hydrogels often exhibit poor mechanical properties due to their low polymer density in the swollen state. To address this issue, another polymer is incorporated into the hydrogel matrix, thereby increasing the total polymer density [25]. Nanocellulose (NC) materials are a good solution due to their exceptional properties, such as transparency at high clay content and uniform structure. NC gels have a low modulus and high stretchability yet high tensile strength comparable to industrial rubber, making them soft, flexible and challenging [68]. A new platform for generating and isolating cancer spheroids using a hydrogel microwell array has also been introduced. This platform, which is an alternative to expensive and difficult 3D cell culture methods, is thermoresponsive PNIPAM-based, allowing for spheroid growth at 37°C and easy isolation at room temperature. The researchers have conducted a drug response study using DOX hydrochloride, which showed anticancer efficiency in MG-63 cell spheroids that was dependent on concentration and time. The PNIPAM-based hydrogel microwell array used in this study is a novel model with potential to improving the application of 3D cell culture and in vitro drug screening and disease modelling [69]. Recently, the elaborate organization of PNIPAM–DNA-based hydrogels by cryostructuring resembling organism respiration systems has been synthesized. They have functional DNA-tethered PNIPAM networks and macroporous channels. These hydrogels potentiality could undergo a fast and reversible shrinking/swelling cycles, hence facilitating the movement of the substance [70]. One of the innovations in the field of responsive materials can be considered the development of conductive hydrogel that is photoresponsive and anisotropic known as PMD hydrogel. For fabrication of this hydrogel, there was applied directional freezing, and this method was improved by MXene-based PNIPAM. The features of the PMD hydrogel include fast response time and high driving strength [71]. This has resulted in a bilayer hydrogel and an anisotropic structure of PNIPAM hydrogels with an augmentation of a polyvinyl alcohol (PVA) polymer film. Few hydrogen bond interactions exist between PNIPAM and an active layer, and the formation of a semi-interpenetrating network structure at their interface is observed. When PVA is incorporated within the hydrogel material, the tensile stress is found to be enhanced from 23.6 to 62.6 kPa; this is substantially higher, which directly suggests an increase in mechanical robustness [72]. Growing polyaniline (PANI) NPs creates the PNIPAM/PANI hydrogel within a Pluronic F127 diacrylate (F127DA) cross-linked PNIPAM hydrogel matrix in situ. The hydrogel matrix exhibits extraordinary properties, including high stretchability (730%) and rapid deswelling upon heating. With the efficient photothermal conversion of embedded PANI, the hydrogel can achieve a substantial temperature increase of approximately 50°C within 150 s and a significant volume reduction of 65% upon near-infrared (NIR) light irradiation. The hydrogel’s unique combination of properties allows it to function as an underwater actuator capable of programmable deformations, such as hand-like gestures and Venus flytrap–like locomotion under local NIR irradiation [73]. The viscoelastic properties of hydrogels made of PNIPAM cross-linked with glutaraldehyde were investigated by researchers through small-amplitude oscillatory and steady shear experiments, with a focus on dependencies of frequency and shear rate. The researchers observed that the monomer-to-glutaraldehyde cross-linker ratio strongly influenced the properties. The rheological properties of the hydrogels were tuned by leveraging the thermosensitivity of PNIPAM, using temperature changes as an external stimulus. The experimental viscosities and linear viscoelastic moduli were analysed using a schematic mode-coupling ansatz, which integrated a rescaled F12 model for interpretation [74]. A hybrid hydrogel was created by combining PNIPAM and acrylolsobutyl polyhedral oligomeric silsesquioxane (MAPOSS) through radical polymerization. The hydrogel was synthesized with the addition of PEG to act as a pore-forming agent and help achieve the desired mechanical properties and swelling behaviour. The hydrogel containing 8.33 wt% MAPOSS exhibited stable and sustained drug release, making it a promising candidate for drug delivery applications and as a carrier of 5-fluorouracil (5-FU) [75]. The study focuses on DDSs based on PNIPAM microgels. It uses molecular dynamic simulations and theoretical models to investigate the behaviour of different molecules within water–PNIPAM mixtures at different polymer volume fractions. The study shows that phenol and 5-FU have a strong affinity for the PNIPAM surface, irrespective of temperature relative to PNIPAM’s LCST. However, a temperature-dependent switch in the ΔG trans sign for methane underscores the complexity of interactions within these mixtures [76]. Adding CS enhanced the tissue adhesion of PNIPAM-based hydrogel dressings, composed of NIPAM, sodium alginate and graphene oxide (P/SA/GO) [77]. A hydrogel was created by combining a copolymer of PNIPAM-co-acrylamide (PNIPAM-AM) with oxidized succinoglycan (OSG). The incorporation of OSG into PNIPAM-AM networks led to hydrogels that exhibited improved thermal stability, increased elasticity, newfound self-healing capability and a fourfold increase in tensile strength when compared to PNIPAM-AM hydrogels alone. Furthermore, OSG/PNIPAM-AM hydrogels loaded with 5-FU demonstrated effective temperature-/pH-responsive drug release. The cytotoxicity experiments confirmed that OSG/PNIPAM-AM hydrogels were not toxic. These hydrogels displayed differential swelling behaviour with temperature variations and exhibited greater pore size and swelling ratio at higher OSG concentrations than PNIPAM-AM gels [78].
3.3. PNIPAM Micelles
Micelles are structures which are formed when the amphiphilic molecules, for instance, surfactants or block copolymers, aggregate in aqueous media. They possess a lipophilic core and a hydrophilic shell and can encapsulate and deliver hydrophobic drugs and improve their bioavailability [79]. Polymeric micelles, which incorporate PNIPAM, have emerged as a versatile drug delivery platform, demonstrating enhanced stability, bioavailability and controlled release of therapeutic agents. These micelles are monodispersed and thermoresponsive and can encapsulate number of drugs like flavonoids, for instance, icariin (ICA) [80], DOX [81] and PTX [82]. If ICA is encapsulated with micelles of PEG–poly(L-lactic acid) (PLLA) and poly(d-lactide) (PDLA)–PNIPAM, it leads to the slow release of ICA and enhanced bioavailability of ICA. Similarly, it has been proved that therapeutic efficacy of DOX integrated with micelles is better than the free DOX [81]. Similar to previous reports in cancer therapy, DOX–gold nanorods (GNRs)–PNIPAM@PEG–polylactic acid (PLA) micelles named DAPP were found to have elevated cell toxicity against melanoma cells and better tumour-suppressing ability on exposure to NIR light [83]. The new material PNIPAM-g-Cell is a cellulose graft copolymer synthesized via photoinduced metal-free ATRP. Above 5% in water, this copolymer creates a thermoresponsive hydrogel containing micelles. They have good biocompatibility based on the body and can maintain the DOX release of not less than 10 days [84]. A newly developed injectable hydrogel to be adapted particularly in matrix metalloproteinase (MMP)–rich conditions like in cancer tissues known as HyMic has also promised intracellular drug delivery [85]. Creating a PEGylated macroiniferter by combining methylene diphenyl diisocyanate (MDI), tetra-p-phenylethylene derivative (TPED) and polyethylene oxide (PEO) through polycondensation has also been reported. The macroiniferter reacts quickly with acrylamides to form ABA block copolymers responding to temperature changes which acts as a drug carrier and facilitates the controlled release of hydrophobic drugs and ideal for biomedical applications. The process is eco-friendly as compared to other polymerization techniques [86]. Moreover, the temperature-dependent phase behaviour of mixtures of PNIPAM microgel colloids and a triblock copolymer surfactant (PEO–polypropylene oxide [PPO]–PEO) allows for the formation of different structures depending on the temperature history of the sample [87]. Amphiphilic diblock copolymers such as PChM-PNIPAM and poly(2-methacryloyloxyethyl thiocticcarboxylate)-block-PNIPAM (PMAOETC-b-PNIPAM) have shown promise in drug delivery applications. These copolymers self-assemble into micelles driven by hydrophobic interactions, forming a hydrophilic shell below the LCST [88]. Thermoresponsive diblock copolymers have also shown promise in palladium-catalysed coupling reactions in water. With micelle formation at 50°C and dissolution at room temperature, these copolymers enable efficient Mizoroki–Heck reactions in water, achieving high turnover numbers (TON) with specific palladium complexes [89]. Cholesterol end-capped PNIPAM self-assembles into micelles and exhibits a molecular weight–dependent phase transition temperature in water. They have demonstrated toxicity against glioblastoma cells, with the cholesteryl moiety acting as a cell-penetrating agent [90]. Multifunctional micelles, poly(N,N-dimethyl-N′-undecylurea) and carboxymethyl dextran-coated magnetite (Fe3O4) NPs (PNDU)/carboxymethyl dextran-coated Fe3O4 NPs (CM-Dex Fe3O4), exhibit pH-dependent temperature response and magnetic properties. These micelles were synthesized by grafting hydrophilic CM-Dex Fe3O4 onto PNDU. Hesperetin, an anti-inflammatory drug, was encapsulated within these micelles using membrane dialysis [91]. A novel amphiphilic block copolymer, PNIPAM–Ge(C6F5)2–poly(2,2,3,3-tetrafluoropropyl methacrylate), was synthesized through a double successive chain transfer reaction to bis-(pentafluoro phenyl) germane groups. Despite subphase acidity, this copolymer exhibits high collapse pressures (πmax = 48–61 mN/m) [92]. Researchers created thermoresponsive polymeric micelles to deliver zinc protoporphyrin (ZnPP) to prostate cancer cells. These micelles provide improved stability and decreased drug leakage. The ZnPP-containing micelles had an average size of 148 nm and displayed temperature-sensitive drug release. In PC3 cells, they demonstrated a 36% lower inhibitory concentration 50% (IC50) compared to the free drug, suggesting enhanced toxicity. The drug-laden micelles were more successful in triggering apoptosis in PC3 cells than the free drug [93]. Table 2 outlines temperature-responsive polymer compositions suited for increased biological applications. These include polymeric micelles for controlled drug release, leveraging PNIPAM’s thermosensitivity to improve bioavailability and stability and new techniques including theranostic applications using NPs for simultaneous therapy and diagnosis.
| Sr. No. | Composition | Drug | Preparation technique | Key features (observation) | References |
|---|---|---|---|---|---|
| 1 | PEG–PLLA/PDLA–PNIPAM | Icariin (ICA) | Formation of polymeric micelles from poly(ethylene glycol)–poly(L-lactic acid) (PEG–PLLA) and poly(D-lactic acid)–poly(N-isopropylacrylamide) (PDLA–PNIPAM) | This study has yielded promising results, demonstrating uniform nanosize distribution and high stability for 48 h. In vitro testing revealed sustained release of ICA, while in vivo studies showed improved bioavailability and stability, with reduced drug metabolites and decreased first-pass effects. Temperature-sensitive PNIPAM proved effective in preventing ICA hydrolysis by intestinal bacteria. These findings suggest potential for improving the bioavailability and stability of compounds facing similar challenges | [80] |
| 2 | Fe3O4 nanoparticles coated with PNIPAM | Vincristine sulphate | Surface-initiated ATRP | Very tiny dimensions, with the ability to carry a high amount of medication; release of medication is dependent on pH and temperature; has superparamagnetic properties for MRI contrast; used for both therapy and diagnosis (theranostic application) | [52, 53] |
| 3 | DOX–GNRs–PNIPAM@PEG–PLA | Doxorubicin | Self-assembly of micelles named DAPP comprising gold nanorods (GNRs) and DOX encapsulated within a core–shell PNIPAM@PEG–PLA matrix | This technology has the potential to be used in melanoma therapy as it allows for the controlled release of drugs in response to specific stimuli. The drug release is regulated, and temperature-sensitive PNIPAM helps to ensure that the drug is released only when needed. When exposed to near-infrared (NIR) light, it is more effective in killing melanoma cells and can strongly inhibit tumour growth | [83] |
| 4 | PNIPAM nanoparticles containing acetaminophen | Acetaminophen | Photopolymerization, integration into gelatine hydrogels | Reduced swelling, antibacterial, controlled drug release | [8] |
| 5 | PNIPAM hydrogel with acrylolsobutyl polyhedral oligomeric silsesquioxane (MAPOSS) | 5-Fluorouracil | Radical polymerization with addition of PEG | Stable, sustained drug release; promising candidate for drug delivery application characteristics | [75] |
| 6 | PNIPAM-AM/OSG hydrogels | 5-Fluorouracil | Radical polymerization with addition of OSG and PEG | Enhanced heat resistance, flexibility, self-repair capability; drug release controlled by temperature and pH; nontoxic; distinct swelling characteristics | [78] |
| 7 | β-CD-star-(PMAA-b-PNIPAM) in PHEMA-g-(PCL-BM) solution | Doxorubicin | Fabrication of temperature and pH-sensitive micelles with low CMC using β-CD-star-(PMAA-b-PNIPAM) dissolved in DMSO and added to a PHEMA-g-(PCL-BM) solution | Micelles exhibit temperature and pH sensitivity, with a lower critical solution temperature (LCST) range of 40−41°C. They enable effective drug release in aqueous environments while being biocompatible. They have been shown to possess superior anticancer activity compared to free DOX and are suitable for use in controlled drug release systems | [83] |
| 8 | PMAOETC-b-PNIPAM | Paclitaxel | Development of polymeric micelles for PTX delivery using PMAOETC-b-PNIPAM | The drug has a high loading capacity and remains stable. It releases significant PTX in cancerous tissue while causing minimal toxicity against HCT-116 cells. Tumour volume is reduced when compared to free PTX treatment. This provides an opportunity to enhance therapeutic outcomes in drug delivery systems | [82] |
| 9 | PChM-PNIPAM | Unspecified | Synthesis of amphiphilic diblock copolymers PChM-PNIPAM for potential drug delivery applications | The hydrophobic interactions drive the formation of water-soluble polymer micelles below LCST. The hydrophilic shell is formed by PNIPAM. The thermal cycles do not affect its behaviour, which makes it a suitable candidate for controlled drug release systems | [88] |
| 10 | MDI-TPED-PEO/ABA block copolymers | Doxorubicin | Creation of PEGylated macroiniferter for synthesizing temperature-sensitive micelles through polycondensation | Cancer cells can be safely targeted with biocompatible copolymers with low toxicity. These copolymers deliver DOX highly effectively, and their therapeutic efficacy is superior to free DOX. This suggests that these copolymers have potential as drug carriers for cancer treatment | [86] |
| 11 | PNIPAM-g-Cell | Doxorubicin | Creation of PNIPAM-g-Cell hydrogel via photoinduced metal-free ATRP, forming thermos-responsive micelles suitable for biomedical applications | A hydrogel composed of micelles that are responsive to thermos has been developed. This hydrogel can also release DOX in a sustained manner. It is highly biocompatible and can be injected, making it suitable for biomedical applications. The approach used for organic photocatalysis eliminates the requirement for transition metal catalysts | [84] |
| 12 | PNIPAAm or PDEAAm/PSSNa or PAMPSNa | Unspecified | Utilization of thermoresponsive diblock copolymers for palladium-catalysed coupling reactions in water | The extraction efficiency is excellent, and purification is easy. Coupling reactions are efficient with high numbers of turnovers. There is potential for environmentally friendly coupling reactions in aqueous media | [87] |
| 13 | Cholesterol end-capped PNIPAAm | Unspecified | Development of effective thermoresponsive drug delivery systems via RAFT polymerization | Micelle formation in water depends on molecular weight and has a phase transition temperature. They are nontoxic to fibroblasts but toxic to glioblastoma cells. Micelles can function as drug carriers and be used in synergistic therapy | [90] |
| 14 | Block copolymers through ATRP | Unspecified | Introduction of injectable hydrogel ’HyMic’ for drug delivery applications, particularly in MMP-rich environments like cancer tissues | Micelles have the potential to deliver drugs into cells. Targeted drug delivery strategies can be effective through the controlled release of therapeutic agents in environments rich in MMP. Cells’ uptake of micelles shows promise for intracellular drug delivery | [85] |
| 15 | PNDU/CM-Dex Fe3O4 | Hesperetin | Synthesis of multifunctional micelles exhibiting pH-dependent temperature response and magnetic properties | The substance’s temperature sensitivity and biocompatibility vary depending on the pH level. In vitro experiments have shown that it significantly reduces inflammation. It has potential for use in biomedical applications to deliver anti-inflammatory drugs | [92] |
| 16 | Poly(N-isopropylacrylamide)–Ge(C6F5)2-poly (2,2,3,3-tetrafluoropropyl methacrylate) | Unspecified | Synthesis and characterization of a novel amphiphilic block copolymer with colloidal properties suitable for various applications | Langmuir monolayers exhibit high collapse pressures. Morphological analysis suggests the formation of micelles. The self-organization at the interface of water and air is influenced by the acidity of the subphase and the presence of methanol. The self-assembly of amphiphilic block copolymers can provide insights into this phenomenon | [93] |
- Abbreviations: ABA, atom transfer radical addition; ATRP, atom transfer radical polymerization; CD, carbon dot; CMC, carboxymethyl cellulose; DMSO, dimethylsulfoxide; MDI, methylene diphenyl diisocyanate; MMP, matrix metalloproteinase; MRI, magnetic resonance imaging; OSG, oxidized succinoglycan; PAMPSNa, poly(2-acrylamido-2-methyl-1-propanesulfonic acid) sodium salt; PDEAAm, poly(N,N-diethylacrylamide); PEO, polyethylene oxide; PLA, polylactic acid; PMAOETC-b-PNIPAM, poly(2-methacryloyloxyethyl thiocticcarboxylate)-block-poly(N-isopropylacrylamide); PNDU, poly(N,N-dimethyl-N′-undecylurea); PNDU/CM-Dex Fe3O4, poly(N,N-dimethyl-N′-undecylurea) and carboxymethyl dextran-coated magnetite (Fe3O4) nanoparticles; PNIPAM, poly(N-isopropylacrylamide); PSSNa: poly(sodium 4-styrenesulfonate); RAFT, reversible addition–fragmentation chain transfer; TPED, tetra-p-phenylethylene derivative.
3.4. Other Innovative PNIPAM-Based Delivery Platforms
Nanospheres are one form of microgel particles that exhibit a cross-linked structure that renders them highly swollen and hydrophilic; this provides them the ability to encapsulate more of the drug and release the drug when certain conditions like temperature and pH are met [94]. NPs of PNIPAM are generally smaller in size and are different in structure and hence capable of adsorbing on surfaces and penetrating through different layers [95]. PNIPAM nanospheres possess thermosensitive characteristics and contain a swollen and hydrophilic microgel core to increase drug viability and sensitivity to external stimuli. They can deliver the drugs in a better way in the acidic conditions which prevail in cancer cells, thus enhancing the efficiency of the treatment. This makes PNIPAM nanospheres a good candidate for a better advancing DDS particularly in cancer treatment [96]. Researchers synthesized nanospheres of PNIPAM@PAA, which is a core of PNIPAM polymer and can load efficiently the drug DOX. The leakage of these nanospheres is extremely low at a neutral pH, while it is more at a low pH and thus sensitive to tumour conditions [97]. According to Fick’s first law, the release rate of DOX is of sustained release mechanism by diffusion. The in vitro studies also revealed that the cytotoxicity of DOX encapsulated in these nanospheres was higher than the free DOX, suggesting the possibility of using them in dual stimuli-responsive anticancer drug carriers [98]. Microgels of PNIPAM for veterinary vaccines were synthesized through precipitation polymerization. The prepared nanogels were highly biocompatible for the cells, and the cellular uptake rate was found to be very efficient. They were employed to transfer the antigen OmlA for porcine pleuropneumonia intranasally to the mice, eliciting a rather encouraging immunity [99]. A thermoresponsive hydrogel microwell array (PHMA) made from PNIPAM by lowering temperature enables stress-free formation and capture of cancer cell spheroids and also accurately mimics native cell anatomy, giving information about cancer physiology and pharmacology. The PHMA’s cost-effectiveness and efficiency suggest it could replace existing 3D cell culture methods for preclinical drug screening and cancer research [69]. Formation of responsive photonic devices that change colour with temperature and light, due to the photothermal properties of the gold (Au) core and the thermoresponsive PNIPAM shell, was created by coating Au-PNIPAM NPs by AuNPs. Uniform-sized microgels of Au@PNIPAM show faster performance and more stable spectra compared to those with AuNP-doped PNIPAM microgels [100]. In this work, lipid cubic NPs to be used as DDSs and stabilized through PNIPAM-based block copolymers are investigated. Their effectiveness in delivering a chemotherapy drug, camptothecin (CPT), to human cancer cells which include the colon and bladder T24 cells is examined in the study. It was indicated that the enhancement in CPT absorption is due to the cubosomal formulation and stabilization by poly(N,N-dimethylacrylamide)-block (PDMA-b)-PNIPAM polymers instead of Pluronic. Similar to two-dimensional (2D) and 3D cancer cell cultures, the B20K-stabilized cubosome formulation has higher CPT loading and more prominent anticancer properties than free CPT or Pluronic formulations [101]. Studies are devoted to the fabrication of nanofibers with the incorporation of GNRs and drugs for regulating the drug release by employing NIR light. New composite nanofibers with the GNRS dispersed in the PNIPAM nanofibers were synthesized. This arrangement helps in achieving a gradual discharge of drugs any time there is an exposure to NIR light; this is due to the heat from GNRs that causes the PNIPAM to shrink and thereby release the drug. The nanofibers provide a large surface area for the drug delivery application, and it can incorporate both hydrophilic and hydrophobic drugs. Cell studies have also confirmed the biocompatibility and controlled drug delivery exhibitions with the help of nanofibers [102].
4. Targeted Drug Delivery Strategies
4.1. Active Targeting
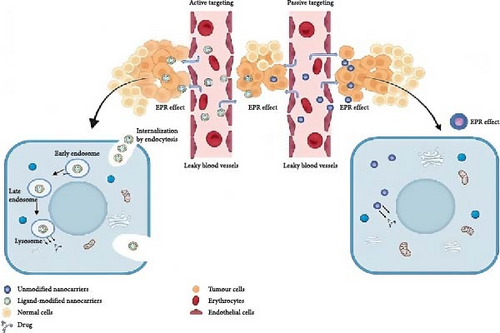
Copolymers synthesized from PNIPAM have attracted much attention in the controlled delivery of drugs to cancerous cells. These copolymers have to have LCST which is lower than the body temperature to allow drug release based on temperature difference. For example, DOX–PNIPAM-co-acrylic acid graft copolymer conjugate for the targeted delivery of anticancer drug to breast cancer cells has been prepared [103]. Other topical approaches that have been tried include the active targeting of PNIPAM-based copolymers with the purpose of desired drug release selectivity. This includes the process of attaching targeting ligands to the polymer to attach to receptors overexpressed in cancer cells [104]. For instance, microgels synthesized from PNIPAM have been conjugated with folic acid (FA) which directly targets the receptor that is overexpressed in the cancer cells and DOX. In another study, for cancer cells, a nanocarrier through covalent conjugation of P(NIPAM)-co-5%AA with a targeting group and DOX as the anticancer drug using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and N-hydroxysuccinimide (NHS) chemistry was synthesized [105]. Another variant is the FA-conjugated PNIPAM-based lipopolymers for the drug delivery application. These lipopolymers have property of temperature-sensitive controlled release, and the interaction with FA receptor enhances their anticancer efficacy [106]. Literature review has also been done to improve the stands of the plant sterol, β-sitosterol (BS), with potential anticancer properties by incorporating an anticancer delivery system for epidermal growth factor receptor (EGFR) and methionine enkephalins (MET) receptor cross-talk. For enhancing targeting efficiency of BS to the target site, the surface properties of BS were altered by conjugating SPIONs, PEG and PNIPAM coatings [107]. New core–shell particles have been synthesized to reach out to the melanoma cancer cells through multifunctional biocompatible core–shell particles (MBCSPs). These particles have a magnetic core composed of magnetite NPs incorporated in poly(lactic-co-glycolic acid) (PLGA) and a thermoresponsive shell composed of PNIPAM-acrylamide-allylamine. To attach to α(5)β(3) receptors of the melanoma cell, MBCSPs were linked with glycine-arginine-glycine-aspartic acid-serine (GRGDs) peptides [108]. A novel strategy in the form of multifunctional dual drug-loaded NP (MDNP) that binds with folate receptors (FRs) has been developed to overcome the problems related with late-stage lung cancer and for enhancing the efficacies of the existing treatments. For shell made of PNIPAM–carboxymethyl CS and a PLA core, the MDNP delivers inflated localized chemoradiotherapy for the lung cancer treatment [109]. A new 2D polymeric network formed by cross-point connections of one-equivalent PNIPAM chains by a four-arm polyglycerol has been developed and studied. The synthesized material had the tested porous structure to enable loading of antibiotic like tetracycline and amoxicillin [110]. Researchers have developed a novel strategy for designing protein kinase A (PKA)-triggered supramolecular assemblies with anticancer activities. They utilized PKA activity to trigger the assembly process selectively in PKA-upregulated MCF-7 cancer cells, disrupting cytoskeletal structures and sensitizing cancer cells to DOX treatment [111]. Hybrid bionanoporous peptides loaded onto PNIPAM-co-butyl acrylate (PNIPAM-co-BA) coatings have been designed and obtained via the matrix-assisted pulsed laser evaporation (MAPLE) technique. The goal was to incorporate cationic peptides magainin (MG) and melittin (Mel) to target synergistic anticancer and antibacterial activities while minimizing toxicity to normal mammalian cells [112]. Active drug delivery targeting strategies involve conjugating ligands/molecules to carrier moieties, enhancing the target-to-nontarget ratio for cell-specific killing. This is tailored to tumour types using specific targeting moieties, either chemically conjugated or physically attached to nanocarriers, showing success in vitro and in vivo. There are two main categories: receptor-mediated endocytosis and stimuli-responsive intracellular delivery based on pathological microenvironment changes. Cancer cells overexpress various receptors, making them distinguishable. Conjugating complementary ligands on NPs allows selective targeting, leading to rapid internalization via receptor-mediated endocytosis. Specific receptors include FR isoforms (FRα, FRβ, and FRγ), transferrin receptor, EGFR, human epidermal growth factor receptor 2 (HER2), carbon dot (CD) receptors, estrogen receptor and αvβ3 integrin receptor [113]. Multifunctional nanocapsules for tumour-targeted drug delivery have been developed using the Pickering emulsion (PE) route. PNIPAM-co-AA NPs were employed to stabilize the nanocapsules, which were then cross-linked with cystamine and coupled with cell-surface molecule markers (cRGDfK) to enable controllable drug release and enhance targeted antitumour effects [114]. Figure 3 illustrates two approaches to improve the administration of drugs in cancer chemotherapy by utilizing nanocarriers. Active targeting is the attachment of ligands to nanocarriers that selectively bind to receptors on tumour cells. This promotes the internalization of the nanocarriers through endocytosis and enables accurate drug delivery to the tumour. Passive targeting exploits the enhanced permeation and retention (EPR) phenomenon, whereby nanocarriers spontaneously gather in tumour tissues as a result of their permeable blood vessels and inadequate lymphatic outflow. This enables medications to be administered directly into the tumour environment. Both approaches result in an elevation in medication concentration specifically at the tumour location so improving the effectiveness of the treatment while minimizing any negative effects on healthy tissues.
4.2. Antibody–Drug Conjugates
Using proteins, antibodies and antibody fragments to decorate NPs allows for precisely targeting specific cells, thereby enhancing the delivery and effectiveness of therapeutics and diagnostics [115]. Mesoporous silica NPs (MSNs) when conjugated with PNIPAM copolymer make the MSN responsive to the tumour microenvironment in terms of pH and temperature. It has been proved that PNIPAM allows providing the delivery of DOX into tumour cells only and minimizes the influence on healthy cells. It was also evident that when integrated with anti-HER2 antibodies, the NPs adequately home in and invade HER2-positive breast cancer cells. In furtherance of these effects, PNIPAM favours self-concentration of the NPs within the tumour site due to its acidic and warm temperature which results into enhanced drug delivery [116]. There is a technique of isolating certain cell types with the help of membrane created from PNIPAM-graft-polypropylene (PNIPAM-g-PP) that is coated with antibodies to adhere to the given cells. The antibody binds to the membrane at 37°C and debonds at 4°C which helps the cell to attach and deattach to the surface. Target cell recovery can be obtained when the membrane is coated with a specific antibody [117]. Conjugation of peptides to antibodies represents a new strategy in cancer immunotherapy epitome known as antibody–peptide epitope conjugates (APECs). These conjugates redirect the T cell’s viral immunity to the tumour cell, eliminating them with the help of CD8+ T cells. Followed this work on ovarian cancer, researchers developed a library of APECs and selected epithelial cell adhesion molecule, matrix metalloproteinase 7, and cytomegalovirus (EpCAM-MMP7-CMV) APEC or epithelial cell adhesion molecule-modified carrier (EpCAM-MC) as the immunotherapy. This paper shows that APECs hold promise in the treatment of ovarian carcinoma [118]. It is one of the forms of another malignant disease causing noncurable changes in the plasma cells of the human body. Antibody–drug conjugates (ADCs) have become some of the new treatment tactics developed due to advances in treatment methods. These ADCs involve the B cell maturation antigen (BCMA) and are safe and effective and specifically used for relapsed and refractory multiple myeloma [119]. The subject researchers established a workflow for generating APECs that are specific to patient populations with ovarian carcinoma. They designated a potential APEC named EpCAM-MMP7-CMV (EpCAM-MC) for ovarian carcinoma immunotherapy. This paper laid solid foundation for addressing future researches in individualized cancer therapies [118].
4.3. Ligand-Modified PNIPAM Nanocarriers
PNIPAM is a crucial element in ligand-modified DDS because of their temperature sensitivity. This property gives a good control on both drug entrapment and its subsequent release in its therapeutic use [120]. Due to the higher temperatures in the tumour microenvironment, PNIPAM can quickly release the encapsulated drugs and improve the drug concentration at the tumour area for effective therapy [121]. It plays a critical role of ensuring drug-loaded carriers retain their therapeutic profile in circulation within the bloodstream. Based on the stimuli-responsive PNIPAM platform, active targeting may be achieved by conjugating PNIPAM-based nanocarriers with specific ligands that can engage overexpressed receptors on cancer cells. This targeted delivery approach minimizes side effects of drugs and enhances the general therapeutic ratio by ensuring greater molar concentration of drugs at the target site. This behaviour along with ligand modification ability categorizes PNIPAM as a versatile component for the nanocarrier system [122]. PNIPAM nanocarrier modified with ligands is one of the most developing methods for drug delivery and diagnosis recently. Coating the surface of nanocarriers with specific ligands increases the contact with the target cells and tissues which in turn increases the uptake and intracellular delivery of the nanocarriers [123]. This leads to the enhancement of efficiency, concentration and efficacy in the healthcare processes and its deliverance, hence the results [124]. Ligand-mediated NPs in cancer treatment therefore provide distinct targeting and intracellular delivery of drugs even those that are in the multidrug resistance group. The properties of ligands which include density, charge, hydrophobicity and shape affect cell–particle interactions and the uptake by the cell and the result affects treatment of cancer [125]. Thus, an effective DDS has been developed using a β-CD-PNIPAM coating at MSN-SS-Fc via host–guest interaction. It is multiresponsive to temperature changes as well as to glutathione (GSH) and hydrogen peroxide (H2O2) concentrations. The nanocarriers designed here are responsive to overexpressed GSH and H2O2 in tumour cells, the external temperature and PH [126]. In diagnostics, there exists a tool that provides diagnosis of corneal ulcers, a sight-threatening condition in developing countries. The concept of the tool is based on identification of the infectious agent by employing a ligand-functionalized PNIPAM hydrogel. The hydrogel prepared has been functionalized with ligands like vancomycin, polymyxin B and amphotericin B which are selective for different pathogens so they get recognized [127]. The present research focuses on the behaviour of two polymer types with vancomycin, a strong antibiotic to determine the interaction of these polymers with bacteria, S. aureus. The highly branched polymer (HB-PNIPAM-van) effectively aggregated S. aureus, whereas the linear (L-PNIPAM-van) counterpart did not [128]. They are ‘smart’ micro-/nanofiltration membranes using inverse colloidal crystal (ICC) membranes made from silicon dioxide (SiO2) particles and polymerized with N-isopropyl acrylamide (NIPAM). The resulting ICC membranes have uniform pores and high porosity [129]. Various physical and biochemical cues influence human mesenchymal stem cell (hMSC) differentiation into the osteogenic lineage. Researchers investigated the osteogenic differentiation of hMSCs cultured on semi-interpenetrating networks (sIPN) with low moduli based on PNIPAM, modified with the integrin-engaging peptide bone sialoprotein-arginine-glycine-aspartic acid (bsp-RGD) (15). They evaluated cell adhesion, proliferation and osteogenic differentiation through markers like alkaline phosphatase (ALP), runt-related transcription factor 2 (RUNX2), bone sialoprotein-2 (iBSP) and osteocalcin (OCN) protein expression. Interestingly, a high-affinity adhesive ligand can compensate for the lack of rigidity and promote osteogenic differentiation within compliant and low-modulus substrates [130]. Using thermoresponsive surfaces, specifically PNIPAM-grafted surfaces, can modulate the affinity binding between ligands and cellular receptors by changing the temperature. Heparin-functionalized thermoresponsive cell culture surfaces can control the multivalent affinity binding between heparin and heparin-binding proteins like essential fibroblast growth factor (bFGF). This allows for the detachment of cultured cells as a contiguous cell sheet upon lowering the temperature to 20°C [131]. Metal coordination platforms (MCPs) have gained attention as effective drug delivery carriers in nanomedicine due to their uncomplicated preparation process and ability to preserve the activity of natural ligands. Supramolecular MCPs exhibit unique physicochemical properties and can be modified to be surface-modified, drug-encapsulated or environmentally responsive [132]. Using nanolipid-based carriers (NLBCs) for drug delivery is promising, but targeted delivery and detection remain challenging. This review focuses on using ligands to decorate NLBCs for targeted drug delivery to cancer cells [133]. A tumour-targeting polypeptide cyclic RGD (cRGD)-modified erythrocyte membrane (eM-cRGD) was assembled onto zeolitic imidazolate framework-8 (ZIF-8) NPs encapsulating DOX. The nanoscale-sized targeting DDS facilitated NP accumulation in tumour tissues via EPR effects, with active targeting ligands directing NPs to endosomes [134]. Hepatocellular carcinoma (HCC) is a top cause of global mortality and is often diagnosed at advanced stages with low survival rates. Nanomedicine offers promising solutions to currently limited treatment options [135]. This study involves the immobilization of CdSe/CdS semiconductor NPs onto PNIPAM microspheres. A ligand-exchange procedure initially transfers reactive NPs into an aqueous solution. The interaction between the NPs and the PNIPAM-based system depended on the nature of the ligands and the chemical composition of the microspheres. NPs capped with amine- or mercapto-polyethylene oxide ligands interacted with PNIPAM–PS beads, while only amine-capped ones showed a clear tendency to interact with PNIPAM containing acid groups, resulting in high NP coverage. The resulting fluorescent composites exhibited nonspecific binding to fibroblasts, demonstrating potential for use in drug delivery and specific targeting of cells [136]. This study focuses on the modification of PNIPAM onto anodic aluminium oxide (AAO) membrane surfaces using the ATRP method. PNIPAM-modified AAO membrane demonstrates reversible variations in ionic conductance, highlighting its robustness, stability and controllable properties, particularly in discriminating between hydrophobic and hydrophilic ions under consecutive temperature changes [137].
4.4. Passive Targeting
Passive targeting is a DDS based on the physiochemical attributes of NPs to improve the delivery effectiveness of therapeutic agents to certain localized regions in the body [138]. This strategy is realized by adjusting the properties of NPs to address the specific pathophysiologic properties of the targeted tissues/organs [139]. The mechanism of passive targeting can be said to work mostly through the EPR effect which is the permeability and retention of the NPs in the tumour [140]. Tumour and inflamed tissues have been known to possess irregular vasculature, and hence, NPs tend to accumulate in these areas. This allows for a preferential uptake of NPs, the drug distribution in affected tissues is much better than in healthy tissues [141]. Diblock copolymers containing PNIPAM and poly(ε-caprolactone) (PCL) were created and analysed in a study. The goal was to prepare micelles for drug delivery which will be thermosensitive and biocompatible. The critical micelle concentration (CMC) value of the copolymers was found to be decreasing with increasing the length of PNIPAM chain. The organic micelles were observed to form a gel layer on the shell above LCST, thus showing a delayed and a controlled release mechanism of carboplatin, a chemotherapy drug. Carboplatin was found to have lower release rate at 37°C as compared to the release rate at 25°C; hence, the micelles were confirmed to have temperature-sensitive drug release method. In vivo studies on the biodistribution of the micelles was done on rats through bioimaging. The outcomes of the study showed that the micelles of this size could passively target the lungs, and therefore, the accumulation was quite impressive. Additionally, due to micelles’ ability to minimize the recognition of reticuloendothelial system (RES) organs, particularly the spleen, systemic clearance was also minimized. Compared to a control, micelles increased carboplatin concentration in the lungs and reduced the concentration in the heart and kidneys [142]. CS-grafted PNIPAM NPs (CS-g-PNIPAM-PTX) coated with l peptides provide a dual stimuli-responsive DDS with tumour selective as well as minimized off-target action. These NPs with size <300 nm and polydispersity index (PDI) < 0.45 through the EPR effect accumulate passively in the tumour. It has a high drug payload and interaction time of the drug particle which provide slow release of PTX at physiological pH and temperature, however, has quick release under the tumour microenvironment stimuli. Especially, l-CS-g-PNIPAM-PTX has high selectivity to breast cancer cells with high expression of GRP78 and displays higher cytotoxicity both in vitro and in vivo to free PTX, leading to a better therapeutic performance and higher cancer cell killing efficiency [143]. EPR effect is a critical factor in the treatment of cancer; it improves the concentration of the molecules or NPs in the tumour tissues. This is a result of peculiarity of the tumour blood vessels and the microenvironment in which they exist [144]. Particles of sizes 100–200 nm are received to have the best ability to penetrate and accumulate within the tumour. Surprisingly, irregular forms, like ellipsoids or cylinders, prove to be superior in terms of their ability to accumulate within the tumour tissues because they can easily move within the tumour mass [145]. The particles that can be deformed and degraded enable a tenant release of medication within a tumour and hence improved efficacy. Regulation of blood flow within tumour can drastically increase a drug concentration in tumour tissues [146]. Other approaches include the use of atom in favour of augmenting blood flow, for instance, through the activation of angiotensin II. In addition, the employment of other large animal models, especially canine cancer models, gives clear picture on nanomedicines’ efficiency in human tumour than the usual small animal models [147]. Polymeric NPs allow the slow release of the drug over several hours to a few days under mild hyperthermia, which correlates well with clinical conditions. The effect of heating is affected by hydrophobic components added to copolymers where it brings the LCST of the copolymers to a lower value. This makes them more appropriate to be used for heat-controlled drug delivery [148]. Nevertheless, getting to clinical usage is not without its hitches. Peculiarities such as the heterogeneity of tumour, physiological barriers and the poor penetration of nanocarriers within the tumour also present large challenges [149]. Thus, optimization of the blood flow to the tumour sites, distribution within the tumour and enhanced intracellular uptake are required to enhance the therapeutic efficacy of nanomedicines utilizing EPR effect [150]. It has also been demonstrated that PNIPAM NPs containing anastrozole had better drug delivery efficiency in cancer treatment. To demonstrate the controlled release of anastrozole over 48 h at pH 7, the drug release profile is provided. Additionally, the profile shows enhanced release at pH 5.0, which is similar to the pH found in tumor microenvironments [151]. Copolymer micelles are characterized to have spherical core–shell-like structures in aqueous solution especially when containing PNIPAM graft chains. These structures with the small size distribution are beneficial when it comes to using the EPR effect to facilitate accumulation in tumour tissues due to leaking vasculature. One of the major advantages of these micelles is their capability of loading appreciable amount of hydrophobic CPT into their core. This process offers the advantages of stabilization and maintenance of a required release rate, thus preventing a burst release of the drug. This mechanism is in agreement with our aim to improve the EPR effect; these micelles are proficient in drug delivery [152]. The enhancement of EPR effect by using MSNs is capped with PNIPAM. This characteristic of tumour tissue results from both leaky vasculature and an inability of lymphatic drainage in tumour, making it suitable for trapping substances at the sight of tumour. The thermosensitive polymer (PNIPAM) that caps the MSNs plays another role of extending the controlled release mechanism of the NPs so that the drug is released upon signals. This reduces drug release prematurely in the bloodstream and causes a build-up of the drug concentration at the tumour site. The presence of GSH and the high temperature prevailing in tumour tissues facilitate the release of the said drug when the system is triggered by the two mentioned stimuli. This is even much more significant in order to strengthen the EPR effect and deliver the drug in the highest rate to the tumour with least side effect [153]. A thermosensitive block copolymer of PNIPAM-b-lauryl acrylate was synthesized by RAFT polymerization with low CMC and prepared stable nanomicelles. The micelles effectively loaded an anticancer drug, docetaxel, through Van der Waals forces, and the formulation experienced temperature-dependent and sustained drug release in vitro [154]. The synthesis of a new block copolymer based on renewable hydrophobic fatty acid block and thermoresponsive PNIPAM block was accomplished using microwave-assisted RAFT polymerization technique. These copolymers can self-assemble into spheres with diameter of about 30 nm and has low CMC and used in the study of hydrophobic model drug carbamazepine showing temperature-sensitive drug release. The DDS present in the micelles has some advantages like ease in preparation, stability and size that help in passive targeting of tumour through EPR effect. The study also identified the fact that the size of the micelles can be controlled according to the degree of length of the hydrophobic block and the molecular weight of the copolymer that the hydrodynamic diameter of the nanocarriers falls between 27 and 31 nm [155]. A thermoresponsive polymeric nanocarrier with the copolymer of PNIPAM-co-acrylamide was synthesized by RAFT polymerization [156]. They switched the configuration by hydrazine and conjugated DOX with acid-cleavable Schiff base linkage of the nanocarrier to adapt the functional change pursuing temperature and pH [157]. The release patterns were studied in the normal and carcinogenic environment mimicking, and it was observed that there occurred a controlled release at the pH value of 7.4 and 5 (37 and 42 °C). In experiments using MCF-7 breast cancer cell line, the ability of the nanocarrier in cancer therapy was established using cytotoxicity analysis [158]. In conclusion, EPR effect’s capability to translocate big molecule drugs to tumour tissues and then use the miscellaneous extravasation and retention mechanism seen in solid tumour is a revolution. The ability to understand and control such factors in order to enhance the delivery of drugs to the tumour tissues offers hope of enhancing the treatments outcomes and reducing the undesirable side effects [159]. Figure 4 depicts PNIPAM NPs in cancer drug delivery. Following intravenous injection, NPs target tumours using the EPR effect and release medicines under mild heat. Further, by intravascular approach, at somewhat higher temperatures, drugs can be infused directly into the bloodstream and then enter tumour cells. Both approaches optimize selective drug delivery, improving cancer therapy outcomes by concentrating treatment on tumour locations.
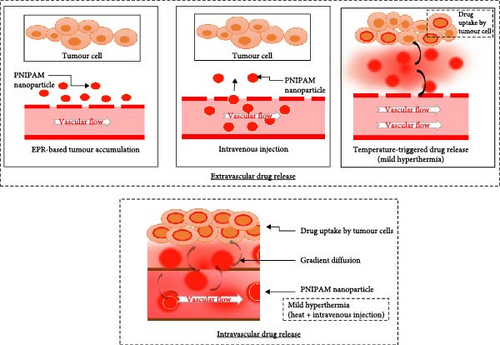
Table 3 illustrates numerous ways employing PNIPAM for targeted medication delivery. These include active targeting via FR and antibody conjugates, functionalized NPs for stimuli-responsive release and utilizing the EPR effect in cancer therapy.
| Description | Reference |
|---|---|
| Active targeting | |
| Poly(N-isopropylacrylamide) (PNIPAM)-based copolymers with lower critical solution temperatures (LCSTs) enable temperature-responsive drug release, such as conjugates with doxorubicin (DOX) for breast cancer cells | [103] |
| Functionalization of PNIPAM-based microgels with folic acid (FA) targets folate receptors overexpressed by cancer cells, enhancing DOX delivery | [105] |
| PNIPAM-based lipopolymers modified with folic acid show temperature-responsive controlled release and improved anticancer activity | [106] |
| Surface modification of beta-sitosterol (BS) with superparamagnetic iron oxide nanoparticles (SPIONs), polyethylene glycol (PEG) and PNIPAM enhances BS delivery to cancer cells | [107] |
| MBCSPs target melanoma cells via α(5)β(3) receptors, conjugated with GRGDs peptides | [108] |
| MDNP targets folate receptors for lung cancer treatment, combining chemoradiotherapy | [109] |
| PNIPAM-based two-dimensional polymeric network efficiently loads antibiotics like tetracycline and amoxicillin | [110] |
| Hybrid bionanoporous peptides on PNIPAM-co-BA coatings show synergistic anticancer and antibacterial activities | [112] |
| Antibody–drug conjugates | |
| Mesoporous silica nanoparticles (MSN) modified with anti-HER2 antibodies selectively target breast cancer cells, releasing doxorubicin in response to tumour conditions | [116] |
| Poly(N-isopropylacrylamide)-graft-polypropylene (PNIPAM-g-PP) membrane coated with antibodies selectively captures target cells | [117] |
| APECs redirect T-cell immunity towards ovarian cancer cells, showing promise for immunotherapy | [118] |
| Anti-BCMA ADCs offer safe and effective treatment for multiple myeloma, particularly in relapsed and refractory cases. | [119] |
| Ligand-modified PNIPAM nanocarriers | |
| Nanocarriers functionalized with ligands exhibit targeted drug delivery, utilizing stimuli-responsive mechanisms | [123] |
| Temperature-responsive polymeric nanocarriers release docetaxel under stimuli, showing promise for cancer therapy | [154] |
| Passive targeting | |
| PNIPAM–PCL copolymers form thermosensitive micelles for controlled drug release and passive lung targeting, reducing systemic clearance | [142] |
| Stimuli-responsive nanoparticles target GRP78-overexpressing breast cancer cells, showing potent antitumour effects | [143] |
| PNIPAM-coated MSNs enhance EPR effect, with controlled drug release in tumour microenvironments | [151] |
| Copolymer micelles encapsulating camptothecin exploit EPR effect for tumour accumulation and sustained drug release | [152] |
| MSNs capped with PNIPAM improve EPR effect, with stimuli-responsive drug release for enhanced tumour targeting | [153] |
| Polymeric nanocarriers release docetaxel in response to temperature and acidity changes, showing potential for efficient cancer treatment | [158] |
- Abbreviations: ADCs, antibody–drug conjugates; APECs, antibody–peptide epitope conjugates; BCMA, B cell maturation antigen; EPR, enhanced permeation and retention; GRGDs, glycine-arginine-glycine-aspartic acid-serine; HER2, human epidermal growth factor receptor 2; MBCSPs, multifunctional biocompatible core–shell particles; MDNP, multifunctional dual drug-loaded nanoparticle; MSN, mesoporous silica nanoparticles; PCL, polycaprolactone; PNIPAM-co-BA, poly(N-isopropylacrylamide-co-butyl acrylate).
5. PNIPAM in Cancer Therapy
Cancer treatment requires experimental breakthroughs and development of more targeted drugs and techniques than what have been used in the past 10 years with shifting towards personalized treatments based on the patient’s specific tumour characteristics. This new development includes the use of mRNA cancer vaccines especially in treatment of melanoma [160], combined treatment regimens consisting of immunotherapy with surgery or chemotherapy and has been effective for diverse forms of cancer [161]. One issue is inherent lack of selectivity, and conventional chemotherapy agents tend to cause harm to both malignant and healthy tissues and organs, consequently high toxicity. These side effects interfere with the patients’ quality of life leading to treatment discontinuation [162]. Due to tumour heterogeneity, various tumour cells can react differently in response to a particular therapy making it difficult to work towards the formation of comparable treatment effects [163]. Further, drug resistance becomes a significant challenge in cancer therapy and thus the constant search for new treatment approaches [164]. PNIPAM due to its thermoresponsive properties and LCST (32°C) help in delivering drugs in a way that is constrained and specific; the anticancer drugs are released mainly at the tumour site increasing its accuracy in drug delivery, bioavailability and the constant release of drug with minimizing systemic toxicity [165]. Furthermore, incorporation of PNIPAM with photothermal therapy (PTT) will improve therapeutic outcomes since it provides personalized treatment with less side effects [166].
5.1. Chemotherapy Enhancement With PNIPAM
In attempts of improving cancer treatment, researchers created a smart polymer nanoplatform for drug delivery. To do this, they used thermosensitive polymer; PNIPAM prepares nanogel particle containing photosensitizer (PS) indocyanine green (ICG) generating heat and reactive oxygen species (ROS) along with anticancer drug 5-FU. This provided for a synergetic treatment of cancers with drug and PTT/photodynamic therapy (PDT) [167]. PDT produces ROS which induce cellular damage and cell death. PTT increases blood flow which enables more oxygen to reach the tumour site, improving the efficacy of PDT. PDT/PPT increases oxygen supply and elevated ROS production, while the incorporation of PNIPAM allows for precise temperature-controlled drug release [168]. The incorporation of nanogels enhanced the bioavailability of the drug and controlled release. The nanogels generated heat and reactive oxygen when irradiated by NIR enhancing cell uptake and killing cancer cells. In vitro experiments that involved using HeLa cells showed that results of the absorption of drugs enhanced, and the anti tumour impacts increased [167]. This is the reason that advances in nano-DDSs encourage the treatment of bladder cancer. A paper described a new nanomaterial called GNPS@PNIPAM-POPC-DOX (GPPD) that was designed specifically for the treatment of bladder cancer. GPPD combines linear AuNPs, thermosensitive PNIPAM and DOX-loaded POPC. It has complimentary actions, both PTT and chemotherapy. Using GNPs’ photothermal effect under NIR lighting, the system tailored the generation of heat in the site containing drug–PNIPAM conjugate [169]. The study aims to conduct research on the application of starlike copolymers referred to as D-g-PNIPAM as nanocarriers for chemotherapy medications cisplatin (Cis-Pt) as well as DOX. These copolymers which had Dextran core and PNIPAM grafts improved cytotoxicity of both drugs on both sensitive and resistant cancer cells. Among all the synthesized copolymers, D6-g-PNIPAM copolymers have better drug delivery profile due to their rod-like molecular structure which minimize the chance of aggregation. Figure 5 depicts tumour-targeted DOX distribution by PNIPAM NPs coupled with FA. Injected intravenously, these NPs use the EPR effect to aggregate in the tumour. FA receptors on tumour cells control their absorption. NIR laser light elevates the temperature above PNIPAM’s LCST, causing DOX to be released and inducing tumour cell apoptosis, effectively curing cancer [170]. The researchers developed nanosystems with the help of temperature-sensitive copolymer PNIPAM and magnetic material to deliver 5-FU and oxaliplatin (OXA) for colon cancer therapy effectively. They synthesized magnetic NPs of Fe3O4 and have functionalized with various comonomers. Fe3O4 is used as the magnetic core providing the system with magnetic properties allowing the microgels to be manipulated by external magnetic fields, which is particularly useful for targeted drug delivery. Then, they coated the above-said NPs with the temperature-responsive polymer PNIPAM. In their experiments concerning the colon cancer cells, the researchers noted that the nanoformulations (NFs) greatly hampered cell division. However, these nanosystems was responsive to temperature variation, and this upregulated the releases of the drugs when the temperature tends to rise [171]. Researchers have come up with a new strategy to synthesize a DDS where they named it as D-PPy@PNAs. This system involves conjugating chemotherapy with PTT to improve the cancer’s treatment process. They employed such a thermosensitive polymer and referred to it as poly(acrylic acid-b-N-isopropyl amide-b-acrylic acid) PNA to enhance the formation of polypyrrole (PPy) during its production. This has led to the synthesis of new injectable hydrogel that can respond to changes in temperature, and it has great photothermal property. This hydrogel can have the ability to encapsulate the DOX, and the drug can be released in the system for longer time period. When NIR laser light is applied on the hydrogel, the DOX uptake is enhanced by the cancer cells while increasing the penetration into the tumour [172]. Dextran sulphate (DS) can be grafted with NIPAM. Depending on the temperature, and these modified DS molecules; they can form small particles that can be used in drug delivery. Among them, a certain copolymer, DS-g-3PNIPAm, was stable in water and selectively bound to leukaemia cells but had no toxicity to normal cells. These were then used to capture methotrexate (MTX), enhance its solubility and reduce side effects associated with chemotherapy while enhancing the drug’s efficacy. Comparing with different carriers for MTX, it can be observed that DS-g-PNIPAM had better performance in releasing 79% of MTX within 48 h and had a great prospect in eliminating cancer cells [173].
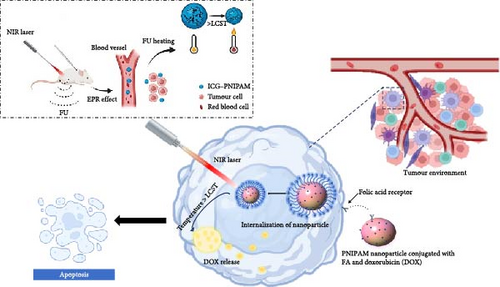
5.2. PTT
PTT is among the modern treatment methods in which photothermal agent (PTAs) are used to create localized hyperthermia through the conversion of irradiated NIR light into heat. This method is well applicable in treating different diseases and undesirable thermal impact to the adjacent healthy tissue. Compared with other hyperthermia techniques, more effective temperature management is available with PTT; thus, it is a noninvasive treatment with decreased risk for patients [174]. A promising strategy with regard to cancer treatment with inorganic nanomaterials is that the latter are functionalized with ligands selective to tumour cells [175]. Among different kinds of nanomaterials, copper sulphine (CuS) NPs are reported to be especially effective agents for the targeted PTT [176]. In the course of PTT, these NPs have been designed to trap the chosen tumour antigens and work as signals to the immune system in order to effectively fight the tumour [177]. Coadministrating the molecules AAc, PPy NPs and FA with PNIPAM also amplifies the effectiveness of PTT. AAc controls the stability of the nanocarriers, thus increasing LCST to 42°C, and PPy NPs enhances the photothermal efficiency and cancer cell killing [178]. There was a thermosensitive hydrogel that was synthesized from PNIPAM and starch, and it had shown sol–gel transition that is occasioned by temperature differences. The thermoresponsive behaviour of PNIPAM enhanced the value of the hydrogel’s photothermal property and the observed high cytotoxicity under the NIR laser. The phase transition at the near body temperature of PNIPAM improved the enhanced photothermal effect for the concentration of the ’iodine–starch’ complex within the hydrogel [179]. The application of the PNIPAM dendrimers has been used as the link between the PSs and the AuNPs that provide better plasmonic photosensitization and the internalization of the cells. Improvement in cancer cell killing is achieved in this strategy, especially in combined PTT–PDT [180]. Similarly, PNIPAM grafted copper sulphide NPs have also exhibited results like the controlled release of copper ions, efficient ability of converting light into heat and bacterial trapping, as well as the elimination of bacteria growth for treating infected skin and promoting wound healing [181]. Amongst all the nanogel systems, PNIPAM/GO nanogels have come closer to meeting the criteria required for a controlled drug-releasing system for cancer therapy. These nanogels harness the features that are associated with large surface area and targeted delivery capabilities of GO. Based on the good interaction between PNIPAM and GO, these nanogels have a small size compared to PNIPAM. They have revealed mild toxicity to the cells after the first 24 h of exposure, but the cell viability was restored after 48 h. These nanogels are capable of providing a sustained and localized drug release and localized heat generation for cancer theranostic applications. Nevertheless, acquired GO in the nanogels is less suitable for the PTT compared to RGO. Furthermore, integrating the photothermal properties reveals that the selection of the comonomer can effect a change. The DOX-modified nanogel composites can become the therapeutic agents for future cancer treatments [182]. A new photoluminescent amphiphilic block copolymer has been newly prepared by the researchers, and it is called as PVB-T/I. Specifically, P2 block copolymer includes poly(N-isopropyl acrylamide) T/I-PVB, and it was examined for micelle formation. The formed micelles were able to emit blue fluorescence resulting to charge transfer in the T and I group. Photoluminescence was also influenced by pH or metal ions such as Pd2+ as the latter showed a significant quenching ability polymer micelles acted as optical sensors for Pd(II) ions; thus, the application of the micelles can be extended to analytical purposes, and core–shell micelles were shown to be sensitive to light, heat, pH and metal cations from a block copolymer [183]. Scientists prepared a complex by conjugating PNIPAM to glycyrrhizin receptors or glucocorticoid receptors (GRs) and incorporating anticancer drug DOX. This complex facilitated the controlled release of said drugs by exposure to NIR laser. They used cancer cells to perform cancer research; the complex did release the drug and when placed under laser had fairly good anticancer activity [184].
5.3. PDT
New star-shaped tetra-hydroxy-phenyl porphyrin (THPP)-cored double hydrophilic-b-poly(methyl acrylamide glucose) block copolymer was prepared, and its response to thermal stimuli was investigated. These copolymers were synthesized by RAFT polymerization and self-assemble micelles above 37°C. These micelles can be utilized to incorporate antitumour drug for their release in proximity to tumour cells. While the copolymers show high toxicity under light irradiation, the toxicity is relatively low when no light is applied; therefore, the compounds possess the potential of the PDT PS. Copolymers can thus be concluded to be biocompatible more so and highly phototoxic to HeLa cells according to in vitro investigations and hence the bright prospects for application. Also, the copolymers can selectively bind to concanavalin A (Con A) that makes active targeting for drug delivery [185]. Four star-shaped copolymers with thermosensitivity, THPP-(PNIPAM-b-poly(oligo(ethylene glycol) methyl ether methacrylate) [POEGMA])4, grow from a tetra-entate 5,10,15,20-tetrakis-(4-hydroxyphenyl)-21H,23H-porphyrin (THPP) core and four arms of PNIPAM. It is apparent that the LCSTs of THPP-(PNIPAM-b-POEGMA)4 are quite low, although they depend on the molecular weight: 37.5°C, 39.9°C and 41. In all cases, the LCST values ranged from 9 to 21°C, with the lower values corresponding to polymer samples with higher hydrophilic POEGMA content of the copolymers. The copolymers with the values of temperature above the LCSTs could form the micelles of different types. Methylthiazolyldiphenyl-tetrazolium bromide (MTT) investigations showed that THPP-(PNIPAM-b-POEGMA)4 and THPP do not possess lethality to HeLa and L929 cells. In addition, THPP- (PNIPAM-b-POEGMA)4 possessed photodynamic activity under light irradiation, resulting in higher phototoxicity to HeLa cells than THPP [186]. Nowadays, the multifunctional NFs are used to enhance the efficacy of the PDT and assess the biodistribution of the PSs. These NFs conjugate PS with NIR fluorescent organic dyes (NIRFDs) encapsulated in polymeric NPs having a bicoated architecture. Specifically, the polymeric shell of the particles is synthesized from poly(NIPAM-co-AA). The hydrophobic molecules are kept apart in water, fluorescence is minimized, singlet oxygen is controlled, and thus, PDT effectiveness is achieved. The developed hierarchy of NFs reveals good possibilities for PDT that can be controlled using fluorescence imaging in visible and other spectral bands, including NIR-I and short-wave infrared (SWIR). Further studies on these NFs will seek to maximize the efficiency of the PDT therapy in a live organism [187]. The investigation aims at solving issues hindering cancer therapy through undergoing synthesis of thermoresponsive PNIPAM and CD hybrid nanogels. These nanogels are expected to be selectively enriched in tumour and, upon the treatment with deep-red-light, generate heat and ROS to eliminate cancer cells via PTT and PDT. Nanogels also have characteristics that promote their accumulation to cancer cells and easy elimination from the body, thus increasing its antitumour efficacy and decreasing side effects [188].
5.4. Gene Delivery and Therapy
A nonviral gene delivery system being formed from cationic block copolymer, where in a study, depicted high nuclease tolerance and resistance to protein absorption [189]. Another research work done involved a polymer known as poly(2-(dimethylamino)ethyl methacrylate-co-(cis-butenedioic anhydride-poly((N-isopropylacrylamide)-co-(butyl methacrylate)))) which is abbreviated as PDMNIB. This polymer had thermosensitive characteristics and had a specific action with DNA for enhancing the transfection of genes [190]. There is especially promising news that therapies based on something called small interfering ribonucleic acid (siRNA) can be used to treat cancer. Research indicates they home in on and suppress cancer-promoting genes, thereby reducing or stopping cancer progress without compromising the normal cells [191]. In a study, siRNAs were administered with the help of thermoresponsive block copolymers that has NIPAM element. A study made on a human fibrosarcoma cell line stated that system’s efficiency was dependent on the polymer’s cloud point, which is the temperature at which aggregation or precipitation is more preferred to solubilization [192]. Investigation of PnBA-b-PNIPAM-b-PDMAEA terpolymers and quaternized PnBA-b-PNIPAM-b-PDMAEA, the polyplex formation with DNA, was investigated. Temperature changes altered the properties of polyplexes in quaternized and nonquaternized terpolymers desirably. Moreover, in the case of terpolymers, there was a possibility of their use as drug delivery agents as curcumin (Cur) solubility and fluorescence intensity at temperature for bioimaging purposes were improved by the said terpolymers [193]. On a similar note, other research employed PNIPAM nanogels for the enhancement of clinical gene delivery through nonviral vectors for the delivery of DNA. Various properties of nanogels were assessed including their efficiency for DNA condensation, protection of DNA from degradation and their potential for intracellular delivery and endosomal release. Altogether, it was noted that biodegradable dendritic polymers (NGs-BDD) was more effective in endosomal escape and gene transfection in HEK293T cells irrespective of pH. Aliphatic chains of sufficient length in NGs-BDD were claimed to be responsible for their membrane perturbing effects [194]. An injectable drug/gene delivery system was developed with active targeting and pH-sensitive GO drug delivery characteristics for the treatment of glioblastoma multiforme (GBM). It was developed to create a hydrogel for a site-specific extended drug release of therapeutics [195]. Appropriate polymeric NPs were synthesized to transfect siRNA specific to PLK1 to the cancer cells since Polo-like-kinase 1 (PLK1) is a protein kinase that is overexpressed in cancer cells. The obtained nanogels had good loading capacity for siRNA and thermoresponsive properties, while PNIPAM-co-NIPAm nanogels were able to retain siRNA better than PNIPAM-co-AA nanogels, both nanogels were biocompatible, and they were internalized through endocytosis pathway. Moreover, PNIPAM-co-NIPAm nanogels possessed enhancement in endosomal escape capacity and higher silencing efficiency to PLK1 without affecting the off-target genes. The results of this study emphasize the need to establish how these cells interact with the material for the enhancement of siRNA delivery and potential future cancer treatment [196]. To enhance the stability and the tumour-targeting ability of the mRNA delivery vehicles, the mRNA was conjugated with cRGD-PEG-PLys (thiol) and PNIPAM-PLys (thiol), yielding the formation of an ionic complex core that has a redox-responsive disulfide linker. This eliminates biological degradation of mRNA through cross-linked structure noticed in this nanocarrier. PNIPAM helps in circulation by preventing nucleases such as RNase degrading the nucleic acids, and since it is thermosensitive, it becomes more hydrophobic at temperature without compromising on the circulation time a particular agent needs. When cRGD ligand is incorporated, cellular internalization and endosome trafficking improves, thereby increasing the tumour retention and gene transduction [197]. Thermosensitive block copolymers (BCPs) prepared from PNIPAM and poly(2- (diethylamino)ethyl methacrylate) (PDMAEMA) were investigated as the carrier systems for DNA. These BCPs formed self-assembled NPs at a critical temperature, which compacted the DNA into spherical particles, approximately between 200 and 300 nm in diameter, which are perfect for transfection. It was observed that the copolymers and DNA were capable to interact with each other based on the temperature; the loose complexes were identified at the temperatures below critical temperature; however, after the collapse of PNIPAM chains in the aqueous medium, the complexes become tight at temperatures above critical temperature. That is why it can be supposed that the thermosensitive polymers could help to compact the DNA into NPs of nanosizes [198]. The study synthesized two kinds of temperature-sensitive liposomal siRNA carriers through the introduction of different polymers. The PNIPAM-co-DMAPAM-modified liposomes have shown enhanced siRNA transfection compared to PNIPAM-co-DMAM-modified liposomes with less cytotoxicity. Using cell viability assays, it was found that thermoresponsive liposomes had better cell viability than the nonmodified liposomes as well as Lipofectamine RNAiMax. In the same study, the downregulation of vascular endothelial growth factor (VEGF) using PNIPAM-co-DMAPAM-modified liposomes in HeLa cells was also explored whereby the suppression of the same was achieved at 42°C as compared with 37°C [199]. Various thermally sensitive microgels regarding charge and hydrophilicity of the surface and functional groups on it were synthesized to design an optimal environment for cardiac stromal cell (CSCs) for the treatment of acute myocardial infarction (MI) [200]. A new strategy of synthesizing hybrid microcapsules of bovine serum albumin (BSA), metal ion clusters and PNIPAM nanoconjugates was explained. Hybrid block BSA–AuNCs–PNIPAM has desirable photostability [201] to deliver biotherapeutics to the target diseases’ affected tissues, minimally affecting the other healthy tissues through ultraviolet (UV) photopolymerization for the fabrication of the nanogels, which are efficient and safe as they employ bioactive materials and are applicable in broad fields such as oncology and gene delivery [202].
6. PNIPAM in Advanced Imaging
6.1. PNIPAM-Based Contrast Agents
PNIPAM and ICG NPs are found to be the most suitable contrast enhancing agents for biomedical fluorescence imaging microscopy and a 2D and 3D analysis. These NPs are stable in biological milieu and sensitive to temperature changes; the release of drugs is used to improve contrast in imaging. The relative chemical stability and longevity in storage, stability in biological environments and the confirmed selectivity and binding affinity towards murine breast tumour and spleen make them ideal candidates for potential targeted use in diagnostics and therapies in cancer treatment [203]. The size of PNIPAM NPs is determined in the tumour targeting and biomarker formation processes. ICG-PNIPAM NPs had much scattering and were cleared through the kidneys in the 2-h period postinjection. The results revealed that the hydrodynamic size of PNIPAM NPs is dependent on the SDS concentration and the viscosity of the solution decreases with the increase in the amount of SDS. A study on tumour sizes showed that the tumour-to-normal tissue ratio was constantly rising over 24 h for all malignant sizes, indicating the improvement in the targeting of NIR fluorescence in tumour [204]. ICG-PNIPAM has issues such as cytotoxicity and the shift of the excitation and emission wavelengths [205]. When employing the PNIPAM nanogels with optical coherence tomography (OCT), for 3D optical coherence thermometry in biomedical image, the sensitivity is increased, and molecular imaging becomes possible. They showed temperature sensitivity at their LCST, and due to this characteristic, they can be used for thermal applications in OCT to obtain 3D thermal maps of tissues. They have shielded cytotoxicity, and therefore, they can be categorized as Rayleigh scattering units that are nonabsorbing in biomedical purposes. The merge of OCT with PNIPAM nanogels is utilized to monitor temperature in tissue-simulating phantoms and model systems. This exemplifies their capacity for clinical implementations like the tracking of thermal impacts to tissues within the body, for example, laser-induced brain hyperthermia [206]. It has also been established that ultralow-cross-linked (ULC) PNIPAM microgel particles contain the ability to be deformed with the help of an ultrasound stimulation depending on the frequency, amplitude and particle concentration [207]. Manganese-doped ferric oxide NPs, Gd3+ chelates and PNIPAM were used to synthesize a nanoscale system. The structure incorporated a magnetite NP doped for T2 contrast and Gd chelates for T1 contrast. The design is triggered on the thermoresponsive characteristic of PNIPAM that can collapse to as low as 5% of its volume when the temperature of the external environment are raised from 20 to 40°C. This change in the size of the PNIPAM shell affects the spatial relationship between the paramagnetic and superparamagnetic components of the nanoplatform and optimizes the toggling of the T1 and T2 MRI signals, hence increasing the MRI contrast [208]. Hypoxia-sensitive SPION–PNIPAM NP formation is considered useful in the future pharmaceutical and MRI contrast media production. These NPs have calibration efficiency for drug, pH and temperature sensitivity along with biocompatibility. They can release drugs such as DOX at a faster rate under certain circumstances which augurs well for drugs delivery at the tumour site. The NPs have the property of contrast agents in MRI imaging and hence have role in being a theranostic agent. Cellular studies have shown that the NPs containing DOX can lead to DNA damage and induce apoptosis in cancerous cells [52]. GNRs and CuS nanospheres were employed in designing of photoacoustic contrast agents. GNRs have large optical cross-section and own progressive absorption peaks. Yet, they can undergo the change of absorption due to plasmonic coupling. To attend to this, core nanoparticles (CNs) were encapsulated in PNIPAM nanogels to fabricate dynamic agents based on contrasting densities. The study also identified two principal phenomena of NP clustering and nanogel deswelling which include clustering enhancement and Hoberman enhancement; the study concluded that PNIPAM-AuNR solution photosensitivities far exceeded that of pure AuNR solutions. The ex vivo animal model of the study also confirmed the high photoacoustic signals of PNIPAM-CuS nanoconstructs [209]. The method to synthesize barium sulphate (BaSO4) in situ within PNIPAM is microfluidic droplet techniques. With this innovative technique, functionalized microgels with BaSO4 nanocrystallites can be achieved, which increase the radiopacity of the microgels when exposed to X-ray radiation for the purposes of diagnosis. Due to the homogeneous distribution of BaSO4 nanocrystallites in the microgels, these materials can be used in different fields of biomedical purposes such as endovascular embolization and visible implantation materials [210].
6.2. PNIPAM-Based Fluorescent Probes
The thermoresponsive property of PNIPAM enables temperature-dependent control and detection, making PNIPAM ideal for developing fluorescent probes, and its biocompatibility makes it suitable for medical diagnostic applications. Functionalization with phenylboronic acids, crown ethers and pH-responsive elements further expands its ability to detect various stimuli [211]. PNIPAM-co-glycidyl methacrylate (GMA)-Lys hydrogel modified with fluorescein isothiocyanate (FITC) produce green fluorescence in MDA-MB-231 cells with increasing concentration indicating cell internalization making hydrogels potential for bioimaging applications. The hydrogel’s exhibited dual pH- and temperature-responsive drug release, with high encapsulation efficiency and complete drug release at 45°C/pH 4.0. Cytocompatibility tests confirmed the hydrogel’s great potential for drug delivery [212]. Fluorescent potassium ion (K+) sensor was named as P2 that has been synthesized by incorporating PNIPAM and a small-molecule K⁺ fluorescent sensor called KS. P2 alters size between temperatures of 35 and 42°C, and for this transition, an LCST was established in 38°C. This transition affects the nanostructures of the polymer as well as the KS microenvironment leading to proportional increase or decrease in the fluorescence intensity of P2 as well as the response to K+ ions. The level of selectivity and sensitivity P2 is very high regarding K+ ions; the response ranges from 1 to 20 mM which makes it possible to investigate extracellular K+ levels. When the bacteria Escherichia coli and B. subtilis 168 were treated with lysozyme, the effects on K+ concentration were measured using the sensor in a 96-well plate high-throughput format. In the same study, it was established that the release patterns of the K+ ions were cell species-dependent [213]. Research was carried out to produce NPs known as poly(3-octylthiophene-2,5-diyl) (P3OT) nanoaggregate-embedded PNIPAM nanogel composites (POPNs) from the combination of PNIPAM and a fluorescent polymer, P3OT, in order to measure the change in fluorescence with respect to variations in temperature. The amount of P3OT that can be incorporated can be controlled by altering the concentration of the PNIPAM network. It is possible to see that these POPNs increase greatly the fluorescence intensity at 33°C and above, probably due to the changes in the environment of the P3OT chains. This characteristic that depends on temperature reveals that POPNs could be beneficial in biomedical applications such as imaging and sensing [214]. Likewise, it is relevant to mention that silicon nanocrystals (Si NCs) are quantum dots that can have tuneable fluorescence and are biocompatible. Those conjugated with FA for better recognition of cancer cells and encapsulated in PNIPAM nanospheres with the temperature-sensitive feature. The resulting terminal structure of Si NCs–FA–PNIPAM shows thermosensitive fluorescent property. If Si NCs–FA was incorporated into the PNIPAM network through the free radical copolymerization at different temperatures, the biocompatible nanospheres might have been formed for biomedical uses. These nanospheres could be temperature-sensitive fluorescent probes used for identification and tracking of cancer cells [72]. An analysis of the results of fluorescence microscopy provided evidence of Cur drug treatment’s impact on cell death. To distinguish between the live and dead cells, hepG2 cells were double-stained with acridine orange which has green fluorescence and propidium iodide which has red fluorescence. The images depicted that cells with only PNIPAM-co-PAAm hydrogel appeared green with very intense fluorescence suggested that the cells were mainly alive. However, the cells treated with Cur-loaded PNIPAM-co-PAAm@Cur hydrogel were red which demonstrated that approximately 90% of the cells were dead [215]. The process of preparing a new luminescent diblock copolymer, PNIPAM (MAh-4)-b-P4VP, otherwise known as PN4P, has been analysed to show various pH and thermoresponse behaviours that come with dual emission-dependent enhanced fluorescence (DDED) and aggregation-induced emission (AIE). PN4P is able to self-assemble into micelles in the aqueous state and has fully reversible fluorescence transition from pH 2–11 and temperature from 28 to 40°C. To be specific, pH and temperature sensitivity of hydrophobic–hydrophilic balance in the micelles forms the core–shell inversion combined with tuneable emission property. This work focuses on AIE as the primary process responsible for altering these fluorescence values and positions PN4P as a good candidate for biosensing and biomedicine applications [216]. PNIPAM microgels with both core–shell and dense-core structures are synthesized and characterized for the purpose of the study. The process involves the polymerization of NIPAM monomers containing fluorescent and cross-linking agents under nitrogen atmosphere and then later washing, freeze-drying and then storing in a desiccator. The microgels are characterized using a light and confocal microscopy for the visualization of the fluorescence in swollen and collapsed microgels. This technique offers a better understanding of the microgels properties and its uses in pharmaceutical and biomedical field [217].
7. PNIPAM in Tissue Engineering
7.1. PNIPAM-Based Scaffolds for Tissue Regeneration
Tissue engineering is a captivating field of biomedical engineering that involves the creation of functional human tissues through the use of cells, biomaterials and biochemical cues [218]. The main objective of tissue engineering is to develop novel therapies for various diseases and injuries, such as heart disease, bone fractures and spinal cord injuries. Scaffolds are crucial in tissue engineering as they guide cell behaviour and promote tissue regeneration. Their functions include transporting seeded cells to specific locations, promoting cell adhesion and interaction with materials and transporting nutrients and factors to support cell viability and growth, as well as specialization. As the tissue regenerates, the scaffold gradually degrades. PNIPAM holds significant promise in tissue engineering due to its tuneable properties as it provides adhesive surface for cell attachment and proliferation above LCST and subsequent cell detachment when temperature is lowered below LCST [37]. Chemical methods create PNIPAM using initiators or catalysts, but electrochemical polymerization (ECP) directly oxidizes NIPAM at the anode without the need for such additives. This disparity makes ECP-produced hydrogels more suitable for tissue engineering applications in biological systems. The physical properties of these hydrogels can regulate nutrient and waste flow to affect cell growth [219]. A stimuli-responsive gel is developed by combining C6-OH allyl-modified CS (OALCS) and PNIPAM, which undergoes a structural change at around 32°C. The gel can be formed into a solid shape by adding a photo initiator and using UV light. It can change its size when factors like pH, temperature and the amount of OALCS and PNIPAM are changed [220]. The study was centred on establishing new sorts of hydrogels that are biodegradable and in addition to that have sensitivity towards temperature. These hydrogels referred to as thermoresponsive biodegradable hydrogels (TBHs) were formulated from NIPAM, poly(ε-caprolactone)dimethacrylate (PCLDMA) and bis(acryloyl)cystamine (BACy). It was identified that the properties of the TBHs were sensitive to the temperature and the ratio of PCLDMA to BACy. Hydrogels were capable of swapping a drug over time by liberating it at a slow pace when it was heated and quickly when cooled. Also, it was demonstrated that TBHs slowly deteriorated in GSH under basal conditions. Hydrogels have been reported to have regular pore-sized interconnected and degradable network. These hydrogels are also stimuli-sensitive to temperature change which make them useful in tissue engineering scaffolds [221]. Osmanthus fragrans extracts can enhance bone formation, and they are involved in autophagy during this process as indicated in the study. By applying the analysis, it was possible to pinpoint 11 genes, associated with osteogenesis, autophagy and O. fragrans at the same time. Furthermore, of these genes, the top three proteins as per the degree value returned were TP53, CASP3 and SIRT1. In this research, autophagy was investigated in relation to the differentiation of osteoblasts using the autophagy inhibitor, 3-MA. The findings established that with the inclusion of 3-MA, ALP activity as well as mineralized nodule formation was suppressed showing that autophagy inhibition counteracted the osteopromoting potential of the OF/NIPAAM hydrogels. Therefore, this study demonstrates the facade that Osmanthus fragrans extracts, as well as OF/NIPAAM hydrogels, can effectively support osteogenic differentiation, possibly through TP53 and autophagy [222]. Chicken eggshell membranes (ESMs) are applied in research to form a scaffold for a wound and to regenerate tissues. The ESMs have been functionalized through grafting of a thermosensitive polymer and also the dispersion of silver NPs (AgNPs) to cause controlled drug releases. ESM-PNIPAAm (AgNP) thus formed are biocompatible and have suitable cell attachment, cell spreading and cell proliferation. It is possible that alteration in ESM constructs can be used in defining wound dressings in clinical practice and as the sensitive structure material scaffolds in drug delivery applications for global health benefits in regenerative medicine [223].
7.2. Dentoalveolar Tissue Engineering
CS is blended with PNIPAM to obtain 3D printable hydrogels for dentoalveolar tissue regeneration. In this work, using the sol–gel synthesis process, new hydrogels based on PNIPAM and CS with N,N′-methylenebisacrylamide (MBA) as a cross-linking agent were synthesized to obtain materials having important characteristics necessary for tissue engineering. These characteristics have high swellablity, biodegradability and big pore size by which the scaffolds needed for the growth and viability of the DPSCs can be developed [224]. Figure 6 shows stem cell–based cartilage healing with PNIPAM hydrogels. Stem cells are identified and grown before being cultivated on 2D or porous 3D PNIPAM scaffolds infused with growth agents. These biodegradable hydrogels promote tissue regeneration and are appropriate for minimally invasive implantation, assisting in cartilage repair and maybe bone and heart tissue repair, hence improving recovery and functionality.
7.3. Scaffolds for Cartilage Regeneration
Articular cartilage is pliable; it has chondrocyte cells sourced and encased in the ECM. In conventional 2D, flat scaffold design, chondrocytes may lose significant proteins necessary for cartilage formation. However, in 3D design, chondrocytes do not dedifferentiate, hence making 3D design more preferable. Precise porosity design such as microporous structures (mean pore size was 300 µm) [225] has increased chondrogenic gene expression as compared to the nanoporous structure that forbids cell development [226]. Chondrocytes are difficult to retain in their chondrogenic phenotype especially when cultured in conventional 2D tissue culture dishes coated with tissue culture polystyrene (TCPS) for the primary reason for their low metabolic activity as well as vulnerability to dedifferentiation. Although there are supplements produced from growth factors that can help in redifferentiation, this procedure may not always be effective [227]. Possible strategies for cartilage repair are made available in the thermosensitive PNIPAM/HA copolymer and blending cross-linked HA with triblock copolymers forms a hydrogel that is conducive for the deposition of chondrocytes [228]. Copolymers formed by PNIPAM-grafted glycosaminoglycan that is thermo- and pH-sensitive shows sustained released of dexamethasone phosphate with no sign of toxicity to ocular cells; therefore, it can be used as a drug carrier [229]. PNIPAM hydrogels exhibit hydrophobicity above their LCST, aiding in cell adhesion and division, but cause cell detachment below LCST. PNIPAM-co-AAc hydrogels enhance chondrocyte proliferation and maintain their phenotype while supporting chondrogenesis when combined with growth factors. Despite these benefits, concerns about biocompatibility and immunogenicity persist, necessitating further in vivo studies. PNIPAM-based formulations, like PNIPAM (PEG-b-PNIPAM), offer versatility for encapsulating mesenchymal stem cells. Bioabsorbable thermoresponsive hydrogels, such as those made with PNIPAM, MAPLA and HEMA, show promise due to their lack of cytotoxicity, improved mechanical properties and gradual resorption, making them suitable for various biomedical applications [37]. Also, chondrocyte-derived exosomes incorporated in an injectable hydrogel, formed by cross-linking of hyaluronic acid (HA) and Pluronic F-127, can afford damaged site by chondrocyte-derived exosomes and prevent cartilage destruction in osteoarthritis by controlled release [230]. HA-modified thermosensitive PNIPAM hydrogels promote the chondrogenic differentiation of rabbit adipose–derived stem cells (rADSCs). Both HA-modified hydrogels enhance cell survival, the expression of chondrogenic marker genes and sGAG synthesis in comparison with unmodified PNIPAM. Of them, HA-PNIPAM-CL exhibits the highest efficiency in promoting rADSC chondrogenic differentiation both in vitro and in vivo, which indicates its possibility for articular cartilage repair [231]. The effect of PNIPAM hydrogels was studied by making modifications in mechanical properties and porosity to check its effect on cell viability and differentiation. In comparison to poly(N-tert-butyl acrylamide) (PNTBAM) hydrogels, PNIPAM hydrogels showed greater porosity resulting in higher mineralization and better support for chondrogenic differentiation and found suitable for 3D osteochondral scaffolds in tissue engineering [232].
7.4. Cardiac Regeneration
PNIPAM NPs effectively reduces cell death by delivering small and macromolecule drugs to skeletal myogenic cells, suggesting its potential for cardiac disease treatment by preventing cardiac cell apoptosis and thus showcase promise for tissue engineering [94].
7.5. IVD Degeneration
The intervertebral disc as a structural and functional model keeps apoptosis and anabolism in the tissue in check. During degeneration, there is qualification in NP cell behaviour that enhances more catabolic tendency possibly due to increased cytokine synthesis, changed matrix quality and turnover, better cell death and senescence and ingress of nerves and blood vessels [233]. Table 4 shows the adaptability of PNIPAM in regenerative medicine and tissue engineering. Hydrogels for cartilage and bone regeneration, DDSs for ophthalmic treatments and cytocompatible scaffolds for tissue engineering, such as bone and cartilage, are examples of varied biomedical applications.
| Polymer formulation | Method of preparation | Application | Reference |
|---|---|---|---|
|
Copolymerization method | Adipose-derived stem cell (cartilage regeneration in rabbit) | [231] |
| HA-PNIPAAm-CP hydrogel | Lyophilization | Adipose-derived stem cell (cartilage regeneration in rabbit) | [231] |
| (PNIPAM-co-DMAc) hydrogel | Cross-linked | IVD degeneration (low back pain) | [233] |
| PNIPAM and polyethylene glycol (PEG) | Copolymerization method | Encapsulation of mesenchymal stem cells is used in the process of tissue engineering for the creation of cartilage tissue | [224] |
| PNIPAM-HAp scaffolds | Electrochemical polymerization | Engineering of bone tissue, with a focus on maintaining the viability of MG63 cells for the controlled release of antibiotics | [219] |
| OAL–CS/PNIPAM hydrogel | RAFT polymerization | Hydrogel exhibits no cytotoxicity towards human bone marrow mesenchymal stem cells. Additionally, histological analysis indicates no signs of inflammation in subcutaneous tissue after 5 days of injection in vivo | [220] |
| PNIPAM-grafted-CHI | Layer-by-layer technique (chemical cross-linking) | Chitosan has been modified with PNIPAM moieties to create PCHI with a cloud point of 31°C. The PNIPAM-modified multilayers have improved cell adhesion with murine stem cells. Cross-linked PNIPAM-modified multilayers are cytocompatible for tissue engineering applications | [234] |
| Hep-g-PNIPAM, CS-g-PNIPAM | RAFT polymerization | The low critical solution temperatures were affected by temperature, pH and polymer concentration. To achieve sharp phase transitions in sensitive nanogels, the terminal dodecyl trithiocarbonate groups in copolymers were converted to thiols. These copolymers are a viable option for ocular drug delivery applications as they enable sustained release of dexamethasone phosphate at 37°C | [229] |
- Abbreviations: CHI, chitosan; CL, caprolactone (often refers to ε-caprolactone, a cyclic ester used in polymer chemistry); co-DMAc, copolymers of N,N-dimethylacetamide; CP, chitosan–PVA composite; CS, chitosan; CS-g, chitosan-grafted; HA, hyaluronic acid; HAp, hydroxyapatite; HEP-g, heparin-grafted; OAL, octanoyl lactic acid; PCHI, poly(chitosan); PNIPAAm, poly(N-isopropylacrylamide); RAFT, reversible addition–fragmentation chain transfer.
8. Future Directions
The current biomedical study utilizes the polymer PNIPAM temperature-sensitive characteristic. In DDS, PNIPAM-based hydrogels are being employed for the enhancement in the bioavailability of the drug and its extended release [235], which can be highly useful in treating patients with dry eye disease [236], oral ulcers [1] or cancer [66]. PNIPAM hydrogels reveal stimuli sensitivity in terms of temperature, pH and ROS and antibacterial activity in wound healing [237]. CS thermogels, along with 3D-printed scaffolds embedded with antimicrobial agents, promote blood clotting and inhibit bacterial growth to enhance proper wound healing [238]. In the field of bioseparation and bioanalysis, several studies have reported the proficient separation of proteins and cells by employing PNIPAM-based system’s retention and elution characteristics depending on temperature [239, 240]. New diagnostic tools use PNIPAM in conjunction with plasmonic NPs and Raman spectroscopy for the noninvasive identification and monitoring of implants and prostheses, which improve specificity in bioimaging and detection of biomarkers [241]. Moreover, many biomedical devices containing PNIPAM for heater–cooler, smart DDS, wound healing and diagnostic fields are being developed to expand the PNIPAM’s role and capabilities in biomedicine.
The potential of PNIPAM for biomedical applications is in the next generations of Smart Material for Precision Medicine. Scientists are now working on the combination of PNIPAM with other sophisticated nanotechnologies that include CRISPR-Cas9 delivery systems, where, besides target drug delivery, there is also gene editing within the cancer cells. Another emerging prospect is about the development of supramolecular, stimuli-responsive platforms that is capable of reorganization of structure and functionality in response to physiological stimuli: PNIPAM-based systems capable of real-time response to more than one physiological signal, inclusive of pH, enzyme or biomarker signals in addition to temperature. In addition, the creation of novel biohybrid materials based on the incorporation of PNIPAM and living cells or biomorphic architectures may open up new prospects in regenerative medicine where synthetic tissues/organs that self-heal in response to the principles of tissue/organ regeneration in the human body might be developed. Rapid advancements in the ongoing research will entail applications of PNIPAM more than the traditional drug delivery domain to include programmable theranostic, personalized nanomedicine, complex tissue engineering and other novel fields which bring vanishingly close futuristic highly individualized adaptive healthcare. Table 5 describes the various applications of PNIPAM in personalized cancer treatment, such as targeted drug delivery to breast cancer cells, thermoresponsive NPs for controlled drug release and novel therapies such as chemophotothermal therapy and gene delivery for pancreatic cancer treatment.
| Application | Description | References |
|---|---|---|
| Targeted drug delivery | Poly(N-isopropylacrylamide) (PNIPAM)-based copolymer conjugates with doxorubicin (DOX) for targeted delivery to breast cancer cells | [66] |
| Thermoresponsive nanoparticles | Nanospheres containing PNIPAM@PAA for the purpose of releasing DOX in a controlled manner when exposed to low pH and increased temperatures | [98] |
| Chemophotothermal therapy | Nanocomposites based on PNIPAM for combined chemophotothermal therapy of cancer for synergistic effect | [178] |
| Triblock polymer | PDMAEMA-co-PNIPAM-co-PMPC is an innovative polymer blend designed for the targeted delivery of siKRAS as part of gene therapy for pancreatic cancer treatment | [242] |
| Three-dimensional (3D) tumour spheroid culture | PNIPAM hydrogel microwell arrays for high-throughput 3D cancer spheroid culture and drug screening | [69] |
| Theranostic nanoparticles | Nanoparticles based on PNIPAM that encapsulate both imaging agents and chemotherapeutics for combined diagnosis and treatment | [243] |
| Combination therapy | PNIPAM-based nanocarriers for combined delivery of chemotherapy and immunotherapy agents for enhanced anticancer effects | [244] |
| Tumour-penetrating peptides | PNIPAM-based nanoparticles modified with tumour-penetrating peptides to enhance tumour accumulation and facilitate drug delivery | [245] |
| Stem cell delivery | PNIPAM/HECS/t-GO hydrogel allows for precise control over encapsulated stem cell environments, enhancing cell viability and cytocompatibility and also enable temperature-tuned encapsulation and 3D culturing of human bone marrow mesenchymal stem cells (hBMSCs), promoting high cell viability | [246] |
| Cancer-associated fibroblast targeting | The inclusion of HA–PNIPAM into collagen I bioink enhanced HA retention, maintaining breast cancer characteristics and cancer-associated fibroblasts (CAF). By using this approach, potential toxicity was reduced, and consistency was improved, leading to better reproducibility of breast cancer models. The collagen–HA bioink enabled the simultaneous culture of breast tumour cells and CAFs, accurately replicating in vivo tumour structure and characteristics | [247] |
- Abbreviations: HA, hyaluronic acid; HECS, hydroxyethyl cellulose; PAA, polyacrylic acid; PDMAEMA, poly(2-(diethylamino)ethyl methacrylate); PMC, polymer-metal composite; siKRAS, small interfering RNA targeting KRAS; t-GO, thermally reduced graphene oxide.
9. Conclusion
This review highlights the utilization of PNIPAM in drug delivery and cancer therapeutics as an exciting interdisciplinary domain that can offer better treatment to the patient. The features of PNIPAM as a thermally sensitive polymer have permitted the establishment of this material as fundamental in the conception of new concepts of DDSs where the alteration of the temperature triggers the liberation of the drug. This characteristic is particularly attractive in cancer treatment, where it becomes possible to achieve greatly improved therapeutic index by focusing the delivery of chemotherapeutic agents to the tumour mass. For instance, in the case of drugs like PTX and etoposide, applying NPs based upon PNIPAM provide a long-lasting and targeted delivery so that the drugs can affect cancer cells without harming the healthy ones. The incorporation of targeting ligands on the other hand brings in specificity, and improved targeting of breast cancer tissues is illustrated by the use of the SILY peptide. This makes the treatment to be more effective apart from protecting the patient’s quality of life since it has less side effect on noncancerous cells. Furthermore, for emerging developments in the preparation of PNIPAM systems, new polymerization strategies have enhanced the stability, efficiency and the coproduct range of these systems. By incorporating materials like CNTs and CS, the mechanical properties of PNIPAM-based hydrogels and micelles have also improved over time and envisage applications in biomedical imaging and tissue engineering in addition to drug delivery. Furthermore, for veterinarian vaccines, PNIPAM microgels have been used. Moreover, the hydrogel microwell array applies to the capture of cancer cell spheroids, which all prove that the material can be widely used in many biomedical sectors. Not only does this help in improving the probability and success of treatments but it also helps in better simulation of diseases and high-throughput screenings. PNIPAM in other molecular imaging and medical diagnosis especially when conjugate with contrast and fluorescent agents enhanced the prospective of real-time monitoring and diagnosis and therefore integrate therapeutic and diagnostic approach called theranostic. These novel trends, however, are not devoid of certain limitations concerning the elucidation of the thermoresponsive PNIPAM systems stability and biocompatibility in animal models or their degradation kinetics for a more sustainable approach. However, these challenges can only be solved by maintaining a robust interdisciplinary relationship between chemists, biologists and clinicians to get the best out of PNIPAM in therapeutic applications. Further into the research, it will, therefore, be crucial to continue pairing novel materials with real medical needs to transform strategies for treating cancer and other diseases and providing patients with better, particular and versatile treatments.
Nomenclature
-
- 5-FU:
-
- 5-Fluorouracil
-
- ABTS:
-
- 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
-
- ATP:
-
- Adenosine triphosphate
-
- CCK-8:
-
- Cell Counting Kit-8
-
- CT:
-
- Computed tomography
-
- DAPI:
-
- 4′,6-Diamidino-2-phenylindole
-
- DLS:
-
- Dynamic light scattering
-
- DMEM:
-
- Dulbecco’s Modified Eagle Medium
-
- DMF:
-
- Dimethylformamide
-
- DNA:
-
- Deoxyribonucleic acid
-
- DOX:
-
- Doxorubicin
-
- DTA:
-
- Differential thermal analysis
-
- EC50:
-
- Effective concentration 50%
-
- EDC:
-
- 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide
-
- EDX:
-
- Energy dispersive X-ray spectroscopy
-
- EPR:
-
- Enhanced permeation and retention
-
- ESR:
-
- Electron spin resonance
-
- FBS:
-
- Fetal bovine serum
-
- FDA:
-
- Food and Drug Administration
-
- FTIR:
-
- Fourier transform infrared spectroscopy
-
- GMA:
-
- Glycidyl methacrylate
-
- GPC:
-
- Gel permeation chromatography
-
- GSH:
-
- Glutathione
-
- HDL:
-
- High-density lipoprotein
-
- HPLC:
-
- High-performance liquid chromatography
-
- HRP:
-
- Horseradish peroxidase
-
- HSA:
-
- Human serum albumin
-
- IC50:
-
- Inhibitory concentration 50%
-
- ICP-MS:
-
- Inductively coupled plasma mass spectrometry
-
- LC-MS:
-
- Liquid chromatography–mass spectrometry
-
- LCST:
-
- Lower critical solution temperature
-
- LDL:
-
- Low-density lipoprotein
-
- MRI:
-
- Magnetic resonance imaging
-
- MTT:
-
- Methylthiazolyldiphenyl-tetrazolium bromide
-
- MWCNT:
-
- Multiwalled carbon nanotube
-
- NHS:
-
- N-Hydroxysuccinimide
-
- NMR:
-
- Nuclear magnetic resonance
-
- NPs:
-
- Nanoparticles
-
- PAMAM:
-
- Polyamidoamine
-
- PBS:
-
- Phosphate-buffered saline
-
- PCL:
-
- Polycaprolactone
-
- PDT:
-
- Photodynamic therapy
-
- PEG:
-
- Polyethylene glycol
-
- PEGylation:
-
- Polyethylene glycol modification
-
- PGA:
-
- Poly(glycolic acid)
-
- PLGA:
-
- Poly(lactic-co-glycolic acid)
-
- PLLA:
-
- Poly(L-lactic acid)
-
- PNIPAM:
-
- Poly(N-isopropylacrylamide)
-
- PTT:
-
- Photothermal therapy
-
- PTX:
-
- Paclitaxel
-
- QCM:
-
- Quartz crystal microbalance
-
- QD:
-
- Quantum dot
-
- qRT-PCR:
-
- Quantitative real-time polymerase chain reaction
-
- RBC:
-
- Red blood cell
-
- RNA:
-
- Ribonucleic acid
-
- ROS:
-
- Reactive oxygen species
-
- SDS:
-
- Sodium dodecyl sulphate
-
- SEC:
-
- Size exclusion chromatography
-
- SEM:
-
- Scanning electron microscopy
-
- siRNA:
-
- Small interfering ribonucleic acid
-
- SMCC:
-
- Succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate
-
- TEM:
-
- Transmission electron microscopy
-
- TGA:
-
- Thermogravimetric analysis
-
- TLC:
-
- Thin-layer chromatography
-
- TPP:
-
- Triphenyl phosphate
-
- UV–Vis:
-
- Ultraviolet–visible spectroscopy
-
- VEGF:
-
- Vascular endothelial growth factor
-
- XPS:
-
- X-ray photoelectron spectroscopy.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Siddhi Throat, the first author, drafted the manuscript, conducted material preparation and performed data collection and analysis. Sankha Bhattacharya, the second author, respectively, contributed to the study’s conception and design, writing, editing and supervising. Sankha Bhattacharya reviewed and approved the final manuscript. All are responsible for the paper’s content and writing.
Funding
As this is a review article compilation, no funding was available for this work.
Acknowledgments
The authors express gratitude to Dr. R.S. Gaud, Advisor to the Chancellor, SVKM’s NMIMS Deemed-to-be University, for the outstanding research facilities and unwavering encouragement during this project.
Open Research
Data Availability Statement
This review article is based on previously published studies and does not involve the generation of new data. Therefore, no new datasets are available.




