Challenges and Proven Recommendations of Praziquantel Formulation
Abstract
Background. Schistosomiasis, ranked second to malaria as one of the crucial parasitic infections in the world, infects close to 240 million people as at 2019. Praziquantel, an oral anthelmintic, is the first-line drug for the treatment of schistosomiasis. Although the drug is safe and effective, the formulated tablets come with some limitations such as low bioavailability and bitter taste. This literature review aims to provide information on how to improve the issues of solubility, low bioavailability, and bitter taste associated with the praziquantel formulation and, subsequently, to be helpful in improving patient’s compliance. Materials and Methods. For gathering all pertinent data in this review on improving the praziquantel formulation, the following databases were used: Google Scholar, Science Direct, Scopus, PubMed, Springer Link, Elsevier, and Wiley online library. Results. Literature revealed that in improving the bioavailability of praziquantel, loading the drug with hydrogenated castor oil solid lipid nanoparticles has shown to be effective in prolonging systemic circulation from 7.6 to 95.9 hours after oral administration. Moreover, employing the solid dispersion technique using the fusion method increases the bioavailability of praziquantel about twice as much. Furthermore, incorporating a superdisintegrant or more than one disintegrant to the formulation can enhance the release of praziquantel. The addition of hydroxypropyl-beta-cyclodextrin (HP-β-CD) and sucralose as sweeteners can mask the bitter taste of praziquantel. Conclusion. The formulation approaches outlined in this review can be employed to greatly enhance the solubility, bioavailability, and taste of praziquantel. Although several techniques to improve praziquantel formulation have been widely studied, further studies on the release profile and compatibility studies with other excipients need to be investigated.
1. Background
Schistosomiasis continues to be a public health concern with cases on the rise in sub-Saharan Africa [1]. This neglected tropical disease continues to be the most prevalent parasitic infection in Ghana [2]. According to estimates, 280 million individuals have schistosome infections and 779 million people reside in endemic regions [3]. The life cycle of schistosoma species includes two stages: a sexual stage in humans and an asexual stage in an intermediate host which is often a freshwater snail. Long-term contact with freshwater that has free-swimming cercariae, the parasite’s infectious stage, such as while bathing, swimming, or washing clothes, might result in infection. These enter the subcutaneous tissues, move through the bloodstream to the liver and then the mesenteric and perivascular venous plexuses, before leaving the body entirely. The parasite is eliminated from the body through urine and feces, which enter freshwater. The miracidia then infect their intermediate hosts, where they transform into cercariae, and the cycle repeats [4]. By periodically administering praziquantel to at-risk populations, the World Health Organization’s proposed strategy for schistosomiasis control in 2006 aimed to lessen disease burden by curing mild symptoms and preventing infected people from developing severe, late-stage chronic disease. The adult male and female worms of the genus Schistosoma that cause this infection reside inside the veins of their human host, where they mate and lay fertilized eggs [5]. According to all available data, schistosome eggs, not adult worms, are the cause of morbidity associated with schistosome infections [6, 7]. Praziquantel is the current drug of choice for treating this schistosome infection [8]; however, there is a need for an improved drug formulation. Praziquantel works by causing severe contractions and paralysis of the worms’ muscles due to the rapid calcium (Ca2+) influx inside the schistosome [9, 10]. Morphological alterations such as darkening and damage of the tegumental surface of the adult worm are other early effects of praziquantel [11]. These morphological alterations are accompanied by increased exposure of schistosome antigens at the parasite surface [12]. The worms are then either completely destroyed in the intestine or passed into the stool [13]. Interestingly, praziquantel is relatively ineffective against juvenile schistosomes [14].
Praziquantel is safe and effective against schistosomiasis, but there are some limitations to its use such as issues of reinfection and frequent treatment failures [15]. As a result, there is wide variability in treatment response and poor patient compliance owing to its unpleasant taste coupled with a number of side effects [16]. The commercial preparation is an equal parts racemate mixture of the “laevo” and “dextro” isomers, only the former of which possesses schistosomicidal action in both in vitro and in vivo [14]. Praziquantel is poorly water soluble with very low bioavailability [17]. For the tablet formulation, a high dose must be administered and must be taken three times on an empty stomach. The drug has high intraindividual variability, and there is a wide range of plasma concentration peak values [18]. The main reason for this variability is the slow release of praziquantel from the tablet formulation. This results in low bioavailability and slow drug absorption, which leads to a wide variation in the plasma concentration peak values, particularly in children. A study done by Botros and colleagues proved that there was varied reduction in bioavailability among the generic brands of praziquantel when compared to the pure praziquantel powder affecting antischistosomal potency when those brands were used to treat schistosome-infected mice [19].
Drug formulations employed in pharmacotherapy should meet the patient’s physiological conditions and treatment requirements. This approach is very preliminary but key in administering an accurate and safe dose as well as improving the well-being of the patient. If a better drug formulation is developed, the limitations can be overcome, subsequently enhancing the effectiveness and patient compliance. This literature review seeks to provide information on some recommendations in mitigating the challenges, particularly with the bitter taste and low bioavailability associated with praziquantel formulation.
2. Dose and Administration of Praziquantel
Praziquantel is administered through the oral route. To treat Schistosoma mansoni and Schistosoma haematobium infection, the recommended dose is 40 mg/kg body weight. However, Schistosoma japonicum and Schistosoma mekongi infections require a higher dose of 60 mg/kg body weight, which is typically divided into two administrations spaced a few hours apart [8]. To calculate the appropriate dosage of tablets, a dose pole is used in the field [20]. For the treatment of young children, crushed tablets are mixed with orange juice before administration [5].
2.1. Pharmacokinetics of Praziquantel
2.1.1. Absorption
Praziquantel is rapidly absorbed with an absorption rate of about 80% within 2 hours and 36 minutes; however, it exhibits low systemic bioavailability which varies substantially among individuals. Drugs administered orally have greater pharmacokinetic variability compared with drugs administered intravenously due to the blood flow at the absorption site, as well as the gastric pH [21]. Studies have shown that consumption of food before praziquantel administration may have a positive effect on the drug’s efficacy [18, 22]. A study conducted by Castro and colleagues proved that the bioavailability of praziquantel increases following continuous uptake of food, preferably foods high in carbohydrate content. The area under the plasma concentration curve from 0 to 8 h increased 271% after administration following a high carbohydrate diet [18].
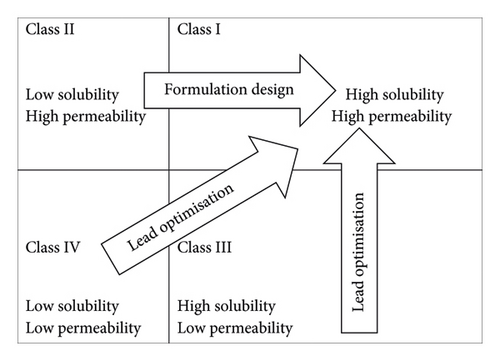
The stomach is normally the first organ in which intense contact between a drug given orally and gastrointestinal (GI) fluids occurs. Although the stomach has a relatively large epithelial surface, its thick mucus layer and short transit time limit drug absorption [23]. The small intestine has the largest surface area for drug absorption in the GI tract, and its membranes are more permeable than those in the stomach [24]. For these reasons, praziquantel is primarily absorbed in the small intestine owing to its ability to cross membranes, which is representative of class II of the drug classification system as shown in Figure 1. The absorption process is mainly due to transcellular transport [4].
2.1.2. Metabolism of Praziquantel
First pass metabolism occurs quickly in the liver; 15 minutes after an oral dosage of praziquantel, 99% of the serum radioactivity was made up of metabolites. The relevant enzyme(s) have not yet been identified with certainty, but according to research using selective inhibition, the lamb and rat are likely to at least contain a member of the P450 3A4 family [25]. There is no description of the significant isozyme in humans. Mono- and di-hydroxy derivatives of PZQ are the main metabolites in both animals and humans. Praziquantel is primarily metabolized extensively in the liver into mono-, di-, and trihydroxylated compounds [26]. The plasma half-life of praziquantel is estimated to be between 1 and 3 hours and more than 80% of the drug is excreted within 24 hours in humans. The systemic bioavailability of praziquantel is therefore very low at less than 20% [17].
2.2. Efficacy of Praziquantel
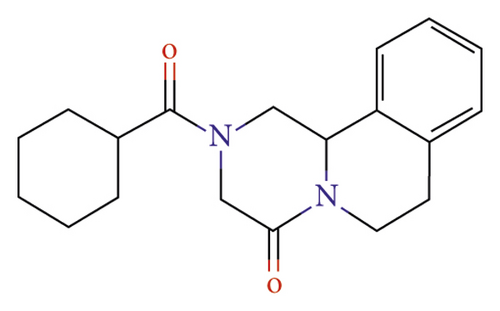
Praziquantel is an effective treatment against all schistosoma species. Therefore, it is the only recommended drug for preventing and treating schistosomiasis despite some observed low cure rates and possible resistance recently reported [21]. The carbonyl functional group of the structure shown in Figure 2 is responsible for the pharmacological action [27]. Studies have shown that praziquantel has a higher efficacy rate against S. haematobium as compared to S. mansoni [28, 29].
Praziquantel administered at 40 mg/kg body weight is the standard dose recommended by WHO for pharmacotherapy in treating schistosomiasis [30]. Articles reviewed mainly reported on the efficacy of different dosages of praziquantel used against both S. haematobium and/or S. mansoni in sub-Saharan Africa. Most studies employed a single dose versus multiple standard doses to see which dose is more effective in managing issues of reinfection [31–33]. The efficacy of praziquantel varied significantly depending on whether it was administered as a single or multiple doses. Moreover, issue of efficacy was influenced by the type of brand of praziquantel administered. A study conducted by Botros and colleagues revealed that there were significant changes in bioequivalence among generic brands of praziquantel [19]. The author mentioned that this could contribute to some treatment failures of mass drug administration (MDA) to control schistosomiasis. The effectiveness of the treatment is also influenced by other factors, including juvenile schistosomes being less susceptible to praziquantel because they are in the systemic circulation, which is evidenced by the drug’s low effectiveness against them [19, 34]. A study conducted by Garba and friends in a highly endemic area in western Niger revealed that administering two 40 mg/kg of praziquantel given three weeks apart was found to be effective [32].
2.3. Side Effects of Praziquantel
Approximately, 30% to 60% of patients experience side effects after receiving praziquantel, but these are typically minor and short-lived, going away within 24 hours [32]. The most frequently experienced side effects include vomiting, abdominal pain, headache, and nausea [33]. The frequency and seriousness of side effects have been repeatedly observed to be a result of the degree of infection, as determined by the quantity of pretreatment eggs [26]. Therefore, it seems that some of the reactions are probably caused by the inflammatory response to schistosome death and the release of their byproducts [14].
3. Challenges and Progress So Far in Improving Praziquantel Formulation
3.1. Bitter Taste
One key drawback associated with praziquantel is the bitter taste which has contributed to noncompliance in the number of mass drug administrations [32]. A preliminary but key step in improving patient drug compliance is making the bitter taste of praziquantel. It is also important to improve the therapeutic efficiency of oral formulations to prevent the occurrence of mucosal irritation. However, the desired masking strategy to be employed in the formulation must not affect the bioavailability of the active ingredient by impeding its release, resulting in poor absorption of the drug [35]. Münster and colleagues employed the electronic tongue and rodent brief-access taste aversion (BATA) model to assess the bitter masking potential of some taste masking agents. From their results, hydroxypropyl-beta-cyclodextrin (HP-β-CD) showed promising taste making abilities for praziquantel [36]. Preliminary studies have been carried out to explore the bitter masking ability of some sweeteners as well as their compatibility with praziquantel, and from the results, sucralose emerged superior [37].
It is also apparent in the literature that there is a liquid suspension of praziquantel (Epiquantel®) manufactured by EIPICO and licensed in Egypt [32]. This is to mask the bitter taste of praziquantel by employing aniseed as the flavoring agent. Epiquantel is packaged in 15 mL dark glass bottles which contains 600 mg of praziquantel per 5 mL. Although there were some advantages to its use in the six-country STUDY, it was found to have no superior properties to crushed tablet substitutes in terms of acceptability and parasitological performance. Again, issues regarding logistics and cost of Epiquantel made it an unattractive alternative to the tablets [30].
3.2. Bioavailability and Solubility
Due to praziquantel’s low hydro solubility and extensive first-pass metabolism, it has a low bioavailability. Reportedly, praziquantel undergoes extensive conversion into an inactive or significantly less potent compound after oral administration. This is one of the drawbacks in fighting this schistosome infection. A study conducted by Xie and his colleagues proved that praziquantel loaded with hydrogenated castor oil solid lipid nanoparticles was able to enhance the bioavailability and prolong the systemic circulation of the drug. The mean residence time of the drug extended from 7.6 to 95.9 hours after oral administration [38].
The drug release of praziquantel from the tablet matrix is very slow, and this is one of the limitations in its fight against schistosomiasis. The drug release of praziquantel has been improved in its use against schistosomiasis by incorporating it in poly(d,l-lactide-co-glycolide) nanoparticles [39]. The solid dispersion technique has the potential to significantly increase the praziquantel dissolution rate and release extension. Gelucire, which is a versatile polymer, was used as a carrier in a 1 : 1 ratio in the specific case of solid dispersion obtained by the fusion method, which allowed for an increase in praziquantel bioavailability of about twice as much [40]. Solid dispersion represents a practical pharmaceutical method for enhancing drug yield through nonmolecular level mixing of substances having a minimum melting point [41]. An in vitro study conducted by Bagade and friends indicated that praziquantel formulated by solid dispersion showed the highest drug release (82.1%) among all formulations [42].
In addition, the study found that solid dispersion offers a practical solid dosage form for drugs with poor water solubility. Praziquantel molecules when incorporated into micelles created in the solution of glycyrrhizic acid disodium salt as solid compositions dissolved in water. Interestingly, the composition of praziquantel had an anthelmintic activity that was 4 to 11 fold higher than that of the standard praziquantel in the opisthorchiasis model [43]. In addition, the pharmacokinetic data showed that the composition tripled the bioavailability of praziquantel.
4. Recommendations to Improve Praziquantel Formulation
Since praziquantel falls under class II of drug classification as shown in Figure 1, the bioavailability can be increased using a variety of formulation techniques, such as enhancing the rate of dissolution or delivering the drug in solution and keeping it there throughout the intestinal lumen [44]. Some of these formulation techniques are highlighted in this study.
4.1. Hot Melt Extrusion Technology
The solid state of praziquantel being crystalline, as shown in Table 1, contributes to its poor solubility, subsequently limiting the oral bioavailability. In order to disrupt the crystal lattice of praziquantel to render it amorphous, hot melt extrusion technology should be employed [45]. This involves the use of polymeric materials such as polyvinylpyrrolidone (PVP) or polyethylene glycol (PEG) above their melting point to incorporate the active pharmaceutical ingredient to attain a molecular level mixing [45]. This molecular blending improves the dissolution profile of the drug’s weak water solubility by transforming the components into an amorphous product with a uniform shape and density. Consequently, this technology has been used to mask the taste of some drugs, which will be an added advantage in the formulation of praziquantel, by creating solid dispersions with a polymer that masks taste [46]. These solid dispersions stop bitter drugs from dissolving in saliva and, as a result, inhibit the interaction of the drug molecules with taste receptors. Again, this exciting technology has other advantages such as reduced processing time and benefits the environment because no solvents are used and improved drug delivery [45].
4.2. Disintegrant and Surfactant
The properties of tablets and active pharmaceutical ingredient release may be significantly impacted by bulking agents and tablet disintegrants. The disintegrants aid in the dosage form’s rapid breakdown in the stomach after ingestion so that the active ingredient is made easily bioavailable [47]. Disintegrants tend to speed up the disintegration of tablets when they come into contact with gastrointestinal (GI) fluid. This in turn depends on how easily the drug’s active ingredient dissolves in the GI fluid as it travels through the intestines. The physical form and chemical make-up of the drug affect its capacity to dissolve. However, the disintegration of the tablet affects the rate of drug dissolution in the body’s bio-fluids [48]. Rapid dissolution accelerates the rate of active ingredient absorption by the body, resulting in the desired blood levels. For immediate release tablets like praziquantel, more than one disintegrant could be incorporated in the formulation process to increase the surface area and reduce the binding agent that holds the solid particles together in the tablet matrix [49]. This can promote or improve the drug release of praziquantel. Moreover, the use of superdisintegrants such as croscarmellose, crospovidone, sodium starch glycolate, and magnesium aluminum silicate in the formulation process can significantly improve the drug release of praziquantel [48]. This is because superdisintegrants employ both swelling and water absorption to enhance the wettability and dispersibility of the tablet matrix [50]. It is also apparent in the literature that the addition of surfactants in the tablet formulation process helps in reducing the disintegration time and enhances the dissolution of hydrophobic drugs [35, 51]. Addition of a surfactant such as polysorbate-80 in the praziquantel tablet formulation process may improve the bioavailability of praziquantel [52].
4.3. Enteric Coating
The first pass effect of praziquantel limits its efficacy against young worms already in the systemic circulation. This is because of the low concentration at the larval tissues [19]. Moreover, the extent of the first pass effect varies individually, and this should be considered in determining the appropriate dose. For the correct implementation and maintenance of pharmacological therapy, understanding the clinical importance of the first pass effect is essential [53]. In order to maintain a safe and effective dose of a medicine that experiences the first-pass effect, it is essential to maintain optimum serum concentrations of the drug. Formulation techniques such as enteric coating can be employed to overcome this challenge. This involves the use of unionized polymers such as shellac, cellulose acetate phthalate but not limited to poly(methacrylic acid-co-methyl methacrylate) to control the drug to be released in the small intestine for absorption [54]. This technique has been used to enhance the bioavailability of some anti-inflammatory drugs like nifedipine [55].
5. Conclusion
Even though there are other obstacles to overcome to control schistosomiasis in poverty-stricken areas in most African countries, we are confident that improving the formulation of praziquantel by employing an amorphous polymer-stabilized solid dispersion technique can improve its solubility and bioavailability. The addition of sucralose as sweeteners can mask the bitter taste of praziquantel which will also improve patient compliance. Furthermore, this insight can serve as a linchpin in the broader campaign to eradicate this debilitating disease from the affected communities, thereby fostering a brighter and healthier future for countless lives.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
DNOK carried out the research work and prepared the manuscript. FAA provided guidance and monitored the research. Authors AA and APA contributed in the final drafting of the manuscript. All authors have read and approved the manuscript.
Open Research
Data Availability
The data supporting the findings of this study are included within the article.




