Bioassay and RP-HPLC Method Development for the Analysis of Extracts and Herbal Products Containing Anthocleista nobilis
Abstract
Anthocleista nobilis is a common constituent in numerous conventional medications in West Africa. The stem bark of A. nobilis is known to contain brucine and is used to treat intestinal parasites, stomachaches, gonorrhoea, wounds, etc. The extensive use has led to high market value and adulteration of their herbal products. In this work, the antioxidant properties of extracts of A. nobilis are verified, and a validated RP-HPLC method is used to estimate the brucine content of extracts and products containing A. nobilis. Stem bark extracts from ethyl acetate, 96% v/v ethanol, 70% v/v ethanol, and water of A. nobilis were investigated for phytochemical content using standard methods and their antioxidant activity using DPPH and phosphomolybdenum assays. An RP-HPLC method was developed and validated using brucine as a reference standard. The optimized conditions of the developed RP-HPLC method include a Supelcosil C18 column with dimensions 150 × 4.6 mm and particle size of 3 μm, a mobile phase comprising of MeOH : 0.1% v/v HCOOH (65:35 %v/v ) which was injected at a flow rate of 0.8 mL/min, and an injection volume of 50 μL. The wavelength of detection was 274 nm. The developed method was validated as per ICH guidelines. The common phytochemicals among the various extracts were tannins and alkaloids. All extracts exhibited a reasonable antioxidant activity in the DPPH and phosphomolybdenum assays, with the ethanol extract recording the highest activity of 27.681 μg/mL and 696.7452 μg/g AAE, respectively. The content of brucine in the extracts was determined to be 0.0177–0.1259 × 10–3% w/v, whereas the herbal products tested had a content of 0.8950−2.5013 × 10–3% w/v. These levels were below the toxicity threshold of brucine. The developed method could be used for the routine quality control of A. nobilis extracts and formulations.
1. Introduction
Humans use plants to treat various infectious and noninfectious diseases, making them a reliable therapeutic source. They are a primary, proven, and reliable source of most therapeutic drugs used today. For example, quinine and artemisinin, derivatives from the cinchona and qinghaosu plants, remain relevant treatment options in malaria [1]. Antioxidants are chemicals that help the body get rid of free radicals. Taking in foods containing abundant antioxidants has immense health benefits [2]. The relatively low cost and health benefits associated with herbs and natural products have pushed over 70% of the global population into focusing on such products. Contrary to the idea that these products are safe, several studies have indicated that some natural products contain harmful substances, even in very low amounts. The toxicity index of plant and natural products has often been associated with large doses and the presence of chemical adulterants that are in themselves toxic.
Anthocleista nobilis G. Don is known as hororoho in Fante and awudifoakete or bontodi in Asante Twi. It is known to grow widely in West African countries. The decoction of its bark is used in Ghana and Nigeria as an alternative treatment for piles, worms, gastrointestinal disorders, microbial infections, constipation, and jaundice [3]. One of the known major constituents of A. nobilis is brucine, an indole alkaloid and potent pain relief and anti-inflammatory agent. It has also recently been reported as a possible antitumor agent [4, 5]. Despite its beneficial pharmacological profile, its therapeutic window is narrow, with a reported 50% lethal dose (LD50) of 50.10 mg/kg, making its clinical use delicate [6]. The content of this agent in therapeutic formulations must therefore be controlled.
Data on biological activity and phytochemical constituents of A. nobilis are available [2, 7], and these are useful for verification. However, information on quantitative analysis of constituents remains lacking. Data on the qualitative and quantitative analysis of constituents in such plants must be established to help manufacturers and regulatory bodies set the appropriate dosage limits while seeking to enjoy the therapeutic benefits. This study evaluated the antioxidant activity and developed an RP-HPLC method to quantify brucine, a chemical marker in extracts and herbal products of the stem bark of A. nobilis. This will verify some ethnopharmacological claims and more importantly provide a means of quality control of herbal medicinal products containing A. nobilis.
2. Experimental
2.1. Equipment and Reagents
Water bath (PRM 2850, Halo-Hollen, Denmark), analytical balance (WD140050809, Kern, Germany), rotary evaporator (Buchi Industries, Switzerland), analytical HPLC column (μBondapak, Waters, USA), ultrasonicator (Elmasonic S15, New York, USA), hammermill (Schutte-Buffalo, USA), and pH meter (ION 2700, Eutech Instruments) were used.
2.2. Plant Material and Herbal Product Collection
The fresh stem bark of Anthocleista nobilis was collected from Kumasi and was authenticated by Mr. Clifford Asare at the Department of Pharmacognosy. A sample was deposited at the department’s herbarium and assigned the voucher number KNUST/HM/ANB/2020/B045.
Five herbal products (HBS1–HBS5) were obtained and analyzed for the presence and content of brucine. The selection was based on the label inscription indicating the presence of the A. nobilis plant (bark).
2.3. Plant Material Processing
Foreign materials were removed, washed under running water, and allowed to dry at room temperature. The material was cut, and the pieces were then ground into a coarse powder with a custom-made mechanical grinder. The sample was kept in a paper envelope in a cool, dry place away from light until ready for use.
2.4. Plant Material Extraction
2.5. Characterization of Extracts
The extracts obtained were characterized by their phytochemical composition and antioxidant activity.
2.5.1. Phytochemical Screening
The coarse powdered stem bark was screened for phytochemical constituents following established protocols [8]. Details of the tests are provided in the supplementary data, M1.
2.5.2. Antioxidant Activity
Each sample level antioxidant activity was measured using the EC50 value, the concentration (μg/mL) necessary to inhibit DPPH radical production by 50%, as derived from the graph after plotting scavenging percentage against extract concentration.
(2) Total Antioxidant Capacity (TAC) Assay. It was evaluated with the technique proposed by Prieto et al. [9], with slight modifications in temperature.
0.3995 g of disodium hydrogen phosphate was dissolved in 100 mL distilled water to yield a 28 mM disodium hydrogen phosphate solution. A 0.6 M sulphuric acid was made by measuring 3.324 mL of sulphuric acid into a 100 mL volumetric flask and topped up with distilled water to make the mark. 4 mM ammonium molybdate was prepared by dissolving 0.4944 g of ammonium molybdate into 100 mL of distilled water. Concentrations (1000–31.25 μg/mL) of the plant extracts were prepared. Different ascorbic acid concentrations (100–0.781 μg/mL) were used as standard. 1 mL of each extract at various concentrations was used for the test. Each was given 3 mL of the reagent solution and incubated for 90 min at 95°C. The process was repeated with ascorbic acid, and the absorbance was read at 695 nm. The antioxidant capacity of each gram of extract was determined in milligrams of ascorbic acid equivalent (AAE) [10].
2.6. Identification of Brucine Powder
Anhydrous brucine powder was purchased from Chemiphase, UK. A copy of the certificate of analysis obtained from the manufacturer is shown in the supplementary material. Simple spectroscopic and melting point determinations were used to confirm the identity of the pure sample. Results can be seen in the supplementary information.
2.6.1. HPLC Method Development and Validation
An isocratic RP-HPLC method was developed to accurately identify and quantify brucine, a known chemical marker in the stem bark of Anthocleista nobilis. 10 mg of brucine was dissolved in 25 mL of diluent (methanol : 0.1% formic acid; 65 : 35 v/v) to obtain a 0.4 mg/mL standard stock solution. A 1-in-2 serial dilution was performed from the stock solution to get six solutions (solutions A–F) with concentrations of 0.2, 0.1, 0.05, 0.025, 0.0125, and 0.00625 mg/mL. The solutions were filtered through a 0.45 μm nylon membrane syringe filter and placed in 2 mL vials before injection into the HPLC to obtain their peak areas. The peak areas were used to generate a calibration curve.
The developed method was validated per ICH guidelines for specificity, linearity, range, the limit of detection (LOD), limit of quantitation (LOQ), precision, accuracy, and robustness.
2.6.2. Analysis of Extracts and Market Samples
A 0.1 mg/mL solution was prepared by weighing 10 mg each of the A. nobilis extracts and dissolving in a 25 mL diluent. It was sonicated for about 10 min. Afterward, the solution was topped up to the 100 mL mark using the diluent. The solution was filtered through a 0.45 μm nylon membrane syringe and placed in 2 mL vials to obtain and record peaks.
5 mL of each product was transferred into a 50 mL volumetric flask and made to the mark with the diluent. The 10% v/v solution was filtered using the 0.45 μm nylon membrane syringe and transferred into 2 mL vials before obtaining and recording peak areas.
The concentration of brucine was determined using the calibration curve equation obtained from the calibration curve of brucine.
3. Results
3.1. Plant Material Extraction and Characterization of Plant Extracts
Following the successive extraction of the bark with the solvents, the results of the characterization of the stem bark extracts are summarized in Table 1 (phytochemical screening) and Table 2 (antioxidant activity). The percentage yield of dried extract obtained (w/w) is also recorded in Table 2.
| Test | 70% ethanol | Ethanol | Aqueous | Ethyl acetate |
|---|---|---|---|---|
| Flavonoids | + | + | + | − |
| Saponins | + | + | + | − |
| Triterpenoids | − | − | − | − |
| Sterols | − | − | − | − |
| Glycosides | + | + | + | − |
| Alkaloids | + | + | + | + |
| Tannins | + | + | + | + |
- + = present; − = absent.
| Test | % yield (% w/w) ± SD | EC50 (μg/mL) | TAC (μg/g AAE) ± SD |
|---|---|---|---|
| Water | 9.29 ± 0.03 | 48.37 | 439.34 ± 44.11 |
| 70% ethanol | 18.14 ± 0.08 | 97.69 | 557.04 ± 24.39 |
| 96% ethanol | 18.67 ± 0.10 | 66.66 | 629.60 ± 51.87 |
| Ethyl acetate | 5.60 ± 0.14 | 36.39 | 632.64 ± 23.13 |
| Control (ascorbic acid) | 11.82 |
- All results are means of triplicate determinations.
3.1.1. Phytochemical Screening
Phytochemicals present in the extracts of the stem bark of Anthocleista nobilis are given in Table 1.
3.1.2. Antioxidant Activity
The free radical scavenging capacity of the extracts was examined using DPPH and phosphomolybdenum.
3.2. Identification of Brucine Powder
UV-visible spectra and melting point determinations were performed as a means of confirmation of identity. The results obtained were compared with those in literature (supplementary data: T1, SD1).
3.3. HPLC Analysis
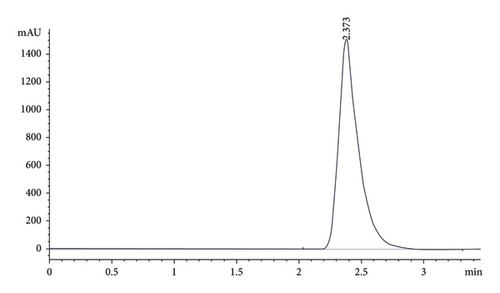
The conditions for the developed HPLC method are shown in Table 3, and the corresponding chromatogram for pure brucine is shown in Figure 1. The HPLC chromatograms using the above methods for the extracts and herbal products are shown in the supplementary materials: SD2–SD10.
| Condition | Value/specification |
|---|---|
| Column | Supelcosil C18 (150 × 4.6 mm, 3 μm) |
| Flow rate | 0.8 mL/min |
| Wavelength of detection | 274 nm |
| Injection volume | 50 μL |
| Temperature | 28°C |
| Mobile phase | Methanol : 0.1% formic acid (65 : 35, v/v) |
| Retention time (mean ± SD) | 2.374 ± 0.0065 min (n = 11) |
| Mode of elution | Isocratic |
3.3.1. Method Validation
The developed method was validated according to ICH guidelines [11]. The results are shown below.
(1) Specificity. The HPLC chromatograms of the extracts and medicinal products showing the brucine peak clearly resolved in each instance verify the specificity of the method developed. The chromatograms can be found in the supporting documents.
(2) Linearity (Calibration Curve). The linearity was verified by plotting a calibration curve. The values used and the graph are shown in Figure 2.
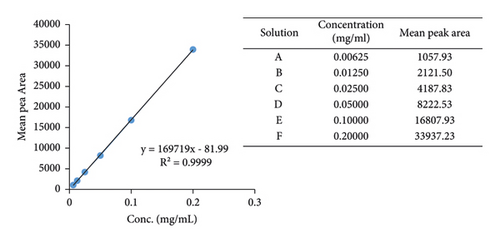
(3) LOD and LOQ. Using σ (standard deviation of the response) = 69.09 and S (slope of the calibration curve) = 169719, LOD and LOQ were calculated as 0.00115 mg/mL and 0.00348 mg/mL, respectively.
(4) Accuracy. The percentage recoveries were calculated after samples of herbal medicinal product were spiked with the analyte (brucine) (Table 4).
Brucine content in product (mg/mL) A |
Amount of brucine used to spike product (mg/mL) B |
Mean brucine content in spiked product (mg/mL) C |
Amount recovered (mg/mL) C − A |
Mean % recovery ± SD (C − A)/B × 100% |
|---|---|---|---|---|
| 0.00895 | 0.08 | 0.08965 | 0.0807 | 100.90 ± 0.07 |
| 0.00895 | 0.10 | 0.10915 | 0.1002 | 100.24 ± 0.07 |
| 0.00895 | 0.12 | 0.12995 | 0.1210 | 100.81 ± 0.07 |
- All results are means of triplicate determinations. Acceptance criteria for percentage recovery: 100 ± 15% [12].
(5) Precision. For intraday precision (repeatability), the percent relative standard deviation was calculated after obtaining the peak areas from six different determinations from six injections of the 0.1 mg/mL working concentration on the same day. For intermediate precision, the percent relative standard deviation was calculated after obtaining the mean peak area of three different determinations made by two different analysts on two separate days (Table 5).
| Intraday | Intermediate | ||
|---|---|---|---|
| (N = 6) | Day 1 | Day 2 | |
| Mean peak area | 16931 | 16734 | 16827 |
| SD | 132.56 | 24.71 | 15.72 |
| RSD | 0.78 | 0.15 | 0.09 |
- Acceptance criteria for RSD ≤2 [11].
(6) Robustness. Robustness was assessed by making variations to temperature and wavelength used for the analysis (Table 6).
| Sample | Temperature (°C) | Mean peak area ± SD | RSD (%) | Wavelength (nm) | Mean peak area ± SD | RSD (%) |
|---|---|---|---|---|---|---|
| Brucine | 23 | 16806 ± 8.19 | 0.05 | 273 | 17373 ± 13.06 | 0.08 |
| 28 | 16981 ± 98.43 | 0.58 | 274 | 16908 ± 46.82 | 0.28 | |
| 33 | 16733 ± 6.56 | 0.04 | 275 | 16180 ± 15.38 | 0.10 |
- All results are means of triplicate determinations. Acceptance criteria for RSD ≤2 [11].
3.3.2. Percentage Content of Chemical Marker (Brucine)
Based on the above calculations, the content of brucine in the A. nobilis extracts and the market samples were determined (Table 7).
| Sample | Mean peak area ± SD | Mean % content (×10–3% w/v) ± SD | Extract | Mean peak area ± SD | Mean % content (% w/v) ± SD |
|---|---|---|---|---|---|
| HBS1 | 699.07 ± 0.78 | 0.8950 ± 0.0046 | Water | 4573.66 ± 9.04 | 0.0324 ± 0.00054 |
| HBS2 | 1564.60 ± 2.95 | 1.4050 ± 0.0174 | 96% ethanol | 5378.05 ± 88.54 | 0.1259 ± 0.01798 |
| HBS3 | 1461.15 ± 4.56 | 1.3440 ± 0.0269 | Ethyl acetate | 1501.88 ± 2.20 | 0.0507 ± 0.00048 |
| HBS4 | 3425.29 ± 14.45 | 2.5013 ± 0.0852 | 70% ethanol | 4530.34 ± 6.55 | 0.0177 ± 0.00022 |
| HBS5 | 2735.73 ± 1.89 | 2.0951 ± 0.0112 |
- All results are means of triplicate determinations.
4. Discussion
4.1. Plant Material Extraction and Characterization
The solvents were added in order of increasing polarity: from ethyl acetate to water. This was done to ensure that a wide array of constituents are extracted due to the constituent compounds [13]. The yield values suggest that ethanol is the best solvent for good yield. This could mean that majority of the compounds present in the plant sample are polar organic compounds.
Phytochemical screening indicates that except for the ethyl acetate extract, all the others contained the same class of phytochemicals, namely, tannins, alkaloids, glycosides, saponins, and flavonoids. The ethyl acetate extract tested negative for flavonoids, saponins, alkaloids, and tannins. The results obtained correspond with results from earlier work on this plant [2, 7]. This indicates that the ethyl acetate extract was the most potent antioxidant. This extract also showed the best free radical scavenging ability (Table 2). From the phytochemical screening (Table 1), the ethyl acetate extract revealed the presence of only alkaloids and tannins, which were also present in all the other extracts. It could be inferred that the antioxidant effect of A. nobilis emanates largely from the tannin and alkaloid content. Tannins are polyphenolic compounds; polyphenols can act as reducing agents and are suitable antioxidants [14]. Some alkaloids, such as N-methylisosalsoline, cepharanthine, and fangchinoline, have also shown antioxidant activity [15–17]. The other extracts also showed a significant level of antioxidant activity as well. The DPPH scavenging method and the total antioxidant capacity assay of the phosphomolybdenum confirm the antioxidant activity of the extracts of the stem bark of Anthocleista nobilis. The phytochemical screening and antioxidant activity tests served to confirm the properties of the plant material used. However, the focus of the study was to develop a tool for the quality control of extracts of Anthocleista nobilis and herbal products thereof, hence the need for the development of the direct HPLC method of analysis using a chemical marker.
4.2. Identification of Brucine Powder
The proof of identity of the purchased brucine powder was provided via the certificate of analysis provided by the supplier. A copy can be found in the supplementary data. Notwithstanding, additional tests (UV-VIS and melting point measurement) were performed to confirm identity and purity. The wavelength maxima obtained from the spectrum for brucine (supplementary data) compared favorably with results obtained by Wang et al. [18]. The melting point range obtained for brucine powder was 177–179°C, which agreed with British Pharmacopoeia, 2013 [19].
4.3. HPLC Method Development
The use of chemical markers for quality control of herbal products has been previously reported [20]. The choice of brucine as a marker compound is based on its reported toxicity. Identifying such toxic chemical markers can serve as good quality indicators for plants and herbal products containing them. This will help to avoid possible adverse effects. The optimised conditions of the method developed were achieved after several trials and adjustments of parameters. The temperature at which the run was carried out was 28°C using a Supelcosil C18 column with dimensions of 150 × 4.6 mm and particle size of 3 μm. The C18 (octyldecyl) column ensured a fair amount of retention of brucine in the stationary phase. The mobile phase composition was made up of 0.1% v/v formic acid and methanol in a 65 : 35% v/v ratio throughout the run. The formic acid used is ideal for helping the dissolution of the alkaloid (a basic compound) in the mobile phase. The mobile phase was pumped at a rate of 0.8 mL/min, and other flow rates experimented with initially did not produce an ideal separation as observed at 0.8 mL/min. The detector was a UV-VIS detector with a detection wavelength set at 274 nm because the marker contains a chromophore and is, therefore, UV-VIS active. The initial wavelength of 264 nm, which was selected (λmax of brucine per the spectrum obtained), did not produce chromatograms with good resolution (results not shown).
Interestingly, a wavelength of 274 nm was determined to be suitable and used for the method development. A 50 μL injection of the sample was determined as the best for each determination after several other volumes gave overlapping peaks with very poor resolution (results not shown). The brucine biomarker eluted at an average time of 2.374 ± 0.0065 min. The elution under 5 min provides an economic advantage as well as a relatively short time of analysis.
4.4. Quantification
The ethyl acetate, ethanol, 70% ethanol, and aqueous extracts revealed brucine contents of 0.0507, 0.1259, 0.0177, and 0.0324 % w/w, respectively. The developed method also revealed that the content of brucine in HBS 1, HBS 2, HBS 3, HBS 4, and HBS 5 was within the range 0.8950–2.5013 × 10–3% w/v, respectively. This implies that every 100 mL of the herbal product contains 0.8950–2.5013 mg of brucine. The permissible level of brucine indicated as safe is 1000 mg daily for adults. From the results obtained, both the extracts and products were safe for consumption [21].
5. Conclusion
Phytochemical analysis and antioxidant activities have been employed to characterize the stem bark of Anthocleista nobilis. These parameters can be used to validate the claims for using the stem bark of Anthocleista nobilis as an antioxidant agent. A simple, robust, precise, and accurate isocratic RP-HPLC method was developed to analyze brucine-containing samples. The developed method can also be used for the qualitative and quantitative analysis of brucine in plants and herbal products that contain it.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Open Research
Data Availability
The primary data used to support the findings of this study are included within the article.




