Minimally Invasive Mitral Valve Repair with Artificial Chordae: Insights from a 6-Year Single-Center Study
Abstract
Purpose. Minimally invasive mitral valve repair (MIMVR) has been demonstrated to be safe and effective, but technical difficulty, outcome variation, and lack of standardized protocols undermine the utility of artificial chordae. This study aims to analyze the midterm outcomes of repair using artificial chordae through right minithoracotomy. Methods. A retrospective cohort study was conducted on consecutive patients who underwent MIMVR using artificial chordae at a single center in Vietnam between April 2016 and April 2022. Valve repairs were separated into two groups based on a previously validated complexity score: simple repair (Group 1) and intermediate-to-complex repair (Group 2). Demographic variables, comorbidities, operative characteristics, surgical outcomes, and follow-up data on survival and mitral regurgitation (MR) grade were analyzed. The learning curve was assessed by comparing the number of procedures with operation time and aorta cross-clamp time. Primary endpoints included survival and freedom from recurrent MR at four years. Results. Ninety patients were identified, including 41 simple and 49 intermediate-to-complex repairs. The mean age was 50.5 ± 12.9 years. Both groups had similar preoperative characteristics. The perioperative and postoperative outcomes were favorable, with no cases requiring mitral valve replacement. The median follow-up time was 30.3 months (18.2–40.4), and there were two (2.2%) cardiac deaths, with one in each group. The Kaplan–Meier survival estimates for Groups 1 and 2 at 12 and 24 months were 97% vs. 100% and 97% vs. 96%, respectively (95% CI = 0.05–12.2, P = 0.850), and estimates for freedom from recurrent MR were 97% vs. 92% and 97% vs. 88%, respectively (95% CI = 0.49–12.0, P = 0.260). There was a negative association between the volume of operations and the duration of operation and aortic cross-clamp time, leading to shorter durations. Conclusion. Based on our single-center experience, MIMVR using artificial chordae via right mini-thoracotomy can be safely and effectively performed in resource-limited countries for patients with MR. This approach has been shown to be applicable for a range of MR complexities, from simple to intermediate-to-complex MV repairs, and has demonstrated promising results in terms of midterm freedom from MR recurrence.
1. Introduction
Mitral valve (MV) repair has better long-term results than MV replacement and has been established as the surgical treatment of choice for mitral regurgitation (MR) when the valve lesions are reparable and the procedure is performed at specialized centers with a high rate of successful repairs [1–3]. Furthermore, minimally invasive mitral valve repair (MIMVR) has been demonstrated to be safe and effective for MV surgery, providing less morbidity, including stroke, atrial fibrillation, renal failure, and length of stay in carefully selected patients [4–6]. Since its introduction, there has continued to be a need to keep developing simpler, more effective, and more durable surgical techniques for MV surgery.
Resectional techniques, including insertion of annuloplasty rings and resection of prolapsing segments, were established by Carpentier [7]. Since then, excellent long-term results have been reported, but their reproducibility and learning curve have shown difficulties [3, 7, 8]. Zussa et al. [9] and Frater et al. [10] introduced the use of artificial chordae and polytetrafluorethylene (PTFE) cords to replace ruptured or elongated native chordae tendineae.
Presently, the use of artificial chordae has become a standard technique for MV repair, supported by excellent long-term results. Nevertheless, MIMVR using artificial chordae carries its challenges, especially the proper length of artificial chordae, adequate placement, technical learning curve, outcome variation, and lack of standardized protocols [1, 8, 11]. This study aimed to analyze the midterm outcomes of MIMVR using artificial chordae through right mini-thoracotomy in a single center.
2. Materials and Methods
2.1. Patient Selection
A single-center, retrospective cohort study of all consecutive patients who underwent MIMVR using artificial chordae (expanded polytetrafluoroethylene) was conducted via right minithoracotomy in Vietnam between April 2016 and April 2022.
The operations are performed by a single primary surgeon specializing in cardiovascular surgery, supported by other assistants. Preliminary results and techniques were presented by our group in 2023 [12]. Valve repairs were separated into 2 groups based on a previously validated complexity score (Anyanwu score) [13]: simple repair (Group 1) and intermediate-to-complex repair (Group 2).
2.2. Surgical Technique
The patients were placed in a supine position. To establish peripheral cardiopulmonary bypass, cannulation of the femoral vessels was done using an open Seldinger-guided technique. A 4-cm incision was made parallel to the anterior axillary line and dissected into the 4th right intercostal space. Subsequently, a video camera port was inserted into the 3rd space, followed by the insertion and clamping of the Chitwood cross-clamp. Custodiol HTK (histidine-tryptophan-ketoglutarate) solution was then administered. A left atriotomy was performed to expose the MV, and a left atrial retractor was employed.
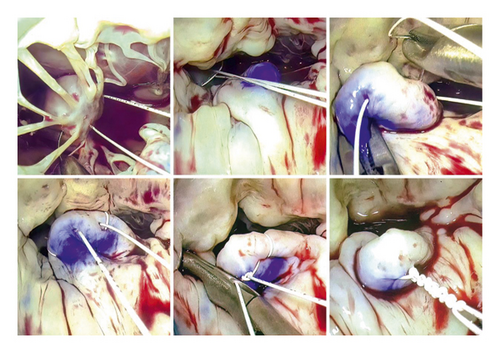
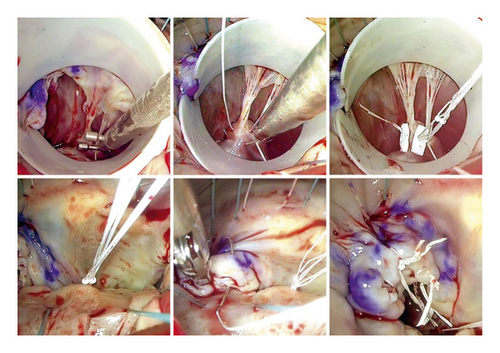
Our MVMVR methodology closely follows the principles outlined by Gillinov et al. [14]. The process begins with the strategic placement of annuloplasty sutures to improve visibility and enables a thorough examination of the valve. The repair technique then closely follows the guidelines established by Carpentier [15], which emphasize the stabilization of the annulus, maintenance of leaflet mobility, and the creation of an ample coaptation surface. Consequently, in all cases, chordal replacement and annuloplasty constituted the exclusive treatment modalities employed. To install the neochords, first, the prolapsing segment was identified and the appropriate papillary muscle was selected. The A1-P1 region or the non-prolapsed segment of the native chords was used as a reference point. Two techniques were utilized: The free-hand chordae technique (Figure 1) involves passing a PTFE CV-5 suture (W. L. Gore & Associates, Inc., Newark, DE, USA) through the fibrous region of the papillary muscle, bringing each end through the prolapse area. The PTFE length was adjusted based on the reference point before tying the suture. The premeasured chordal loops technique (Figure 2) was implemented using Chord-X loop (On-X Life Technologies, Inc, Austin, TX, USA). It utilizes a caliper to measure the length of a reference point, choosing the loop length and then anchoring it to the papillary muscle and attaching it to the edge of the leaflet-free margin in the prolapse area. During ring annuloplasty, the size of the ring is determined very carefully based on the surface area of the anterior leaflet. It is then aligned with pre-positioned annuloplasty sutures. In all cases, the Carpentier Physio II ring (Edwards Lifesciences Corp., Irvine, CA, USA) was used. It is important to note that neochords were only secured after the ring annuloplasty was implemented. Transesophageal echocardiography was utilized to verify the final repair result.
2.3. Statistical Analysis
Demographic variables, comorbidities, operative characteristics, postoperative outcomes (intubation time, ICU time, mechanical circulatory support, peripheral vessel complications, stroke, reoperation), postoperative echocardiographic features, and follow-up outcomes (mortality, freedom from recurrent MR) were assessed and analyzed. Data are presented as mean ± standard deviation for normally distributed continuous variables and as medians with interquartile ranges for non-normally distributed continuous variables, while categorical variables are presented as frequencies and percentages.
A learning curve was analyzed by plotting trend lines on a two-dimensional scatter plot that compared the number of procedures with operation time and aorta cross-clamp time. Primary endpoints included survival and freedom of recurrent MR at 48 months (4 years), secondary outcomes included echocardiogram findings.
The research was approved by the Ethical Board of the University of Medicine and Pharmacy at Ho Chi Minh City, number 166/HDDD-DHYD, on February 26th, 2021.
3. Results
Ninety patients were identified, including 41 cases of simple repairs (Group 1) and 49 cases of intermediate-to-complex repairs (Group 2; 45 intermediate repairs and 4 complex repairs) [13]. The mean age including both groups was 50.5 ± 12.9 years and 70 (77.7%) were men. Table 1 summarizes the clinical characteristics stratified by complexity score, and there were no significant differences between the groups. Preliminary results were presented by our group in 2023 [12].
| All repair (N = 90) | Simple repair (N = 41) | Intermediate-to-complex repair (N = 49) | P value | |
|---|---|---|---|---|
| Age (year) | 50.5 ± 12.9 | 52.3 ± 12.3 | 49.1 ± 13.3 | 0.244 |
| Sex (male) | 70 (77.8) | 33 (80.5) | 37 (75.5) | 0.619 |
| BMI (kg/m2) | 22.7 ± 2.6 | 22.8 ± 2.5 | 22.6 ± 2.7 | 0.660 |
| BSA (m2) | 1.67 ± 0.17 | 1.70 ± 0.17 | 1.65 ± 0.17 | 0.231 |
| NYHA classification | 0.710 | |||
| I-II | 80 (88.9) | 37 (90.2) | 43 (87.8) | |
| III-IV | 10 (11.1) | 4 (9.8) | 6 (12.2) | |
| Atrial fibrillation | 9 (10.0) | 4 (9.8) | 5 (10.2) | >0.999 |
| Hypertension | 43 (47.8) | 21 (51.2) | 22 (44.9) | 0.672 |
| Diabetes | 9 (10.0) | 3 (7.3) | 6 (12.2) | 0.502 |
| Dyslipidemia | 12 (13.3) | 6 (14.6) | 6 (12.2) | 0.765 |
| COPD | 3 (3.3) | 2 (4.9) | 1 (2.0) | 0.590 |
| Euroscore II (%) | 1.12 ± 0.99 | 1.12 ± 1.01 | 1.12 ± 0.99 | 0.998 |
- Data are presented as mean ± standard deviation or n (%). BMI, body mass index; BSA, body surface area; COPD, chronic obstructive pulmonary disease; NYHA, New York Heart Association.
All MR were categorized as Carpentier regurgitation type II, with fibroelastic deficiency being the primary etiology observed in both groups. The mean annular mitral diameter measured 39.2 ± 4.4 mm. A slight difference was observed between Group 1 and Group 2; Group 1 had a smaller MV annulus size compared to Group 2 (37.9 ± 4.0 mm vs. 40.2 ± 4.5 mm, P = 0.012); the preoperative echocardiography characteristics and perioperative features are presented in Tables 2 and 3.
| All repair (N = 90) | Simple repair (N = 41) | Intermediate-to-complex repair (N = 49) | P value | |
|---|---|---|---|---|
| LVEDD (mm) | 59.2 ± 6.0 | 58.4 ± 6.0 | 59.9 ± 6.0 | 0.237 |
| LVESD (mm) | 36.6 ± 5.0 | 36.2 ± 4.7 | 37.0 ± 5.2 | 0.428 |
| Left atrial diameter (mm) | 46.6 ± 8.2 | 45.8 ± 7.8 | 47.3 ± 8.5 | 0.410 |
| Left atrial volume (ml) | 134.8 ± 50.5 | 122.7 ± 37.5 | 144.8 ± 57.7 | 0.057 |
| LVEF (%) | 63.5 ± 7.4 | 63.6 ± 5.2 | 63.4 ± 8.8 | 0.897 |
| sPAP (mmHg) | 38.5 ± 17.0 | 38.0 ± 12.7 | 38.9 ± 20.4 | 0.858 |
| TAPSE (mm) | 25.3 ± 4.1 | 24.7 ± 4.0 | 25.8 ± 4.2 | 0.227 |
| Mitral annulus diameter (mm) | 39.2 ± 4.4 | 37.9 ± 4.0 | 40.2 ± 4.5 | 0.012 |
| Mitral valve prolapse | <0.001 | |||
| Anterior leaflet | 23 (25.5) | 0 (0.0) | 23 (46.9) | |
| Posterior leaflet | 62 (68.9) | 41 (100.0) | 21 (42.9) | |
| Bi-leaflet | 5 (5.6) | 0 (0.0) | 5 (10.2) |
- Data are presented as mean ± standard deviation or n (%). LVEDD left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; sPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion. Data are presented with associated p values, with bold values indicating statistical significance at p < 0.05.
| All repair (N = 90) | Simple repair (N = 41) | Intermediate-to-complex repair (N = 49) | P value | |
|---|---|---|---|---|
| Etiology of mitral valve | 0.791 | |||
| FED | 72 (80.0) | 34 (82.9) | 38 (77.6) | |
| Barlow | 16 (17.8) | 6 (14.6) | 10 (20.4) | |
| Forme fruste | 2 (2.2) | 1 (2.4) | 1 (2.0) | |
| No chordae per patient | 2 [1.3; 3.0] | 2 [1.0; 3.0] | 2 [2.0; 3.0] | 0.688 |
| Type of artificial chordae | 0.472 | |||
| Single | 30 (33.3) | 15 (36.6) | 15 (30.6) | |
| Loop | 50 (55.6) | 22 (53.7) | 28 (57.1) | |
| Single and loop | 10 (11.1) | 4 (9.8) | 6 (12.2) | |
| Annuloplasty ring | 0.235 | |||
| 26 mm | 2 (2.2) | 0 (0.0) | 2 (4.1) | |
| 28 mm | 21 (23.3) | 12 (29.3) | 9 (18.4) | |
| 30 mm | 27 (30.0) | 13 (31.7) | 14 (28.6) | |
| 32 mm | 24 (26.7) | 7 (17.1) | 17 (34.7) | |
| 34 mm | 12 (13.3) | 6 (14.6) | 6 (12.2) | |
| 36 mm | 4 (4.4) | 3 (7.3) | 1 (2.0) | |
| Edge-to-edge repair | 6 (6.7) | 1 (2.4) | 5 (10.2) | 0.214 |
| Commissuroplasty | 17 (18.9) | 4 (9.8) | 13 (26.5) | 0.059 |
| Concomitant procedures | 9 (10.0) | 4 (9.8) | 5 (10.2) | 0.286 |
| ASD closure | 1 (11.1) | 0 (0.0) | 1 (20.0) | |
| Maze procedure | 6 (66.7) | 2 (50.0) | 4 (80.0) | |
| Tricuspid repair | 2 (22.2) | 0 (0.0) | 2 (50.0) | |
| CPB time (mins) | 139.0 [120.5; 158.5] | 137.0 [123.0; 159.0] | 136.0 [120.0; 158.0] | 0.709 |
| ACC time (mins) | 90.0 [80.0; 104.0] | 90.0 [77.0; 101.0] | 89.0 [80.0; 100.0] | 0.874 |
| SAM | 7 (7.8) | 6 (14.6) | 1 (2.0) | 0.044 |
- Data are presented as mean ± standard deviation, median (interquartile range), or n (%). ACC, aortic cross-clamp; ASD, atrial septal defect; CPB, cardiopulmonary bypass; FED, fibroelastic deficiency; SAM, systolic anterior motion.
There were no differences in the left ventricular measurements between groups before and after repair, as shown in Table 4. The median follow-up time was 30.3 months (18.2–40.4), and there were two (2.2%) deaths during this time or one in each group. No reoperations related to the MV were observed during the follow-up period. The midterm outcomes have been demonstrated in Table 5.
| All repair (N = 90) | Simple repair (N = 41) | Intermediate-to-complex repair (N = 49) | P value | |
|---|---|---|---|---|
| Intubation time (hours) | 9.0 [5.3; 16.8] | 9.0 [6.0; 16.0] | 10.0 [5.0; 19.0] | 0.994 |
| Prolonged ventilation time | 9 (10.0) | 3 (7.3) | 6 (12.2) | 0.502 |
| ICU time (days) | 2.8 [1.9; 4.2] | 2.8 [1.9; 4.2] | 2.9 [1.9; 4.0] | 0.685 |
| IABP support | 1 (1.1) | 0 (0.0) | 1 (2.0) | >0.999 |
| Femoral artery stenosis | 1 (1.1) | 1 (2.4) | 0 (0.0) | 0.456 |
| Limb compartment syndrome | 1 (1.1) | 0 (0.0) | 1 (2.0) | >0.999 |
| New onset of atrial fibrillation | 19 (21.1) | 10 (24.4) | 9 (18.4) | 0.606 |
| Stroke with sequelae | 1 (1.1) | 0 (0.0) | 1 (2.0) | >0.999 |
| Reoperation for bleeding | 3 (3.3) | 1 (2.4) | 2 (4.1) | >0.999 |
| Mitral regurgitation | 0.017 | |||
| None/trivial | 80 (88.9) | 40 (97.6) | 40 (81.6) | |
| Moderate-to-severe | 10 (11.1) | 1 (2.4) | 9 (18.4) | |
| Mean transmitral gradients (mmHg) | 2.9 ± 1.6 | 3.3 ± 2.0 | 2.6 ± 1.2 | 0.064 |
| LVEDD (mm) | 49.5 ± 5.8 | 49.1 ± 5.4 | 49.9 ± 6.0 | 0.508 |
| LVESD (mm) | 34.8 ± 6.5 | 35.5 ± 6.2 | 34.2 ± 6.8 | 0.379 |
| LVEF (%) | 55.1 ± 7.4 | 53.8 ± 5.9 | 56.1 ± 8.4 | 0.143 |
| sPAP (mmHg) | 30.5 ± 7.6 | 31.4 ± 7.8 | 29.6 ± 7.5 | 0.369 |
| TAPSE (mm) | 15.2 ± 4.4 | 15.8 ± 4.7 | 14.7 ± 4.0 | 0.304 |
- Data are presented as mean ± standard deviation, median (interquartile range), or n (%). IABP, intra-aortic balloon pump; ICU, intensive care unit; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; sPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion. Data are presented with associated p values, with bold values indicating statistical significance at p < 0.05.
| All repair (N = 90) | Simple repair (N = 41) | Intermediate-to-complex repair (N = 49) | P value | |
|---|---|---|---|---|
| Following time (months) | 30.3 [18.2; 40.4] | 31.0 [15.3; 38.0] | 28.5 [18.8; 43.4] | 0.318 |
| Mortality | 2 (2.2) | 1 (2.4) | 1 (2.0) | >0.999 |
| Mitral re-regurgitation | 0.083 | |||
| None/trivial | 57 (63.3) | 31 (75.6) | 26 (53.1) | |
| Medium | 25 (27.8) | 8 (19.5) | 17 (34.7) | |
| Severe | 8 (8.9) | 2 (4.9) | 6 (12.2) |
- Data are presented as median (interquartile range) or n (%).
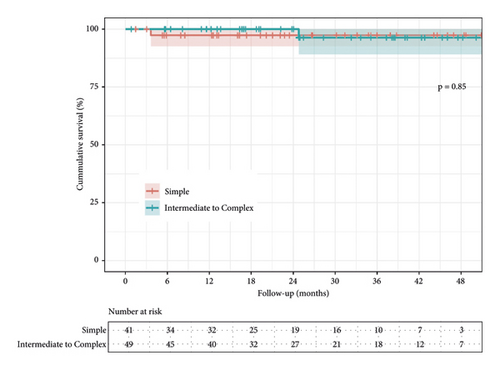
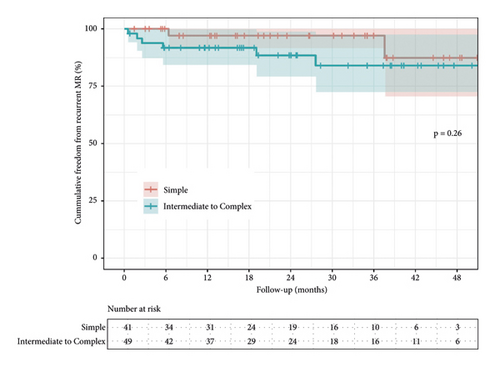
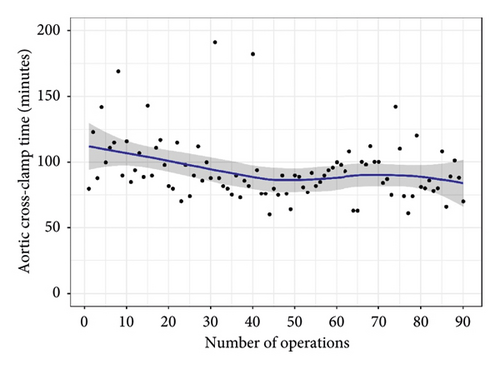
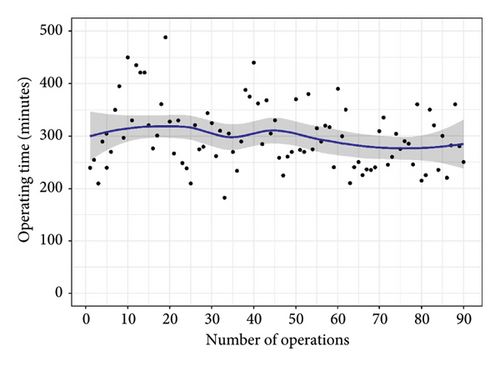
There was no difference between overall midterm survival or recurrent MR. Groups 1 and 2 Kaplan–Meier estimates of survival at 12 and 48 months were 97% vs. 100%, and 97% vs. 96% (95% CI; 0.05–12.2, P = 0.850), respectively (Figure 3). The freedom from recurrent MR estimates were 97% vs. 92% and 97% vs. 88% (95% CI; 0.49–12.0, P = 0.260), respectively (Figure 4). In both groups, there were no reoperations. The analyzed learning curve revealed a negative correlation between the number of operations and the duration, as indicated by the trend lines. Specifically, as the volume of operations increased, the duration of both operation time and aortic cross-clamp time decreased, resulting in shorter durations (Figures 5 and 6).
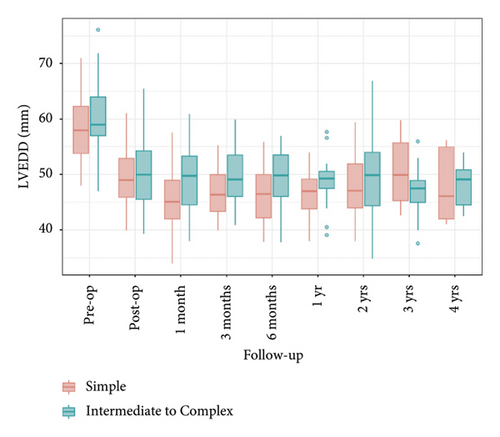
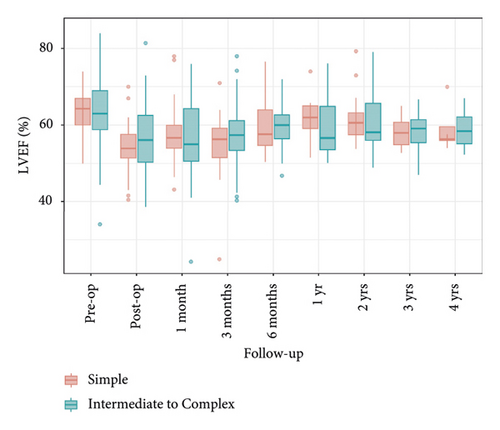
A comparison of left ventricular end-diastolic diameter (LVEDD) and left ventricular ejection fraction (LVEF) between the two groups showed that LVEDD significantly decreased preoperatively and remained below 50 mm during the 4-year follow-up, with Group 2 consistently having higher levels than Group 1. LVEF also significantly decreased preoperatively and postoperatively, remaining relatively stable during the follow-up period, with no significant differences between the groups. These findings indicate similar outcomes for both groups regarding LVEDD and LVEF, suggesting that the surgical intervention was equally effective (Figures 7 and 8).
4. Discussion
Mitral valve repair remains the treatment of choice for MR if the expertise is available, and minimally invasive approaches have been explored over the last two decades due to their potential patient-centered advantages. However, they are also related to technical challenges and require adequate training. Outcomes are associated with the patient’s conditions, surgeon’s experience, and center volume. This study aimed to evaluate and describe the initial experience of a valve surgery program at a single center in a lower middle-income country. The postoperative mortality and stroke rates were 0.0% and 1.1%, respectively. At two years, the mortality was 2.2% due to cardiac deaths and severe MR rates were 8.9%.
Etiology is a crucial consideration when it comes to MV disease and MV repair, especially in Vietnam. In a recent study, Cazaubiel and Iung [16] analyzed 2734 patients who had MV surgery in Vietnam, finding that surgeries that occurred from 1995–2000 were more likely to be classified as rheumatic valvular disease, in contrast to the 15.4% increase in degenerative etiology found in the 2005–2010 era of surgeries. Moreover, the change in etiology could not necessarily be pinpointed in these studies, as there was a significant increase in the degenerative etiologies, specifically within the fibroelastic disease-causing issue. Vietnam, despite being considered resource-limited in healthcare due to financial burdens, unequal resource distribution, and insurance coverage challenges [17], demonstrates a significant uptake of minimally invasive approaches in surgical procedures, with around half of the mitral valve surgeries in the country employing these techniques [18]. This adoption can be attributed to factors such as a patient-centered culture, increased patient awareness, and early recognition and acceptance of these procedures during the initial phases of the disease [18]. Cultural influences significantly impact the acceptance and implementation of new surgical techniques, and Vietnamese patients exhibit a greater receptiveness towards minimally invasive approaches, resulting in their widespread utilization [17, 18]. The rise in degenerative conditions and the strong adoption of minimally invasive approaches have generated a greater demand for minimally invasive mitral valve repair (MIMVR) despite the resource limitations in the country.
To ensure a successful mitral regurgitation (MR) repair, grading methods have been developed to assess the complexity of the repair and to choose appropriate techniques, with expert consultation if needed. One such practical and comprehensive system is the Anyanwu system, which considers echocardiography features, valve tissue calcification, location of leaflet prolapse, and limited leaflet mobility [13]. In this study, we analyzed patients who required MV repair and had a valve degeneracy rating of simple, intermediate, or complex based on Anyanwu’s classification of various MV degeneracies, which has also been used by Nakayama [19]. These results demonstrate the importance of considering etiology and comprehensive grading systems to ensure successful MR repair in degenerative MV disease cases.
The current study demonstrated the feasibility, safety, and effectiveness of MIMVR using an artificial chordae technique. The postoperative mortality and stroke rates were similar to recent data presented by the Mini-Mitral International Registry (mortality 1.7% and stroke 1.7%) [20]. All cases used repair techniques, including ring annuloplasty and artificial chordae, with the latter being the primary technique. Valve replacement was not required in any instance. According to Rankin’s findings, the artificial chordae repair method is an effective solution for managing mitral disease, including complex cases [21]. This technique addresses the fundamental issue of chordae rupture or elongation that is common in mitral valve surgeries. One of the advantages of artificial chordae is their technical simplicity in minimally invasive procedures, while also preserving the valvular tissue that aligns more anatomically compared to traditional leaflet resection methods [21, 22]. However, challenges such as the potential for hemolytic anemia, and longevity concerns arising from PTFE hyalinization or calcification, are noted [8]. In addition, post-repair remodeling of the left ventricle could lead to mismatched chordae lengths, potentially causing a recurrence of MR [23]. Alongside the artificial chordae technique (addresses MV leaflets), annuloplasty ring usage extends repair durability by restoring the mitral annulus’s functional support. This, in turn, preserves leaflet coaptation and prevents future annular dilatation in degenerative MV cases. The integration of ring annuloplasty in the minimally invasive approach has proven to be seamless, particularly as it complements the fundamental step of exposing the mitral valve for effective repair.
While artificial chordae can easily fix simple prolapse, repairing anterior leaflet or bi-leaflet prolapse poses a more significant challenge. Per consensus among surgeons, artificial chordae is recommended for anterior prolapse repair [5]. For bi-leaflet prolapse, a two-step repair approach was followed, starting with posterior leaflet repair, followed by annuloplasty ring insertion and saline testing to assess the extent of regurgitation. Further repair techniques, such as artificial chordae, leaflet resection, and edge-to-edge stitch, were applied as required, as proposed by Castillo et al. [3]. In cases of substantial and even bi-leaflet prolapse, annuloplasty ring repair may be appropriate. In contrast, artificial chordae implantation may be suitable for asymmetrical prolapse, according to Koprivanac [5].
The edge-to-edge technique was primarily utilized in Group 2, as opposed to Group 1 (10.2% vs. 2.4%) due to the higher complexity scores from Anyanwu’s system seen in Group 2, indicating the need for multiple repair techniques for successful MV repair. The technique was employed as a backup solution in cases where repair proved inadequate [24]. Its straightforwardness and efficiency, particularly in challenging circumstances such as those observed in Group 2, have made it a preferred choice. In our practice, we employ the edge-to-edge technique to prevent systolic anterior motion (SAM), especially in mucoid degeneration prolapse with significant posterior leaflet height. We also often create shorter posterior neochordae to limit leaflet tissue movement toward the left ventricular outflow tract during systole, a crucial step when using smaller annuloplasty rings to prevent postoperative mitral stenosis [25]. Likewise, the commissuroplasty technique was more prominent in Group 2 than in Group 1 (26.5% vs. 9.8%). For simpler MV lesions in Group 1, typically a single MV segment prolapse, one or two pairs of artificial chordae were often sufficient without requiring supplementary repair techniques.
The present study evaluated the ICU length of stay and survival rates of patients with both simple and complex cases of MV disease, revealing favorable outcomes in terms of the duration of ICU stay. However, postoperative complications, such as pleural effusion were noted in 3 cases (3.3%) within both groups, which required re-exploration via the same thoracic incision. Out of these cases, 2 had an unclear bleeding source, while 1 case experienced bleeding at the thymus site. A case of postoperative low cardiac output was identified in Group 2, which was caused by low blood pressure and a decline in left ventricular function. An intra-aortic balloon pump was inserted and successfully removed, and no in-hospital deaths were reported in our study. There were two patients with femoral access complications; one case of postoperative femoral artery stenosis requiring thrombectomy and primary repair, and the second case had a postoperative lower limb compartment syndrome associated with a prolonged cardiopulmonary bypass duration requiring fasciotomies. The compartment syndrome presented 10 hours after the surgery and the main symptoms were severe pain, coldness, tenderness, paresthesia, and weak peripheral pulses [26].
In terms of postoperative complications, a higher incidence of moderate-to-severe MV re-regurgitation was observed in patients classified as intermediate-to-severe MV repair cases (Group 2) in comparison to the patients classified as simple cases (Group 1) with a rate of 18.4% vs. 2.4%, respectively. This outcome underscores the need to evaluate the complexity of MR prior to surgery as a means to optimize the outcome of valve repair.
Perioperative SAM was detected via transesophageal echocardiography in 7.8% of all cases and was managed with a step-wise approach consisting of medical therapy, followed by ventricular loading and surgery as the final option. Inotrope was stopped if patients’ hemodynamics permitted or used beta-blockers to reduce heart rate, IV fluids, stopped diuretics, and transoesophageal echocardiogram monitoring. According to Alfieri and Lapenna [27], SAM resolves spontaneously in about 1/3 of cases with cessation of inotropes and increased fluid and improves in 80% of patients reducing heart rate and increasing preload. Surgical repair is rarely needed and encounters anatomical challenges. All cases of perioperative SAM were resolved after medical therapy optimization in our study. This “transient” phenomenon was not detected during follow-ups or on echocardiography at discharge, which is consistent with previous studies [25, 27]. The only statistically significant perioperative complication was the occurrence of transient perioperative SAM, which was more common in Group 1 (14.6%) than in Group 2 (2.0%). The reasons for this discrepancy remain unclear, hinting at a possible underestimation of SAM risk in Group 1 due to the lack of additional repair techniques to prevent SAM. The underlying reasons for this discrepancy remain unclear, suggesting a possible underestimation of SAM risk in Group 1, where additional repair techniques were not applied to prevent SAM. Our results diverged from Nakayama’s study [19], which demonstrated opposite postoperative characteristics. Specifically, Nakayama’s study reported increased MR in patients classified as an intermediate-complex. However, our study shared a similar learning curve and lower mortality rates than those reported in the Society of Thoracic Surgeons database [28].
The postoperative transmitral mean gradient was 2.9 ± 1.6 mmHg. Patients who underwent MV repair for degenerative MR with a mean gradient greater than 3 mmHg had reduced exercise capacity [29]. The use of certain valve repair techniques, such as artificial chordae instead of resection techniques, and the selection of smaller annuloplasty mitral rings, rigid full mitral rings, and excessive utilization of the edge-to-edge technique, were associated with elevated postoperative transmitral gradient and an increased risk of functional mitral stenosis, leading to the long-term hemodynamic deterioration [1, 29].
During the follow-up period, no significant difference in mortality was observed between the two groups, as one cardiac death was recorded in each group. One patient was readmitted after 4 months post-surgery for acute heart failure, and the second patient presented infective endocarditis around the second month postoperative. Other studies have also reported similar findings, and in addition, our study exhibited lower mortality rates when compared to the Society of Thoracic Surgeons database [19, 28].
The Kaplan–Meier survival estimates at 12 and 48 months for Group 1 and Group 2 were 97% versus 100% and 97% versus 96%, respectively. Pfannmueller et al. reported 2134 cases with 1-, 5-, and 10-years survival rates (artificial chordae group) of 98%, 95%, and 86%, respectively. The resection group showed survival rates of 97%, 92%, and 81%, respectively [22]. Additionally, Davierwala et al. reported 2829 cases predominantly utilized [30]. Figure 3 presents the Kaplan–Meier estimates for Groups 1 and 2 regarding freedom from recurrent MR at 12 and 48 months, showing rates of 97% versus 92% and 97% versus 88%, respectively. Notably, no reoperations specifically related to the MV were observed during the follow-up period. This highlights that the rate of reoperation on the MV does not necessarily provide an accurate reflection of the valve’s functional status in patients who have not undergone reoperation. The observed difference in the cumulative incidence of recurrent MR and the need for reoperation suggests that factors such as asymptomatic patients, patient’s refusal for reoperation, or individuals with advanced age and other comorbidities may contribute to this disparity. It is crucial to recognize that the rate of reoperation alone does not indicate a significant occurrence of recurrent MR in these cases [31].
Our study’s learning curve analysis revealed that before reaching 50 cases, the durations of aortic cross-clamping and operating times displayed instability. However, after surpassing this cumulative case threshold, both durations demonstrated a stable decrease, indicating that high-volume surgeons tend to achieve improved outcomes. These findings align with previous research by Li et al. [32], where cumulative sum analysis was used to assess the average incidence of adverse events and operative time, revealing that surgeons typically require 50 to 200 operations to overcome the learning curve associated with mitral valve repair rates. These results provide valuable insights into the procedural efficiency of mitral valve repair using artificial chordae and highlight the significance of surgical experience in achieving favorable patient outcomes.
There are limitations to the current study. First, using a retrospective design introduces potential biases and limitations in data collection. Moreover, the absence of a control group hampers our ability to draw definitive conclusions regarding the effectiveness of the interventions. Additionally, our hospital’s adoption of the minimally invasive approach since July 2014 prevents comparison with the total sternotomy approach. The relatively small sample size and single-center setting raise concerns about the generalizability of the findings to a broader population. Despite the study’s retrospective data over a period of 6 years, the limited number of patients and only nine patients at risk during the 48-month follow-up examination undermine the study’s statistical power. Furthermore, the learning curve analysis was conducted by a single surgeon, potentially introducing biases. To address these limitations, future research should include larger prospective studies involving multiple centers and surgeons to enhance the reliability of the evidence.
5. Conclusion
Based on our initial experience, performing minimally invasive mitral valve repair using artificial chordae through a right minithoracotomy is a viable, safe, and effective option for patients with MR in resource-limited countries. This technique can be used for a range of MR complexities, from simple to intermediate-to-complex MV repairs, and has shown a promising midterm success rate in terms of freedom from MR recurrence. Further studies are needed to evaluate long-term outcomes.
Abbreviations
-
- PTFE:
-
- Polytetrafluorethylene
-
- MV:
-
- Mitral valve
-
- MR:
-
- Mitral regurgitation
-
- MIMVR:
-
- Minimally invasive mitral valve repair
-
- SAM:
-
- Systolic anterior motion.
Ethical Approval
The research was registered and approved by the Ethical Board of the University of Medicine and Pharmacy at Ho Chi Minh City, number 166/HDDD-DHYD, on February 26th, 2021.
Consent
Informed consent was obtained from all participants.
Conflicts of Interest
The authors declare that there are no conflicts of interest in the preparation of this article.
Authors’ Contributions
DHN conducted the surgical procedures. VDAB assisted during the surgeries, provided preoperative and postoperative care, and collected the necessary data. AP, SSB, and HR were responsible for writing. DN, CSI, TT, DHV, and AFA reviewed the study. DV, AH, and NHN provided scientific supervision. All authors of this paper have read and approved the final version submitted.
Acknowledgments
Vinh D. A. Bui was funded by Vingroup JSC and supported by the Master, Ph.D.Scholarship Programme of Vingroup Innovation Foundation (VINIF), Institute of Big Data, code VINIF.2022.TS146.
Open Research
Data Availability
The datasets related to the present study can be obtained from the corresponding author upon a reasonable request.