Antioxidant, Antidiabetic, and Antihypertension Inhibitory Potentials of Phenolic Rich Medicinal Plants
Abstract
Veronica (Plantaginaceae) and Schoenoplectus have a unique chemotaxonomic and phytochemical importance and are widely utilized in Turkish and Traditional Chinese Herbal Medicine (TCM) for treating tonics, influenza, diuretics, expectorants, restoratives, and respiratory diseases, and both are very useful in treating infectious and metabolic disorders as well. This study evaluates two medicinal plant species, Veronica biloba and Schoenoplectus triqueter (L.) Palla; extraction was performed through Soxhlet and maceration methods as well as determination of free and bound phenolics. Evaluated biological screening of (extracts and phenolics) angiotensin-I converting enzyme (ACE), Type-II diabetes (α-glucosidase and α-amylase), and antioxidants potential was performed using modified assays. The angiotensin-I converting enzyme (ACE) 50% inhibition potential in Veronica biloba was found at IC50 = 210.68 μg/mL and in Schoenoplectus triqueter (L.) Palla at IC50 = 229.40 µg/mL, respectively. Meanwhile Type-II diabetes with α-amylase 50% inhibition shown by bound phenolics of Veronica biloba at IC50 = 219.66 µg/mL and its water extract at IC50 = 110.09 µg/mL possesses higher potential, and α-glucosidase potential by free phenolics was found to be active at IC50 = 469.56 µg/mL, while water and ethyl acetate extracts showed higher potential, IC50 = 78.65 µg/mL and IC50 = 97.03 µg/mL, than the standard acarbose, recorded lower. In case of amylase, α-glucosidase showed IC50 = 88.73 μg/mL. Our results showed that both plants possess a direct relationship with the increase in the concentration of extracts and inhibited very strongly angiotensin-I converting enzyme (ACE) and Type-II diabetes (α-glucosidase and α-amylase). The properties of enzyme hindrance may be associated with phenolic compounds and rich phenolic plant antioxidant potential provides a route to the elucidation of natural antihypertension and antidiabetes.
1. Introduction
Natural products are God-gifted, such as extracts from plants that possess a variety of biologically active compounds, and their purification, characterization, and isolation are very helpful for synthesizing a novel drug with chemical diversity to cure a number of health-related diseases utilized as pure compounds or standard extracts [1, 2]. Approximately 10–20% of plants in pharmaceutical studies were revealed positively for harmful diseases including cancer [3]. The extracts from medicinal plants contain several bioactive compounds, and each of them is responsible for any specific bioactivity [4]. According to ecological studies, synthetic drugs have several side effects, while drug or standard extract from medicinal plants shows high and effective results with no or few side effects and is more preferred [5–7]. The Veronica (Plantaginaceae) genus has 79 popular species in a total of 450, and 26 are endemic species present in both temperate and hemisphere regions [8, 9]. This genus has a unique chemotaxonomic and phytochemical importance and is widely utilized in Turkish and Traditional Chinese Herbal Medicine (TCM) for treating tonics, influenza, diuretics, expectorants, restoratives, and respiratory diseases [10]. Previously, we have determined a strong antibacterial and antifungal potential of Veronica biloba extracts [11] as well as phytochemicals and antioxidants comparable with standard acarbose potential [12]. The family Cyperaceae mostly possesses fibrous halophytic plants; one genus of this family is Schoenoplectus present in the river territories of Pakistan, India, Africa, Morocco, and Spain at the extreme Mediterranean [13, 14]; about 49 compounds are extracted from the specie of Schoenoplectus lacustris and evaluated for eutrophic spots; a bioindicator was tested on algae green Selenastrum capricornutum and showed positive potential in comparison with copper sulphate, algaecide [15]. Cyperaceae contains 4231 chromosomes found in only 16% of its species which are helpful for biological activities [16]. Previously, we have reported that Schoenoplectus triqueter (L.) Palla extracts possess potential against both bacterial strains, Gram-positive and Gram-negative, as well as antioxidant potential [17, 18]. Type-II diabetes mellitus represents more than 90% of all elicit diabetes in both developing and developed countries. Around 382 million individuals are viewed as living with diabetes from one side of the planet to the other, and scientific predictions reveal that the number will increase to around 471 million individuals by 2035 [19]. Postprandial hyperglycemia has been embroiled in the advancement of insulin opposition [20], being quite possibly the earliest marker of glucose homeostasis liberation [21]. Furthermore, hypertension, cardiovascular illness, and diabetic neuropathy are linked to this issue [22, 23]. A hyperglycemia therapeutic prevention is carried out by the inhibition of key enzymes such as alpha-amylase and alpha-glucosidase, which are involved in the hydrolysis of carbohydrates and disaccharides to diminish the absorption of glucose. Some commonly available drugs such as miglitol and acarbose are efficient to decrease the glucose level in the blood, but they possess severe long-term side effects; therfore, its uses are very low [24, 25]. According to Bakriset al. [26], hypertension arises mainly due to long-term diabetes and may lead to severe chronic renal failure, a cardiovascular disease [26]. In phenomena of ACE, an important Zn-Metallopeptidase enzyme plays a role in breaking and conversion of bradykinin (vasoconstrictor, vasodilator) and angiotensin-I to angiotensin-II. In therapeutics, the efficient inhibition of ACE (angiotensin-I converting enzyme) is much preferred, which helps in lowering hypertension in normal and hyperglycemic diabetic persons [27]. In medicinal plants, mainly phytochemicals (such as phenolics) show antihypertension and antidiabetes potential [28, 29]. The ongoing hyperglycemia in diabetes stimulates oxidative pressure on organs and tissues [24], which might be constrained by cell reinforcement. Interest in natural antioxidants is presently growing [30–33]. Phenolic compounds address enormous gathering of biologically active phytochemicals which are present in practically all restorative and food plants, as a result of their extremely high environmental pertinence in plant life forms [34–39]. Due to fewer side effects and the high importance of natural antioxidants, phenolics, and phytochemicals, this study evaluates two medicinal plant species, Veronica biloba and Schoenoplectus triqueter (L.) Palla; extraction was performed through Soxhlet and maceration methods as well as determination of free and bound phenolics and investigation of inhibitory activity of phenolic-rich extracts on key enzymes involved in hypertension and diabetes, that is, α-amylase, α-glucosidase, and ACE.
2. Materials and Methods
2.1. Identification and Collection of Plants
The two different medicinal plant species, Schoenoplectus triqueter (L.) Palla and Veronica biloba, were confirmed from various botanical flora databases of plants, a comparison of literature survey, and a botanical export of Government Post Graduate College Mardan, Faculty of Botany. Professor Muhammad Israr confirmed voucher specimen of Veronica biloba (ID: 19-VB.PMI-PGCM) and Schoenoplectus triqueter (ID: 22-ST.PMI-PGCM). The specie Veronica biloba (A-VB) plant used in the project was the whole plant selected. Fresh whole plants in their floweringstage were collected from Sang-e-mar mar, near Par Hoti District Mardan andalso from Surkh Dheri, Rustam, Mardan. The plant collection was done during themonth of February–March. Healthy plants are collected from a fertile land. Whilespecies Schoenoplectus triqueter (L.)Palla (B-ST), only stem part collected in the month of January-February, locations;East 72° 4′ 49″ and North 34° 21′38″ coordinates from river areas of KatlangAsia Mardan 23200 Khyber Pakhtunkhwa Pakistan..
2.2. Drying and Grinding of the Plant
After collection of both plant species, A-VB and B-ST were introduced for surface cleaning first by tap water and then by distilled water slowly for the removal of any small particles of mud and other dust on surface. With the help of scissors and knives, plants were separately cut into smaller pieces and kept in a dust-free protective environment to avoid contamination for 3 weeks without exposure to any light at room temperature. After complete drying of both species, they were ground via a normal grinder to increase the surface area and obtain uniform size particles for a better extraction process in less time with a high yield.
2.3. Extractions
2.3.1. Soxhlet Extraction
The Soxhlet hot continuous method of extraction was followed according to reports [11, 12, 17, 18] for both plants. At first, two sterilized porous bags were manually prepared, in which each possesses 20 gm by weight of the fully powdered plant. 250 ml ethanol was placed in the Soxhlet round bottom (R.B) flask lower section. In the second, porous cellulose bags were kept in the Soxhlet thimble chamber upper section. Additionally, water outflow and inflow were provided to the upper condenser section for the successive extract process and liquid condensation; a fixed temperature in the range of 35–45°C was provided by the Monteux heater. After 14–18 hours, a clear liquid from the Soxhlet siphon arm was obtained without leaving residue in cycling. Each plant extract obtained was fractionated into different solvents, n-hexane, dichloromethane, water, and ethyl acetate fractions under a controllable water bath; each dried fraction was saved and used for further biological analysis.
2.3.2. Maceration Extraction
The maceration method of extraction was followed according to reports [11, 12, 17, 18] for both plants. In this setup, two sterilized Pyrex-glass jars were used, in which each possesses 30 gm by weight of the fully powdered plant; a solvent of 300 mL ethanol for successive extractions was used. At room temperature in shade placed jars for 22 days with air-tight cap, at least twice for 10–15 minutes, daily shaking with stirring was performed to transfer slowly soluble metabolites to solvent. After filtration, each plant extract obtained was fractionated into different solvents, n-hexane, dichloromethane, water, and ethyl acetate fractions under a controllable water bath; each dried fraction was saved and used for further biological analysis.
2.4. Phenolic Extractions
The phenolic contents were extracted according to Chu et al. [40]. Free phenolics were extracted from ethanol fraction by filtration (Whatman filter paper) and evaporation in rotary (<45°C); for stability, dry extract was subjected to lyophilization and then stored (<−6°C). Meanwhile bound phenolics were extracted from the residue of free phenolics and ethyl acetate fractions, with condition of room temperature 22 ± 6°C, NaOH (M = 2) 15 mL for hydrolysis, pH = 2.1 ± 0.1 (adjusted by HCl), and constant stirring of mixture for 45 minutes. Then ethyl acetate was subjected to dryness and evaporation (<45°C) and kept for further processing.
2.5. Determination of Total Phenolics
The content of phenolics was determined as illustrated by Singleton et al. [41]. At first, Folin-Ciocalteu’s reagent (10%, 3 mL) and extracts were taken under Na2CO3 (8%, 2 mL) for oxidation and neutralization and subjected to an incubation period (40°C, 30 min). At last, the absorbance was recorded at a wavelength of 765 nm by UV-spectrophotometer. The standard gallic acid was used for specified values.
2.6. Determination of Reducing Power
The reducing power was determined as illustrated by Oyaizu [42], which is based on the reduction power of FeCl3 solution. At first, K3[Fe(CN)6] (Potassium Ferricyanide) 1%, 3 mL, Na2HPO4 (Sodium Phosphate) having M = 200 mM and pH = 6.5 ± 0.2 amount 3 mL and same amount of samples were mixed and subjected to incubation (40°C, 20 min). Secondly, Trichloroacetic acid (TCA), C2HCl3O2 10%, 3 mL, was added and mixture was obtained at 2600 rpm/10 minute after centrifugation; clear supernatant (5 mL) mixed with FeCl3 (Ferric Chloride) 0.1%, 1.5 mL, in distilled water. At last, the absorbance was recorded at a wavelength of 710 nm by UV-spectrophotometer. The standard ascorbic acid was used for specified values.
2.7. Total Antioxidant Potential
The total antioxidant potential was evaluated as illustrated by Sharifi Rad et al. [43], using ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)). First, ABTS generation was performed by treating ABTS with Potassium Persulfate (K2S2O8); both compositions are 6 mM, and2.3 mM, with duration of 12 hours at dark (to avoid decomposition). After dilution of the extracts with ABTS (amount fixed 0.5 mL+2 mL) solution, at last, the absorbance was recorded at a wavelength of 730 nm by UV-spectrophotometer. The standard trolox was used for specified values.
2.8. Alpha-Amylase
The α-amylase inhibitory potential was evaluated as illustrated by Yousaf et al. [44] using a series of phenolics (free and bound) and A-VB fraction extract dilution in the range of 5–150 µL. First, α-amylase (porcine pancreatic) solution, 0.5 mg/mL, 500 µL buffer (pH = 6.9 ± 0.02) of Sodium Phosphate (M = 0.02) and Sodium Chloride (M = 0.006), was incubated for 15 min at 25°C. Then, individually 1% 500 µL solution of starch was added subsequently in the buffer (pH = 6.9 ± 0.02) of Sodium Phosphate (M = 0.02) and Sodium Chloride (M = 0.006); again the whole mix was incubated for 15 min at 25°C, for countering over, 1 mL DNS (dinitrosalicyclic acid) added. Afterward, the reaction mixture for 15 minutes was transferred to a controllable water bath (containing distilled water) at room temperature range of 22 ± 4°C. At last, for dilution, 10 ml sterilized water was added and the absorbance was recorded at a wavelength of 540 nm by UV-spectrophotometer. The data are illustrated in percent inhibition placed standard acarbose [45, 46].
2.9. Alpha-Glucosidase
The α-glucosidase inhibitory potential was evaluated as illustrated by Sharifi Rad. et al. [47], using a series of phenolics (free and bound) and A-VB fraction extract dilution in the range of 5–150 µL. First, α-glucosidase (1.0 U/mL) solution 100 µL, 500 µL buffer (pH = 6.9 ± 0.02) with Sodium Phosphate (M = 0.1), was incubated for 15 min at 25°C. Second, p-Nitrophenyl-α-D-Glucopyranoside (M = 5 mM) 50 µL solutions were added and the same incubation was repeated. At last, the absorbance was recorded at a wavelength of 405 nm by UV-spectrophotometer. The data are illustrated in percent inhibition placed standard acarbose [48].
2.10. Angiotensin-I Converting Enzyme Inhibition
The ACE (angiotensin-I converting enzyme) inhibition potential determination, using the Cushman and Cheung procedure, was followed as reported in [47]. In the first incubation period (15 minutes at 37°C), the standard ACE 50 µL and diluted phenolics in 5–50 µL were mixed; solution was fixed at 4 mU/mL. Second, Bz–Gly–His–Leu substrate (M = 8.33 mM) supported by buffer pH 8.3 of Tris-HCl (M = 125 mM) in a fixed amount of 150 µL was added for initiation of the enzymatic reaction. In the second incubation period (30 minutes at 35°C), HCl (M = 1) was added with amount 250 µL for countering the reaction. After bond breaking of Gly–His ethyl acetate, 1 mL was added to isolate Bz–Gly by centrifugation and evaporation from reaction. The sterilized water was added for residue analysis in UV- spectrophotometer, calibration, and absorbance recorded at a wavelength of 228 nm. The results were plotted as % inhibition.
2.11. Determination of IC50
The Median Inhibitory Concentration (IC50) was determined for 50% inhibition potential in each case of biological activity α-amylase, α-glucosidase, and angiotensin-I converting enzyme using Excel, Prism, and Origin software.
3. Results and Discussion
The extraction fractionation, phenolics (both free and bound phenolics), ACE (angiotensin-I converting enzyme), α-amylase, and α-glucosidase were determined in both plants, Veronica biloba (A-VB) and Schoenoplectus triqueter (L.) Palla (B-ST), according to the reports of [11, 12, 17, 18, 40–48]. The total phenolics determination results are shown in Table 1. The numbers of bound phenolics found in species Veronica biloba, 62.02 ± 5.2, are higher than those of bound phenolics found in Schoenoplectus triqueter, 41.6 ± 2.5. Meanwhile, for free phenolics, 81.34 ± 0.5, for Veronica biloba and those, 54.11 ± 1.5, for Schoenoplectus triqueter, the results are derived with standard gallic acid mg/100 gm as mean ± standard deviation (having P significantly =< 0.05). That closely resembles previous literature [40, 49–52]. The total antioxidant potential or capacity results are in Table 1 and are determined via standard mmol trolox/100 g; the species Veronica biloba possesses higher potential in both cases of free and bound phenolics, 12.21 ± 1.5 and 16.09 ± 1.2, than Schoenoplectus triqueter which showed 9.8 ± 0.05 and 11.03 ± 0.1, respectively, as mean having P significantly < 0.05, and the higher antioxidant potential is due to phenol content in the plants [24, 48]. The reducing power determination with standard ascorbic acid as mean ± standard deviation (P significantly =< 0.05) is presented in Table 1, with higher values recorded in the case of free phenolics only for Veronica biloba, 14.08 ± 1.5, and Schoenoplectus triqueter, 9.08 ± 2.05. Polyphenols are antioxidants of the plant, which have an important function in breaking up peroxides, neutralizing and absorbing free radicals, and extinguishing singlet and triplet oxygen [53]. Phenolics can be found as conjugated forms or free aglycones in different tissues with glucose as glycosides or other moieties in almost every plant organism [54].
| Samples extracts | Total phenolics | Total antioxidants | Reducing power |
|---|---|---|---|
| Bound phenolic(A-VB) | 62.02 ± 5.2 | 12.21 ± 1.5 | 10.66 ± 2.0 |
| Bound phenolic(B-ST) | 41.6 ± 2.5 | 9.8 ± 0.05 | 7.2 ± 1.5 |
| Free phenolic(A-VB) | 81.34 ± 0.5 | 16.09 ± 1.2 | 14.08 ± 1.5 |
| Free phenolic(B-ST) | 54.11 ± 1.5 | 11.03 ± 0.1 | 9.08 ± 2.05 |
- The statistical data in triplicate (n = 3) are represented as mean ± standard deviation (having P = <0.05); total antioxidant equivalent is determined via trolox standard, and reducing power equivalent is determined via ascorbic acid standard. (A-VB) = Veronica biloba; (B-ST) = Schoenoplectus triqueter (L.) Palla.
The decrease in the antioxidant capacity normally occurs by conjugation present in the phenolic backbone through the hydroxyl groups; hence, the free radical resonance stabilization depends on the free hydroxyl groups present on the phenolic rings. Some lines of evidence suggest that Veronica species possess antioxidant activity marked as insufficient; however, limited studies claimed that few of Veronica species show antioxidant activity [55, 56]. It was observed that the aerial part of Veronica persica contains Persicoside, a phenylethanoid glycoside [57–59]; however, lots of phenylethanoid glycosides have been discovered which possess a wide range of biological activities, containing anticancer and antioxidant properties [60, 61].
The high phenolics containing compounds like phenylethanoids and flavonoids suppress the effect of Veronica species on NO production [62]. It has been investigated that most of the phenolic compounds discovered from Veronica species are beneficial for human health [37, 56, 63]. The antioxidant activity of phenolic compounds is also valuable for delaying or lowering the growth of inflammation [64]. In this way, we identified that Veronica biloba and Schoenoplectus triqueter phenolic extracts showed reducing power in the form of ascorbic acid equivalents (Table 1), exposing that the phenolic extract for bounding had lower reducing power than the free phenolic extract [65, 66].
The therapeutic approaches toward the control or reduction of hypertension and hyperglycemia involve the inhibition of carbohydrates metabolism enzymes that are ACE (angiotensin-I converting enzyme), α-glucosidase, and α-amylase [67–69]. The experimental results of ACE, α-glucosidase, and α-amylase activity of Veronica biloba and Schoenoplectus triqueter (L.) Palla of the extracts, free and bound phenolics, are as shown in Table 2 and Figures 1–8.
| Extracts | 50% median inhibitory potential (IC50 µg/mL) | ||
|---|---|---|---|
| ACE | α-amy | α-glu | |
| Bound phenolic(A-VB) | 210.68 | 219.66 | 608.31 |
| Bound phenolic(B-ST) | 229.40 | 741.19 | >749.35 |
| Free phenolic(A-VB) | 249.05 | 573.39 | 469.56 |
| Free phenolic(B-ST) | 319.59 | >749.52 | 673.05 |
| Water(A-VB) | — | 110.25 | 78.65 |
| Ethyl acetate(A-VB) | — | 121.09 | 97.03 |
| n-Hexane(A-VB) | — | 148.01 | >149.71 |
| Dichloromethane(A-VB) | — | 123.68 | 139.93 |
| Acarbose | — | 138.79 | 88.73 |
- The statistical data in triplicate (n = 3) are represented as mean ± standard deviation (having P = <0.05), calculated by software. (A-VB) = Veronica biloba; (B-ST) = Schoenoplectus triqueter (L.) Palla; ACE = angiotensin-I converting enzyme; α-amy = α-amylase; α-glu = α-glucosidase.
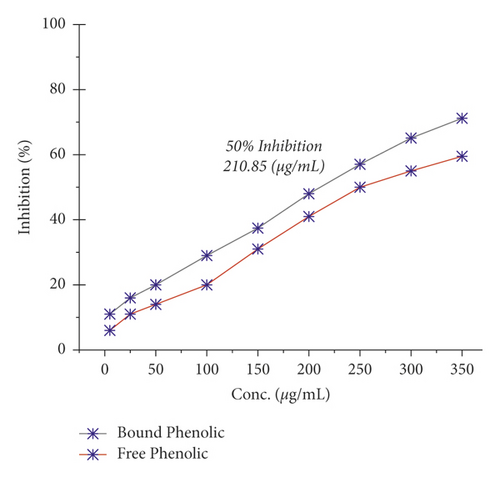
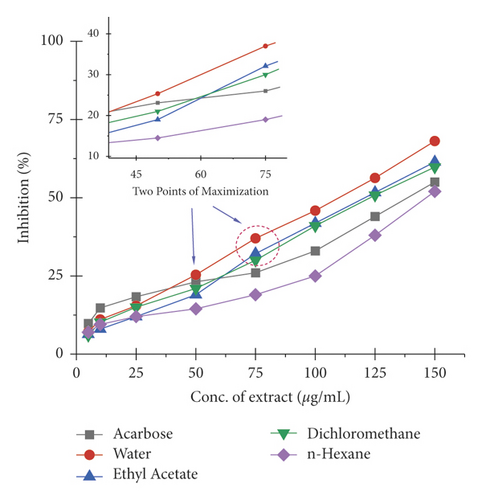
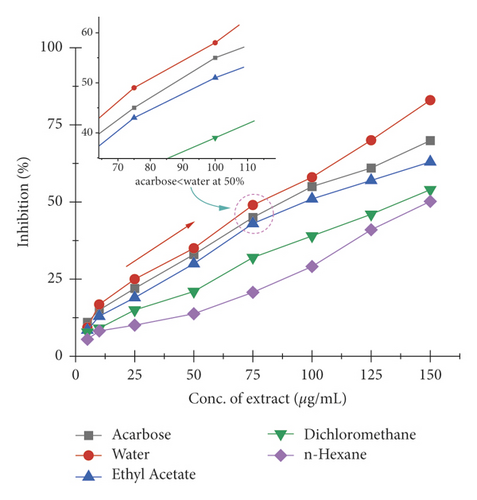
The α-amylase inhibition activity of Veronica biloba fraction extracts showed higher efficiency in the following order: water (110.25 µg/mL) > ethyl acetate > dichloromethane > acarbose > n-hexane (148.01 µg/mL); 50% median inhibition for water fraction appeared at a very lower concentration than standard acarbose and it is shown in Figure 1 that in the range of 50–75 µg/mL there is a direct relation found between inhibition and concentration; the exception was that n-hexane fraction pushed slowly. Meanwhile, in the case of α-glucosidase, the overall activity of water extract was found to be >75% and the results described the following order: water (78.65 µg/mL) > acarbose > ethyl acetate > dichloromethane > n-hexane (149.71 µg/mL); the 50% inhibition for first sequence order appeared at 75 µg/mL as shown in Figure 2.
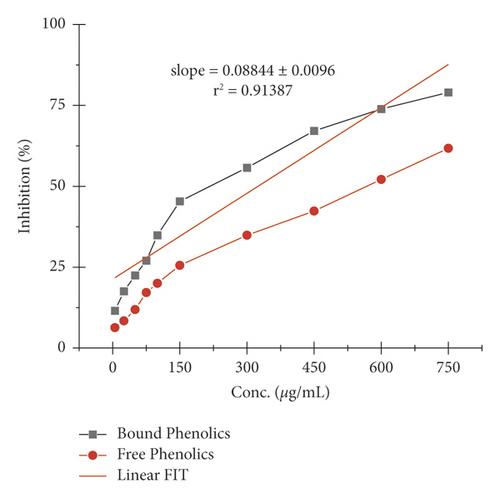
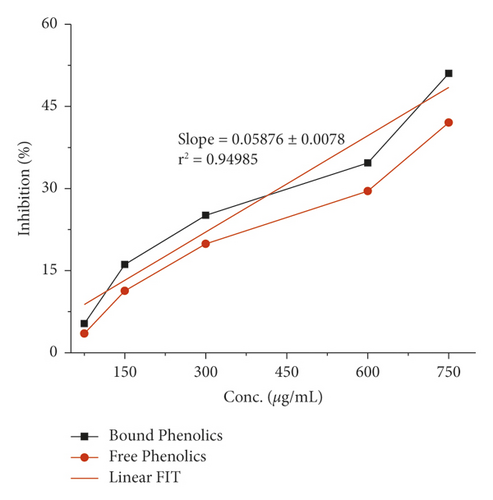
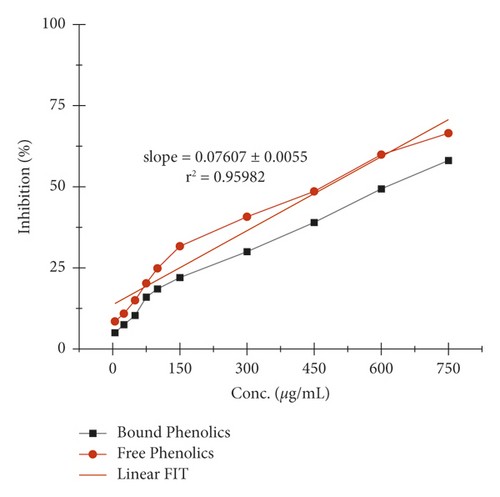
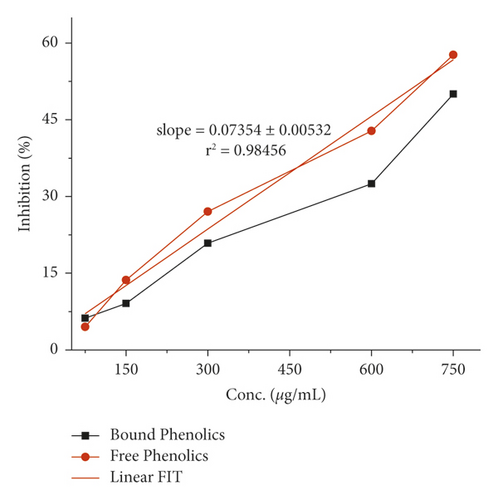
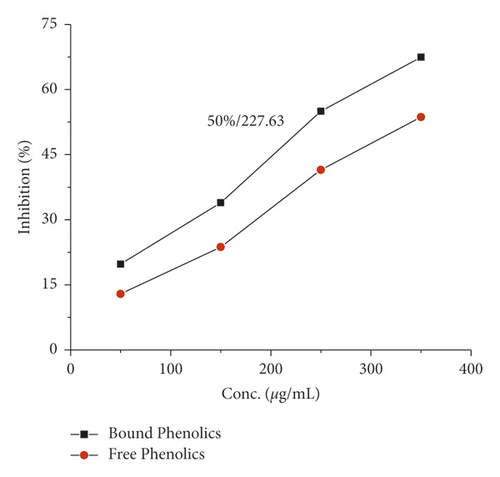
The α-amylase inhibition activity for both plants, Veronica biloba and Schoenoplectus triqueter, and free and bound phenolics in comparison are shown in Figures 3 and 4, respectively; in both plant species, bound phenolics showed 50% activity, while Veronica biloba (219.66/mL) potential was noticed to be higher and more potent with change in concentration. In the case of α-glucosidase inhibition, free phenolics were active for Veronica biloba, 50% = 469.56 µg/mL, and Schoenoplectus triqueter, 50% = 673.05 µg/mL, significantly having P = <0.05, as shown in Figures 5 and 6. Dose-depending antihypertensive activities of both species extracts (bound and free phenolics) are carried out via ACE inhibition in both plants. Bound phenolics showed greater efficiency than free phenolics.
Phenolics of Veronica biloba 50% = 210.68 µg/mL > Schoenoplectus triqueter 50% = 229.40 μg/mL as shown in Figures 7 and 8 and Table 2; in both cases, free phenolics were lower. However, previous studies showed that phytochemicals have lower α-amylase inhibition than α-glucosidase inhibition [49, 50, 70, 71]. The antidiabetic and acarbose possess excess α-amylase inhibition which would avoid the side effects [72]. Thus, plant phenolics with high α-glucosidase and mild α-amylase inhibitory activities have been suggested as suitable substitutes for the clinician to the analogous synthetic inhibitors [67]. Moreover, the antihypertensive potential of phenolic extracts has also been examined through the inhibition of ACE. As shown in Figures 7 and 8, both extracts exhibited a high ACE inhibitory activity in adose-dependent manner. In specific, bounded phenolic extracts (P < 0.05) had very high inhibiting activity on enzymes compared to free phenolic extracts. Further studies on the physical structure of human ACE have given us evidence of the presence of a group of amino acids in the protein molecule cysteine (cysteine is responsible for the formation of disulfide bridges [69]. That is why ACE controlling activity of Veronica persica may be because of the interactions between (bound and free) phenolics and disulfide (cysteines which are oxidized) that are located over the surfaces of macromolecules responsible for functional and structural changes which are in turn associated with enzymes stopping [69]. Our results support the greater α-glucosidase, α-amylase, and ACE reducing activities as shown in Figures 1–8 with bounded extract of phenolics in comparison with the unbounded phenolic extract. The main reason may be associated with the higher water loving of bound phenolics, which are greatly in the form of glycosides. Compared to the free phenolics which are mainly in the form of a-glycones, enzymes (ACE and alpha-amylase) are active in medium containing water, which are inhibitors directing toward positive mean; it increases the interaction between inhibitors and enzymes; the interaction effects of bonded phenolics are greater compared to unbounded phenolics. These studies revealed the inhibition activity of phenolic compounds which might be involved in bridges, that is, disulfide bridges located on the outer layer of α-amylase, thus the enzyme responsible for the modification of the function and structure [68–73]. The reason for the inhibition of ACE, α-amylase, and α-glucosidase potential of both plant species maybe is due to the direct interaction of the phenolics with the disulfide bridges of the enzymes. Furthermore, the activity will be confirmed with the purification of extracts and isolation of phenolics; more consideration is required.
4. Conclusions
It is concluded that both plants, Veronica biloba and Schoenoplectus triqueter (L.) Palla, showed a quiescent hindrance in in vitro ACE (angiotensin-I converting enzyme), α-glucosidase, α-amylase, and antioxidant bioactivities and are helpful in the therapeutic investigation of hypertension and hyperglycemia, linked to Type-II diabetes. The properties of enzyme hindrance may be associated with phenolic compounds. Additional analysis and studies are firmly suggested to clarify the basic phenolics compounds which are moderated by free radicals; the identification and isolation of the components chemical outfit of plants may lead to utilization in clinical trials.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The authors thank Amir Hassan of the Department of Natural Sciences, Novosibirsk State University, 630090, Novosibirsk, Russia, for writing and handling the manuscript and also acknowledge Professor Himayat Ullah for project assessment and evaluation, in addition to experimental credit to Abrar Hussain, Department of Biological Sciences, International Islamic University, 44000, Islamabad, Pakistan.
Open Research
Data Availability
All data used to support the findings of this study are included within the article.