Shear Wave Elastography for the Assessment of Carotid Plaque Vulnerability: A Systematic Review
Abstract
Evidence for the management of asymptomatic carotid stenosis and possibly symptomatic nonstenosing carotid artery disease is limited. In contrary to calcified plaques, soft plaques are considered vulnerable and prone to rupture. Shear wave elastography (SWE), a novel ultrasound technique which uses acoustic wave force to propagate shear wave in tissues, can quantify tissue stiffness through the estimation of Young’s modulus (YM) in kPa or shear wave velocity in meter/second. This systematic review is aimed at evaluating the feasibility of SWE in carotid plaque risk stratification in relation to ischemic stroke (PROSPERO registration: CRD42022309709). 18 studies, obtained via search on PubMed, Cochrane, and Embase from inception until November 1, 2022, assessed SWE’s feasibility in carotid plaque risk stratification in humans (13 studies) and phantom models (5 studies). Human studies showed heterogeneity with respect to SWE devices, acquisition settings, and methodology, which consequently reflected in the between-study variability of YM values used for distinguishing vulnerable/symptomatic (27–52 kPa) and stable/asymptomatic (28–115 kPa) carotid plaques. However, within-study assessment of all human studies indicated SWE’s feasibility in carotid plaque risk stratification. Furthermore, four out of five carotid plaque phantom studies showed the potential of SWE to discriminate tissues of different stiffness comparable to the carotid vessel wall, soft and hard plaques, and with good reproducibility. SWE may potentially offer a bedside risk stratification tool for identifying patients with vulnerable carotid plaques, who may benefit from carotid surgery, stenting, or prolonged dual antiplatelet therapy. Patients with stable carotid plaques could be spared the risks of potentially harmful treatments and complications. However, available data are not enough to facilitate the immediate clinical application of SWE, and therefore, larger prospective clinical are warranted.
1. Introduction
Treatment of carotid artery disease remains controversial, most especially in relation to both asymptomatic carotid stenosis as well as possibly symptomatic nonstenosing carotid plaques, without concrete outlined clinical recommendations [1, 2]. Patients are mostly selected for carotid intervention depending on the extent of carotid luminal stenosis and the presence of clinical symptoms [2, 3]. The occurrence of ipsilateral stroke, however, is not solely dependent on the extent of carotid stenosis, but also on the extent of plaque stability [4]. Carotid plaques which cause ipsilateral stroke are believed to be unstable due to their structure and composition and could therefore be a source of atherogenic emboli [5]. This characteristic of plaques is described as plaque vulnerability. Carotid imaging is therefore desirable, irrespective of the degree of stenosis, for risk stratification which could help in identifying patients with vulnerable plaques and high risk of cerebral thromboembolism, i.e., patients who might benefit from aggressive treatment including carotid surgery, stenting, or prolonged dual antiplatelet therapy. On the other hand, asymptomatic patients with stable carotid plaques could be spared the risks of unnecessary and potentially harmful procedures and treatments including peri-interventional ischemic or hemorrhagic stroke, and even death [3].
Plaque vulnerability can best be assessed histologically through the invasive acquisition of plaque tissue [6]. However, the associated risks render this procedure not ideal for the sole purpose of diagnostics. Contrast-enhanced magnetic resonance plaque imaging (MRPI), which has high diagnostic accuracy in assessing individual characteristics of plaques and carotid plaque vulnerability [4], however, is expensive and not easily available. Likewise, conventional B-mode ultrasound enables evaluating individual characteristics of plaque vulnerability including lipid-rich necrotic core volume, fibrous cap thickness, and intraplaque hemorrhage but is challenged by high interoperator variability [7, 8]. In assessing plaque vulnerability, contrast-enhanced ultrasound (CEUS) may be used to detect intraplaque neovascularization and ulceration of plaques. Neovascularization can also be assessed by superb microvascular imaging (SMI) through visualization of intraplaque microvascular low-velocity blood flow [9]. Computed tomography angiography (CTA) can be used to detect plaque calcification, ulceration, or intraplaque hemorrhage [10, 11]. MRPI, CEUS, and CTA expose patients to contrast agents, which might be inconvenient in patients with contraindications, with the latter additionally exposing patients to radiation (Supplemental Table S1) [12].
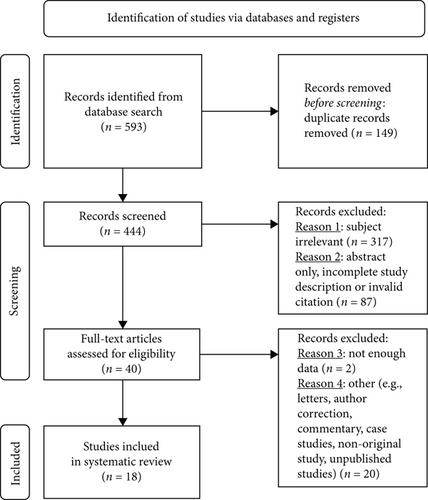
Shear wave elastography (SWE) is a novel ultrasound technique which offers, in addition to the functions of conventional B-mode ultrasound, a multidimensional colorimetric map representation of tissues for the quantification of tissue stiffness [13]. It employs acoustic radiation force impulse (ARFI), which induces the propagation of mechanical shear waves in tissues (Figure 1). The propagated shear wave, which travels orthogonally with respect to the ARFI [14], is then tracked by means of ultrafast ultrasound wave imaging allowing quantitative mapping of mechanical tissue properties in the form of a real-time colorimetric map [15]. The tissue stiffness, expressed in Young’s modulus (YM), based on the formula YM = 3ρc2 (ρ, tissue density; c, shear wave velocity) [16], is proportional to the squared velocity of the propagated shear waves. Quantification of tissue stiffness could therefore be expressed in YM (kPa) or speed (meter/second). Since plaque vulnerability is invariably dependent on the extent of stiffness, quantification of plaque stiffness could therefore predict its vulnerability [17].
This systematic review, compared to previous studies [18, 19], includes more and recent prospective studies and narrowed its scope on evaluating the feasibility of SWE in carotid plaque risk stratification in relation to ischemic stroke (PROSPERO registration number CRD42022309709).
2. Materials and Methods
This study was conducted in accordance with the Preferred Reporting Item for Systematic Reviews and Meta-Analysis (PRISMA). We systematically searched the databases PubMed, Cochrane, Web of Science, and Embase for all published studies from their inception until November 1, 2022, which evaluated the feasibility of SWE in assessing carotid plaque vulnerability (Supplemental Material). Quality assessment was conducted in accordance with the quality assessment of diagnostic accuracy studies- (QUADAS-) 2 tool [20]. Search and quality assessment was performed by two independent reviewers, and disagreements were resolved through a third reviewer. Further details are described in the Supplementary Material. If not specified, plaque stiffness values are presented as mean.
3. Results
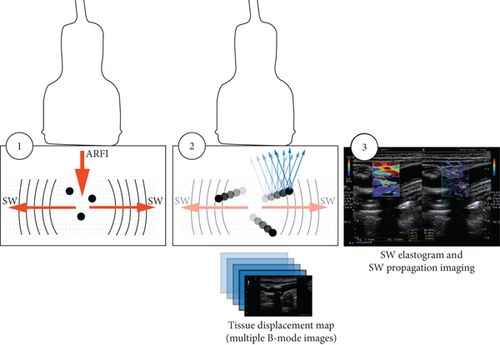
The search yielded 593 studies out of which 18 studies that investigated our primary aim of evaluating the feasibility of SWE in carotid plaque risk stratification were included in the systematic review (Figure 2). The sample size of human studies ranged from 20 to 199 patients. Eleven out of 13 human studies did this by comparing vulnerable/symptomatic with stable/asymptomatic plaques (Table 1). Ten of these eleven studies as well as two additional human studies compared SWE to other plaque imaging modalities (Table 2). In all, five human studies used histology as a standard to assess plaque vulnerability [10, 21–24]. Five studies evaluated the feasibility of SWE in carotid plaque risk stratification by assessing tissues of varying mechanical stiffness in carotid phantom models (Table 3). The majority of the studies showed a low and unclear risk of bias while there seems to be a low risk of applicability concerns across studies (Table 4).
| Literature | Study origin | Sample size | Histology as standard to assess plaque vulnerability | Device | Probe frequency (MHz) | SWE outcomes ∗ |
|---|---|---|---|---|---|---|
| Di Leo et al. [10] | Italy | 43 | Yes | Aplio 500 (Toshiba Medical Systems Corporation, Otawara, Japan) | 5–14 | (i) SWE showed high diagnostic accuracy in identifying vulnerable plaques (sensitivity 87%, specificity 67%, PPV 87%, NPV 67%, and AUC 0.77) |
| Garrard et al. [23] | UK | 25 | Yes | Aixplorer® ultrafast ultrasound system (SuperSonic Imagine, Aix-en-Provence, France) | 5–15 | (i) Vulnerable (50 ± 20 kPa) vs. stable plaques (79 ± 34 kPa), p = 0.027 |
| Goudot et al. [24] | France | 46 | Yes | Aixplorer® ultrafast ultrasound system (SuperSonic Imagine, Aix-en-Provence, France) | 2–10 |
|
| Marlevi et al. [22] | Sweden/USA | 20 | Yes | General Electric (GE) Logiq E9 system (GE Healthcare, Wauwatosa, WI, USA) | 9 | (i) SWE showed moderate to very strong correlation (r = 0.57 to 0.94, p < 0.02) with histological plaque vulnerability parameters (i.e., lipid-rich necrotic core volume, fibrous cap thickness, and intraplaque hemorrhage) |
| Zhang et al. [21] | China | 94 | Yes | Acuson HELX3000, (Siemens Healthcare, Erlangen, Germany) | 4–9 | (i) SWE strongly correlated with type I/III collagen ratio (r = 0.805, p < 0.001) |
| Li et al. [9] | China | 123 | — | Aplio 900 (Canon Medical Systems Corporation, Otawara, Japan) | 5–14 | (i) Symptomatic (52 ± 18 kPa) vs. asymptomatic plaques (78 ± 25 kPa), p < 0.001 |
| Lou et al. [25] | China | 61 | — | Aixplorer® ultrafast ultrasound system (SuperSonic Imagine, Aix-en-Provence, France) | 2–10 | (i) Symptomatic (81 ± 20 kPa) vs. asymptomatic plaques (116 ± 27 kPa), p < 0.01 |
| Ramnarine et al. [26] | UK | 81 | — | Aixplorer® ultrafast ultrasound system (SuperSonic Imagine, Aix-en-Provence, France) | 4–15 | (i) Symptomatic (62 kPa, 95% CI 51–73) vs. asymptomatic plaques (88 kPa, 95% CI 71–105), p = 0.01 |
| Shang et al. [29] | China | 142 | — | Aixplorer® ultrafast ultrasound system (SuperSonic Imagine, Aix-en-Provence, France) | 4–15 | (i) Mean SWV was associated with symptomatic ischemic stroke (B –0.624, offset –0.355, p = 0.004, odds ratio 0.54, 95% CI 0.35–0.82) |
| Sivasankar et al. [27] | India | 60 | — | Acuson S3000 (Siemens Healthcare, Erlangen, Germany) | 4–9 | (i) Symptomatic vs. asymptomatic plaques: proximal 32 vs. 43 kPa, mid 33 vs. 46 kPa, distal 27 vs. 38 kPa, all p < 0.05 |
| Skoloudik et al. [28] | Czech | 97 | — | Aixplorer® ultrafast ultrasound system (SuperSonic Imagine, Aix-en-Provence, France) | 4–15 | (i) Asymptomatic stable (52 ± 30 kPa) vs. asymptomatic progressive (30 ± 18 kPa), p < 0.001, and vs. symptomatic plaques (36 ± 23 kPa), p < 0.033; asymptomatic progressive vs. symptomatic plaques, n.s |
- AUC: area under curve; CI: confidence interval; IQR: interquartile range; NPV: negative predictive value; PPV: positive predictive value; SWE: shear wave elastography; SWV: shear wave velocity. ∗Mean stiffness of vulnerable/symptomatic and stable/asymptomatic plaques is expressed in Young’s Modulus (kPa) or shear wave velocity (m/s).
| Literature | Study origin | Sample size | SWE comparator | Device | Probe frequency (MHz) | Study-relevant outcomes ∗∗∗ |
|---|---|---|---|---|---|---|
| Marlevi et al. [22] | Sweden/USA | 20 | MRPI ∗ | General Electric (GE) Logiq E9 system (GE Healthcare, Wauwatosa, WI, USA) | 9 | (i) SWE could well discriminate vulnerable AHA type VI plaques from the other evaluated AHA plaque types III, IV, and V: longitudinal view 5.8 vs. 4.0–4.2 m/s, p < 0.02; transverse view 7.3 vs. 3.1–3.6 m/s, p < 0.004 |
| Garrard et al. [23] | UK | 25 | Grayscale quantification on B-mode ultrasound ∗ | Aixplorer® ultrafast ultrasound system (SuperSonic Imagine, Aix-en-Provence, France) | 5–15 |
|
| Goudot et al. [24] | France | 46 | Grayscale quantification on B-mode ultrasound ∗ | Aixplorer® ultrafast ultrasound system (SuperSonic Imagine, Aix-en-Provence, France) | 2–10 |
|
| Lou et al. [25] | China | 61 | Grayscale quantification on B-mode ultrasound ∗∗ | Aixplorer® ultrafast ultrasound system (SuperSonic Imagine, Aix-en-Provence, France) | 2–10 |
|
| Ramnarine et al. [26] | UK | 81 | Grayscale quantification on B-mode ultrasound ∗∗ | Aixplorer® ultrafast ultrasound system (SuperSonic Imagine, Aix-en-Provence, France) | 4–15 |
|
| Shang et al. [29] | China | 142 | Grayscale quantification on B-mode ultrasound | Aixplorer® ultrafast ultrasound system (SuperSonic Imagine, Aix-en-Provence, France) | 4–15 |
|
| Zhang et al. [30] | China | 199 | Grayscale quantification on B-mode ultrasound | Aixplorer® ultrafast ultrasound system (SuperSonic Imagine, Aix-en-Provence, France) | 2–10 | (i) Echogenic plaques had higher stiffness than echolucent plaques (proximal 52 ± 16 vs. 16 ± 8 kPa, peak middle 57 ± 17 vs. 11 ± 8 kPa, distal 51 ± 19 vs. 17 ± 9 kPa, all p < 0.01) |
| Skoloudik et al. [28] | Czech | 97 | Grayscale quantification on B-mode ultrasound ∗∗ | Aixplorer® ultrafast ultrasound system (SuperSonic Imagine, Aix-en-Provence, France) | 4–15 |
|
| Carter et al. [31] | USA | 41 | Grayscale quantification on B-mode ultrasound | Mindray Resona 7 (Shenzhen Mindray Bio-Medical Electronics, Shenzhen, China) | 3–9 | (i) Wide range of variation of absolute, mean, and median YM values across each plaque type, however, high correlation between YM and plaque echogenicity |
| Li et al. [9] | China | 123 | SMI ∗∗ | Aplio 900 (Canon Medical Systems Corporation, Otawara, Japan) | 5–14 |
|
| Di Leo et al. [10] | Italy | 43 | CEUS and CTA ∗ | Aplio 500 (Toshiba Medical Systems Corporation, Otawara, Japan) | 5–14 |
|
| Zhang et al. [21] | China | 94 | CEUS ∗ | Acuson HELX3000, (Siemens Healthcare, Erlangen, Germany) | 4–9 | (i) SWE can predict neovascularization of carotid plaques confirmed by CEUS (r = −0.714, p < 0.001) |
- AHA: American Heart Association; AUC: area under curve; CEUS: contrast-enhanced ultrasound; CI: confidence interval; CTA: computed tomography angiography; GSM: grayscale median; MRPI: contrast-enhanced magnetic resonance plaque imaging; NPV: negative predictive value; OR: odds ratio; PPV: positive predictive value; SMI: ultrasound-based superb microvascular imaging; SWE: shear wave elastography. ∗Histology was used as a standard to assess plaque vulnerability. ∗∗Clinical assessment was used as the basis for comparison. ∗∗∗Mean stiffness of vulnerable/symptomatic and stable/asymptomatic plaques is expressed in Young’s Modulus (kPa) or shear wave velocity (m/s).
| Literature | Study origin | Sample size | Device | Probe frequency (MHz) | Tissue stiffness∗ |
|---|---|---|---|---|---|
| Chayer et al. [33] | Canada | 1 | Aixplorer® ultrafast ultrasound system (SuperSonic Imagine, Aix-en-Provence, France) | 4–15 |
|
| Widman et al. [35] | Sweden/Belgium | 8 | Aixplorer® ultrafast ultrasound system (SuperSonic Imagine, Aix-en-Provence, France) | 4–15 |
|
| Widman et al. [34] | Sweden | 3 | Aixplorer® ultrafast ultrasound system (SuperSonic Imagine, Aix-en-Provence, France) | 4–15 |
|
| Widman et al. [36] | Sweden | 6 | Aixplorer® ultrafast ultrasound system (SuperSonic Imagine, Aix-en-Provence, France) | 5–14 |
|
| Ramnarine et al. [37] | UK | 3 | Aixplorer® ultrafast ultrasound system (SuperSonic Imagine, Aix-en-Provence, France) | 4–15 |
|
- SWE: shear wave elastography. ∗Mean tissue stiffness is expressed in Young’s modulus (kPa).
| Study 7 | Risk of bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Carter et al. [31] | ☹ | ☹ | ☹ | ☹ | ☹ | ☹ | ☹ |
| Chayer et al. [33] | ☹ | ☹ | ☹ | ☹ | ☹ | ☹ | ☹ |
| Di Leo et al. [10] | ☺ | ☹ | ☺ | ☺ | ☺ | ☺ | ☺ |
| Garrard et al. [23] | ☺ | ? | ☺ | ☺ | ☺ | ☺ | ☺ |
| Goudot et al. [24] | ☺ | ? | ☺ | ? | ☺ | ☺ | ☺ |
| Li et al. [19] | ☺ | ? | ☹ | ? | ☺ | ☺ | ☺ |
| Lou et al. [25] | ☹ | ? | ☹ | ? | ☺ | ☺ | ☺ |
| Marlevi et al. [22] | ☺ | ☺ | ☺ | ? | ☺ | ☺ | ☺ |
| Ramnarine et al. [26] | ☹ | ? | ☹ | ? | ☺ | ☺ | ☺ |
| Ramnarine et al. [37] | ? | ☺ | ? | ? | ☹ | ☺ | ? |
| Shang et al. [29] | ☺ | ☺ | ☹ | ? | ☺ | ☺ | ☺ |
| Sivasankar et al. [27] | ☹ | ? | ? | ☹ | ☺ | ☺ | ? |
| Skoloudik et al. [28] | ☺ | ☺ | ☹ | ? | ☺ | ☺ | ☺ |
| Widman et al. [35] | ? | ? | ☺ | ? | ☺ | ☺ | ☺ |
| Widman et al. [34] | ? | ? | ☺ | ? | ☺ | ☺ | ☺ |
| Widman et al. [36] | ? | ☺ | ☺ | ? | ☺ | ☺ | ☺ |
| Zhang et al. [30] | ? | ☺ | ☹ | ? | ☺ | ☺ | ☺ |
| Zhang et al. [21] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ |
- ☺Low risk; ☹high risk; ?unclear risk.
3.1. SWE Assessment of Vulnerable/Symptomatic vs. Stable/Asymptomatic Plaques
In predicting vulnerability, SWE showed a strong correlation with histologically assessed type I/III collagen ratio (r = 0.805, p < 0.001) [21] and a moderate to very strong correlation with other histological parameters of plaque vulnerability such as lipid-rich necrotic core volume, fibrous cap thickness, and intraplaque hemorrhage (r = 0.57 to 0.94, p < 0.05) [22] (Table 1). High sensitivity (87%) and moderate specificity (67%) were found for SWE in detecting vulnerable plaques as confirmed by histology after carotid endarterectomy (Table 1) [10]. The stiffness of vulnerable plaques confirmed by histology was lower compared to stable plaques (50.0 ± 19.6 vs. 79.1 ± 33.8 kPa, p = 0.027) [23]. A similar histological study, however, showed no difference in mean plaque stiffness (3.6 [IQR 3.2–4.0] vs. 3.0 [IQR 2.8–4.0] m/s, p = 0.3) (Table 1) [24]. Further results, however, showed the percentage of plaques with a medium stiffness (range 3-5 m/s) to be higher in vulnerable than stable plaques (43 vs. 35%, p = 0.043) [24].
Five out of seven studies which compared plaques of symptomatic and asymptomatic patients reported YM of symptomatic plaques to be lower (27–52 kPa) compared to asymptomatic plaques (38–115 kPa) (p < 0.05) [9, 25–28]. In line with these findings, one study found mean shear wave velocity (SWV) to be negatively associated with symptomatic ischemic stroke (binary logistic regression coefficient –0.624, constant –0.355, p = 0.004, odds ratio 0.54, 95% confidence interval 0.35–0.82) [29]. In the remaining study, SWE could not distinguish between plaques of symptomatic and asymptomatic patients (Table 1) [24].
3.2. SWE Compared to Other Plaque Imaging Modalities
In the only study obtained from our search that used MRPI as a comparator, SWE could well discriminate the particularly rupture-prone American Heart Association (AHA) type VI plaques (7.3 vs. 3.1–3.6 m/s, p < 0.004) [22]. All other evaluated AHA plaque types (Supplemental Table S2), however, indicated similar shear wave velocities (Table 2) [22].
Eight studies compared SWE to grayscale quantification using B-mode ultrasound (Table 2) [23–26, 28–31]. Hyperechoic plaques [32] corresponded to higher YM (51–57 kPa) and higher shear wave velocity (3.9–4.7 m/s), while echolucent plaques on the other hand corresponded to lower YM (11–17 kPa) and lower shear wave velocity (1.5–2.6 m/s) (Supplemental Table S2) [29, 30]. Data from one study showed a strong correlation between YM values and grayscale median (GSM) (r = 0.728, p < 0.05) [25], and three studies found SWE to be superior to GSM in identifying vulnerable or stable plaques using histology as reference [23] and symptomatic or asymptomatic plaques based on clinical assessment [26, 28]. Despite showing a wide range of variation of absolute, mean, and median YM values across each plaque type, one pilot study observed a high correlation between YM and plaque echogenicity [31]. In another study, none of the image texture parameters differed between vulnerable and stable plaques (Table 2), although secondary findings from the same study as previously reported hinted on the feasibility of SWE in carotid plaque risk stratification (Table 1) [24].
In assessing plaque vulnerability, ultrasound-based SMI (odds ratio 4.19, 95% confidence interval 2.09–8.40, p < 0.001) through the assessment of intraplaque neovascularization and SWE (odds ratio 0.95, 95% confidence interval 0.93–0.97, p < 0.001) could both significantly discriminate symptomatic and asymptomatic plaques in the same cohort (Table 2) [9].
SWE showed a strong correlation (r = −0.714, p < 0.001) [21] as well as comparable diagnostic capability with CEUS (sensitivity 87%, specificity 58%, PPV 84%, NPV 64%, and AUC 0.73) in detecting histologically confirmed vulnerable carotid plaques (Table 2) [10]. Similarly, SWE (sensitivity 87%, specificity 67%, PPV 87%, NPV 67%, and AUC 0.77) reported comparable diagnostic capability with CTA (sensitivity 87%, specificity 100%, PPV 100%, NPV 75%, and AUC 0.94) (Table 2) [10].
No comparative studies with SWE were found for ultrasound-based strain elastography, PET, and SPECT.
3.3. SWE Assessment of Tissues with Varying Mechanical Stiffness in Carotid Phantom Models
Data from all five carotid phantom models showed lower and higher YM values to be representative of soft and harder tissues, respectively [33–37]. Results from four carotid plaque phantom studies showed the feasibility of SWE in discriminating vessel wall from soft [33–36] and hard [36] plaques using mechanical testing as standard (Table 3). One additional study indicated the reproducibility of SWE in discriminating vessel wall from both soft and hard plaques (Table 3) [37].
3.4. Reproducibility of SWE in Carotid Plaque Risk Stratification
Data from four studies showed a similar interobserver coefficient of variation in SWE application, depicting a good reproducibility of SWE. These were 0.16 [25], 0.18 [29], 0.22 for the vessel wall and 0.19 for carotid plaques [26], and 0.08-0.20 in a phantom study [37]. Intraobserver coefficient of variation ranging from 0.07-0.22 was reported by one study [37] and 0.14 by another [29]. Further results showed 97% of agreement between two independent observers (p < 0.05) [25].
4. Discussion
In this study, we evaluated the feasibility of SWE in carotid plaque risk stratification in relation to ischemic stroke, which could potentially provide a cheaper and more convenient alternative to guide treatment strategies in patients with carotid artery disease.
Four out of five studies showed a good correlation of SWE with histology, which is the most accurate means of plaque vulnerability assessment, and also the feasibility of SWE to distinguish vulnerable from stable plaques [10, 21–23]. Although the remaining one study could not significantly distinguish vulnerable and stable plaques by mean stiffness values, secondary findings suggest feasibility in carotid plaque risk stratification [24].
Plaques which stand the risk of rupture and distal embolization, and therefore, increase the risk of ischemic strokes are termed “vulnerable” [38]. It should be noted, that the term “symptomatic plaques,” as used in some literature, might be ambiguous since these plaques have been described as symptomatic based on clinical assessment and not on their histological vulnerability. In some cases, the term “symptomatic plaques” may have been related to hemodynamically relevant carotid stenosis or may even have been masked by the stroke of other etiologies, e.g., lacunar infarction. Nonetheless, out of seven human studies which assessed symptomatic and asymptomatic plaques using SWE, five [9, 25–28] showed a significant difference in YM values between these two groups and one [29] reported a significant correlation of lower stiffness values with symptomatic patients (Table 1). A combination of YM with a degree of stenosis could thus improve diagnostic performance and provide more reliable risk stratification [25].
Studies involving human participants, although seemingly similar in study design, reported different YM values for vulnerable/symptomatic as well as for stable/asymptomatic plaques. Nonetheless, YM values differed significantly between vulnerable/symptomatic and stable/asymptomatic plaques within each study, and results were highly reproducible [25, 29, 37]. However, an overlap in mean YM values between studies seems to exist (Table 1). Therefore, the use of the absolute YM values obtained from these studies to distinguish between vulnerable/symptomatic and stable/asymptomatic carotid plaques might not be applicable in clinical practice. Overlap of YM values between studies might potentially be related to differences in SWE devices as well as other technical variables such as acquisition and imaging settings [14, 15], and the type of velocity analysis [14–16, 36]. Aside from technical differences, procedural variations including the type of imaging plane [19] and influences of pulsatile movements of the artery by blood flow [19, 39] or breathing movements [25] might compromise interpretation through the generation of artefacts [10]. We summarize possible means to address these problems in Supplemental Table S3.
Imaging techniques and modalities used for assessing plaque vulnerability are based on their potential to detect one or more factors of plaque vulnerability. These factors, which are most accurately assessed by means of histology, include the thickness of the fibrous cap, the size of the lipid-rich core, and the presence of an inflammatory infiltrate, intraplaque hemorrhage, and/or ulcerations [6]. Due to the higher proportion of lipid core, susceptibility to hemorrhage, and ability to form thrombus, vulnerable plaques are described as “soft plaques.” These are echolucent on conventional B-mode ultrasound and have lower YM values (Figure 3) [23]. On the other hand, stable plaques are more calcified with a higher fibrous content on histology, echogenic on conventional ultrasound, and have higher YM values [40]. Data from six out of eight studies confirm this association between SWE and B-mode ultrasound, by means of grayscale quantification (Table 2) [23, 25, 26, 29–31]. Data from three of these eight studies even hint at the superiority of SWE to GSM in discriminating histologically confirmed vulnerability from stable plaques [23] and clinically symptomatic from asymptomatic plaques [26, 28]. SWE shows a similar diagnostic capability to SMI, which is well known for detecting intraplaque neovascularization, in discriminating symptomatic and asymptomatic plaques in the study population [9]. CEUS and CTA are known to facilitate the assessment of plaque vulnerability through detecting plaque neovascularization as well as ulceration [10, 11, 21]. In detecting plaque neovascularization and ulceration, data show that SWE is equally sensitive as compared to CEUS and CTA [10]. Compared to CEUS and SWE, CTA has a higher specificity [10], probably due to its capability of additionally characterizing plaque vulnerability through the detection of intraplaque hemorrhage, and to some extent, the lipid-rich necrotic core [11].
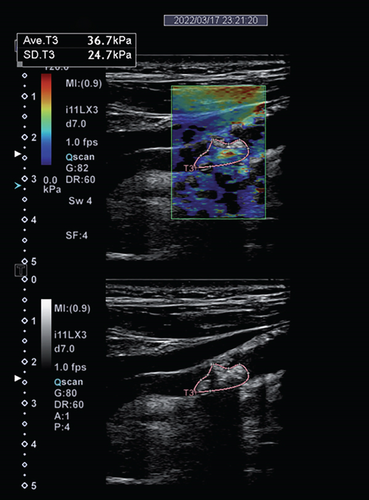
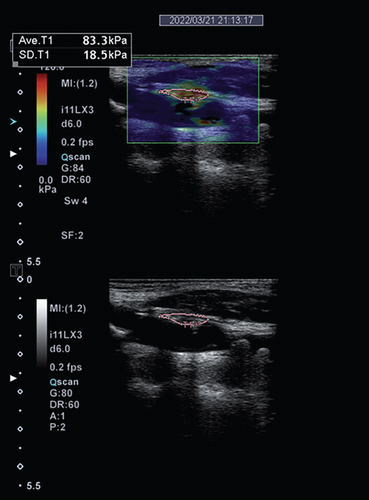
MRPI is currently the gold standard for carotid plaque imaging [4, 5]. With MRPI, carotid plaques are stratified according to the modified AHA classification, which is based on histological hallmark (Supplemental Table S2) [41, 42]. Of most, relevance to carotid plaque vulnerability is AHA type VI plaques, which are complex plaques with possible surface defect and are prone to hemorrhage and thrombus formation [41]. Data shows the feasibility of SWE in distinguishing AHA type VI from other plaque types as assessed on MRPI [22]. Although SWE cannot discriminate between other AHA plaque types [22], for the purpose of carotid plaque risk stratification, this plays no role since the ultimate aim is to differentiate vulnerable from stable plaques.
Supporting the data gathered from real patients with carotid artery disease, studies using SWE in carotid artery phantom models show the capability of SWE in distinguishing plaques of different stiffness as well as the vessel wall itself (Table 3). YM values for vessel walls and soft/hard plaques obtained by either mechanical testing or SWE seemed to vary between studies which is not surprising due to differences in phantom construction. Out of four studies which used mechanical testing as reference, three showed comparable tissue stiffness values to that obtained by SWE, which reflects the potential of SWE for clinical application [33, 34, 36].
As a limitation of SWE, the estimation of YM is based on the assumption that the shear wave is propagated in an incompressible, homogeneous, and isotropic tissue [16]. Most plaques on the other hand are small and anisotropic with irregular morphologies and heterogeneous composition, compromising the conversion of SWV to YM. Thus, reporting SWV rather than YM as an estimation of plaque stiffness may avoid values that are based on incorrect assumptions [24]. Also, access to carotid plaques is hindered by factors such as plaques near the origin of the common carotid artery or plaques in the posterior and medial walls of the internal carotid artery [25], as well as in obese patients, due to excessive signal attenuation by underlying tissues [26]. Available data does not report any safety concerns of SWE, and the force of the wave pulse is known to be a thousand times lower compared to the force of blood pressure on the vessel wall [43]. However, external plaque compression by the transducer during examination just as in any ultrasound-based carotid plaque imaging [44] does not only pose the threat of increased plaque instability and distal embolization especially in vulnerable plaques, but also compromises tissue compression stiffness measurement by increasing the inherent tissue stiffness (Supplemental Table S3) [45–48]. It should also be taken into consideration that the different devices and device programming used in these studies could also be a potential source of variability [15].
5. Limitations of Study
The use of histology as a comparator for plaque vulnerability was reported by five studies. There was heterogeneity in the remaining human studies which assessed the feasibility of SWE by comparing SWE to other modalities of carotid plaque imaging. These comparators have their respective inherent limitation in assessing plaque vulnerability and are, as such, not the most ideal for assessing plaque vulnerability. Three of the studies compared SWE to other carotid plaque diagnostic modalities without using a gold standard as a reference [29–31]. Other sources of heterogeneity in the included studies were with respect to models (i.e., phantom and human) and methodology, for which reason we resorted to conducting a systematic review without a meta-analysis.
6. Conclusions and Future Considerations
Although SWE cannot be used to evaluate individual characteristics of plaque vulnerability, i.e., thin fibrous cap, ulceration, large lipid-rich necrotic core, and intraplaque neovascularization, SWE seems to assess plaque vulnerability through quantification of YM independent of the underlying cause of the vulnerability and for that matter, with good reproducibility. Several studies have hinted on the feasibility of SWE in carotid plaque risk stratification. In spite of the limitations, SWE could potentially facilitate the identification of patients with vulnerable carotid plaques for timely intervention and therapy most especially in institutions where alternatives to MRPI, which is expensive and not readily available, might be needed. On the other hand, patients with stable plaques could be spared possible complications associated with thromboendarterectomy, stenting, and/or dual antiplatelet therapy. However, in order to derive more accurate vascular acquisition settings for the reproducibility of SWE in assessing carotid plaque vulnerability, defining YM thresholds for vulnerable plaques and for predicting restroke, larger prospective multicentric clinical studies would be necessary. These studies would benefit from using histopathology where applicable (i.e., when thromboendarterectomy is originally indicated) or MRPI as comparators to SWE.
Abbreviations
-
- AHA:
-
- American Heart Association
-
- ARFI:
-
- Acoustic radiation force impulse
-
- AUC:
-
- Area under curve
-
- CEUS:
-
- Contrast-enhanced ultrasound
-
- CTA:
-
- Computed tomography angiography
-
- GSM:
-
- Grayscale median
-
- MRPI:
-
- Contrast-enhanced magnetic resonance plaque imaging
-
- NPV:
-
- Negative predictive value
-
- PPV:
-
- Positive predictive value
-
- PET:
-
- Positron emission tomography
-
- SMI:
-
- Superb microvascular imaging
-
- SPECT:
-
- Single-photon emission computed tomography
-
- SWE:
-
- Shear wave elastography
-
- YM:
-
- Young’s modulus.
Disclosure
The research has been performed as part of the employment of the authors, i.e., the University Hospital Tübingen, Tübingen, Germany.
Conflicts of Interest
BB is cofounder and shareholder of AIRAmed (outside the submitted work). UZ received research grants from BMS, European Research Council, German Federal Ministry of Education and Research, German Research Foundation, Janssen Pharmaceuticals, and Takeda and personal consulting fees from Bayer, Cortec, and Pfizer (all outside the submitted work). SP received research support from BMS/Pfizer, Boehringer Ingelheim, Daiichi Sankyo, European Union, German Federal Joint Committee Innovation Fund, and German Federal Ministry of Education and Research, Helena Laboratories, and Werfen as well as speakers’ honoraria/consulting fees from Alexion, AstraZeneca, Bayer, Boehringer Ingelheim, BMS/Pfizer, Daiichi Sankyo, Portola, and Werfen (all outside the submitted work). MW received speakers’ honoraria from Sanofi Pasteur MSD (outside the submitted work). JM, JT, KP, PS, AM, AGE, YW, and UE report no conflict of interest.
Authors’ Contributions
JM and SP conceived and designed the study, and drafted and finalized the manuscript. Literature search and study selection were conducted by JM, MK, KF, and JT. JM, KP, and SP performed the data extraction. Quality assessment was done by JM, JT, and SP. SWE for Figure 2 was measured by JT, PS, and SP. JT, KP, BB, PS, AM, AGE, MK, KF, MW, YW, UE, and UZ reviewed and edited the manuscript. All authors approved the final version of the manuscript. SP supervised all steps of study conduct, had full access to all the data in the study, and had final responsibility for the decision to submit for publication.
Acknowledgments
This study was supported by the Open Access funding enabled and organized by Projekt DEAL.
Open Research
Data Availability
The authors confirm that all data supporting the findings of this study are available within the article and its supplementary materials.




