Synthesized Phosphonium Compounds Demonstrate Resistant Modulatory and Antibiofilm Formation Activities against Some Pathogenic Bacteria
Abstract
A library of six compounds with new hybrids in a single molecule triazole ring attached to the phosphonium salts was synthesized. Click chemistry was, however, used to synthesize the 1-, 2-, and 3-triazole intermediates as a tether for the hybrid phosphonium salts. Their antibacterial activity against Gram-positive bacteria (Staphylococcus aureus and Enterococcus faecalis), Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa), and Mycobacterium smegmatis mc2155 was determined using the HT-SPOTi assay. Compound 2 showed the most effective antimicrobial activity as it inhibited the growth of Pseudomonas aeruginosa and Staphylococcus aureus at 0.0125 µg/mL and 31.25 µg/mL, respectively. From the FICI data, compounds 2ET-TOL (2) and RABYL-TOL (4) successfully modulated the activities of amoxicillin against Pseudomonas aeruginosa and Staphylococcus aureus. All the test compounds exhibited a concentration-dependent biofilm formation inhibition against S. aureus, except P-Z (compound 6). Compounds P-MEOXY (1) and 2ET-TOL (2) exhibited mild activity against P. aeruginosa with compound 4 showing antimycobacterial activity at 500 µg/mL.
1. Introduction
Infectious diseases have over the years threatened the survival of humans globally [1]. As one of the leading causes of mortality, infectious diseases caused by parasites, bacteria, viruses, and fungi result in over one-fourth of deaths worldwide every year [1, 2]. Since these pathogens are ubiquitous and can spread through the air, water, and food, their control and subsequent prevention continue to be a daunting task. To combat these microorganisms, all kinds of antimicrobial agents including antibiotics, disinfectants, and antiseptics have been used [3]. However, the irrational or indiscriminate use of most antibiotics has triggered the emergence of resistant strains of microbes, leading to slow progress in the fight against these pathogens. This continuous insurgence of resistance strains calls for the search for compounds or antibacterial agents with new mechanisms of action. Presently, quaternary ammonium salts, which are positively charged compounds, mostly found in disinfectants have been widely used as antibacterial agents [4]. On the other hand, quaternary phosphonium salts are positively charged lipophilic cations with established antibacterial activities and a better killing rate than their quaternary ammonium counterparts but have been underutilized in pharmaceutical formulations though they can better enhance the antimicrobial efficacy [5]. For instance, various phosphonium salts possessing alkyl chains of different carbon lengths exhibited antimicrobial activities [6]. The linking of different functionalities together has been one of the tools in designing drugs to overcome antimicrobial resistance [7, 8]. This is evident in some hybrid compounds containing a heterocyclic ring and phosphonium salts showing antimicrobial activity [9].
Hence, this study sought to synthesize some hybrid phosphonium salts with potential antimicrobial activity against human pathogens.
2. Materials and Methods
2.1. Chemistry
2.1.1. General
All solvents and reagents for the synthesis of the target compounds were obtained from commercially available sources including, among others, Sigma-Aldrich, Fisher Scientific, Fluorochem, and Alfa Aesar. Solvents were used directly in the reaction flask or as anhydrous via the nitrogen or argon line. Thin-layer-chromatography (TLC) analysis was performed on silica gel plates (E. Merck silica gel 60 F254 plates) and visualized by ultraviolet (UV) radiation at 254 nm. The compounds were purified by gravity column chromatography with silica gel as a stationary phase (Merck 60, 230−400 mesh). Infrared (IR) spectra were recorded in the range 400–4000 cm−1 using a Perkin-Elmer FT-IR spectrophotometer (Japan) which employed attenuated total reflection (ATR) with internal calibration. Samples were run neat. 1H, 13C nuclear magnetic resonance (NMR) and DEPT-135 analysis were performed on a Bruker Ascend FT NMR 500 MHz spectrometer (NM 103508–10, Germany) using TMS (residual solvent) as the internal standard and deuterated dimethyl sulphoxide (DMSO-d6) to prepare the samples and as the lock sourced from Cambridge Isotope Laboratories Inc (United Kingdom). 31P spectra were also obtained for the phosphonium salts after employing a phosphorous probe. Spectroscopic data was assigned with the following abbreviations: br-broad, s-singlet, d-doublet, t-triplet, and m-multiplet. Chemical shifts were recorded in ppm. Coupling constants J are expressed in Hz. High-Resolution Mass spectra (MS) were measured on 6420 Triple Quad with an electrospray ionization source from Agilent Technologies. The purity of all tested compounds was determined by analytical RP-HPLC in tandem with MS using Agilent 1290 infinity series and a Mightysil C18 column (150 mm × 4.6 mm, 15 μm) using a gradient elution system and a flow rate of 1 ml/min at photodiode array detection wavelength at 270 nm. Mass spectra were registered in both ESI (−ve) modes. Uncorrected melting points were determined by an open capillary method using a Gallenkamp (C9759, England) melting point apparatus. All the compounds tested for their biological activity are >90% pure, confirmed with 1H NMR and HPLC-MS. Scanned spectra for all compounds are shown in Figures SM5–SM25.
2.1.2. General Procedure 1: Azides’ Formation
The synthesis of azides in Scheme 3.1 as described by Bock and coworkers was followed [10]. This scheme was applied to the synthesis of nitroaromatic azides. The azides were used without further purification for the ‘click’ reactions.
2.1.3. General Procedure 2: Synthesis of the 1,2,3-Triazoles (‘Click’ Reaction)
The ‘click’ reaction procedure in Scheme 3.3 was adapted based on method reviews described by Bock and coworkers [11].
2.1.4. General Procedure 3: Bromination of the 1,2,3-Triazoles
The bromination process in Scheme 3.4 as described by Zhao and coworkers was followed, replacing the starting material with the ‘clicked’ product in general procedure 2 [12].
2.1.5. General Procedure 4: Synthesis of the Phosphonium Salts
The phosphonium salts synthetic procedure as described by [13] was followed.
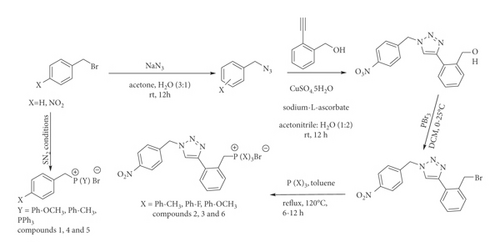
The scheme for the synthesis of the phosphonium salts and a tethered/hybrid phosphonium salt is shown in Figure 1.
Tris (4-methoxy phenyl) (4-(1-(4-nitrobenzyl)-1H-1, 2, 3-triazol-2-yl) benzyl) phosphonium bromide (Compound 1). Following the general procedure 4, the reaction of 4-(2-(bromomethyl) phenyl)-1-(4-nitrobenzyl)-1H-1, 2, 3-triazole (1 eq., 0.139 g, 0.50 mmol) and tris (p-methoxyphenyl) phosphine (1.05 eq., 0.200 g, 0.52 mmol) produced the title compound as a white solid (0.248 g, 76%) after purification. mp > 300°C; IR: νmax/cm−1: 2888 (sp3 C-H), 1455 (N=N), 1592, 1569, 1520 (Ar C=C-H), 531–526 (ArP-C); 1H NMR (500 MHz, DMSO-d6, room temperature): δH 8.30 (1H, s, HAr triazole) 8.28–8.26 (2H, d, J = 10.0 Hz, HAr), 7.53–7.51 (3H, m, HAr), 7.33–7.31 (6H, m, HAr), 7.17–7.16 (6H, m, HAr), 5.72 (2H, s, -CH2), 5.70–5.60 (2H, d, J = 15.0 Hz, –CH2), 3.86 (9H, s, Ar-OCH3; 31P (500 MHz, DMSO-d6 room temperature); δP 21.6; 13C (500 MHz, DMSO-d6 room temperature) δC 160.6 (3C), 144.9 (1C), 142.3 (1C), 134.6 (5C), 134.0 (1C), 130.7 (1C), 128.9 (2C), 123.8 (2C), 114.3 (6H), 110.3 (3C)’; Ar: 57.0 (2C) 55.8 (3C, methyl carbons), 20.7 (2C); HRMS (ESI): m/z calculated for HRMS (ESI): m/z calculated for (M-HBr)+ C37H34N4O5P+, 645.2210, found 645.2210.
4-(1-(nitrobenzyl)-1H-1, 2, 3-triazol-2-yl) benzyl) tri-p-tolyl phosphonium bromide (Compound 2). Following the general procedure 4, the reaction of 4-(2-(bromomethyl) phenyl)-1-(4-nitrobenzyl)-1H-1, 2, 3-triazole (1 eq., 0.180 g, 0.5 mmol) and tri-p-tolyl phosphine (1.05 eq., 0.200 g, 0.75 mmol) produced the title compound as a white solid (0.280 g, 93%) after purification. mp 280–283°C. IR νmax/cm−1: 3032 (sp2 C-H), 1455 (N=N), 1598, 1525, 805 (Ar C=C-H), 565–450 (Ar-P-C); 1H NMR (500 MHz, DMSO-d6, room temperature): δH 8.20–8.19 (2H, d,, J = 5.0 Hz, HAr), 7.57 (1H, s, HAr triazole) 7.48–7.31 (14H, m, HAr), 5.70 (2H, s, -CH2), 5.68 (2H, d, –CH2), 2.40 (9H, s, Ar-CH3); 31P (500 MHz, DMSO-d6, room temperature); δP 23.8; 13C (500 MHz, DMSO-d6, room temperature) δC 144.9 (1C ∗), 142.3 (1C ∗), 138.4 (3C ∗), 134.0 (1C), 130.7 (1C ∗), 133.5 (3C), 129.0 (3C), 128.9 (1C), 128.5 (1C), 123.8 (1C), 115 (3C ∗) Ar, 57.0 (2C), 21.3 (3C, methyl carbons); HRMS (ESI): m/z calculated for (M-HBr)+ C36H32N4O2P+, 597.237, found 597.2374.
Tris-(4-(fluorophenyl) (4-1-(4-nitrobenzyl)-1H-1, 2, 3-triazol-4-yl)benzyl phosphonium bromide (Compound 3). Following the general procedure 4, reaction of 4-(2 (bromomethyl) phenyl)-1-(4-nitrobenzyl)-1H-1, 2, 3-triazole (1eq., 0.186 g, 0.50 mmol) and tris (p-fluorophenyl) phosphine (1.05 eq., 0.180 g, 0.52 mmol) produced the title compound as a white solid (0.223 g, 67%) after purification; mp 256–259°C; IR: νmax/cm−1: 3000 (sp3 C-H) 1591, 1501, 828 (Ar C=C-H), 565–450 (ArP-C); 1H NMR (500 MHz, DMSO-d6, room temperature)): δH 7.62 (1H, s, HAr triazole) 7.60–7.59 (2H, m, HAr), 7.55–7.52 (14H, m, HAr), 5.77 (2H, s, –CH2), 5.75 (2H, d, J = 10.0 Hz, -CH2); 31P (500 MHz, DMSO-d6, room temperature); 31P (500 MHz, DMSO-d6 room temperature): 22.9; 13C (500 MHz, DMSO-d6, room temperature) δC 162.9 (3C), 144.9 (1C) 142.3 (1C), 135.2 (6C), 134.0 (1C), 130.7 (1C), 128.9 (2C). 123.8 (2C), 115.5 (6C), 113.6 (3C) (Ar), 54.1 (2C), 28.7 (2C); HRMS (ESI): m/z calculated for (M-HBr)+ C33H23F3N4O2P, 609.1659, found 609.1659.
Benzyl tri p-tolyl phosphonium bromide (compound 4). Following the general procedure 4, the reaction of p-nitro benzyl bromide (1 eq., 0.186 g, 0.5 mmol) and tri-p-tolyl phosphine (1.05 eq., 0.200 g, 0.75 mmol) produced the title compound as a white solid (0.210 g, 86%) after purification. mp 286–288°C. IR νmax/cm−1: 3014 (sp2 C-H) 1597, 1493, 860 (Ar C=C-H), Ar-C=N (1453), 699–476 (Ar-P-C); 1H NMR (500 MHz, DMSO-d6, room temperature): δH 7.48–7.30 (12H, m, HAr); 7.30–7.25 (3H, m, HAr), 7.24–7.30 (2H, m, HAr), 5.05–5.02 (2H, d, J = 15.0 Hz), 2.45 (9H, d, Ar-CH3).
Benzyl triphenyl phosphonium bromide (compound 5). Following the general procedure 4, the reaction of p-nitro benzyl bromide (1 eq., 0.186 g, 0.5 mmol) and triphenylphosphine (1.05 eq., 0.200 g, 0.75 mmol) produced the title compound as a white solid (0.223 g, 98%) after purification. mp 280–283°C. IR νmax/cm−1: 3049 (sp2 C-H) 1584, 1493, 873 (Ar C=C-H), Ar–C=N (1483), 565–450 (Ar-P-C); 1H NMR (500 MHz, DMSO-d6, room temperature): δH 7.68–7.67 (3H, m, HAr), 7.65–7.32 (12H, m, HAr) 7.30–7.29 (3H, m, HAr), 7.25–7.22 (2H, m, HAr), 5.20–5.17 (2H, d, J = 15.0 Hz, Ar-CH2); 31P (500 MHz, DMSO-d6, room temperature); δP 23.5; 13C (500 MHz, DMSO-d6, room temperature.
Benzyl tri p-meoxy phosphonium bromide (compound 6). Following the general procedure 4, the reaction of p-nitro benzyl bromide (1 eq., 0.186 g, 0.5 mmol) and tris (p-methoxyphenyl) phosphine (1.05 eq., 0.200 g, 0.75 mmol) produced the title compound as a white solid (0.250 g, 93%) after purification. mp 272–275°C. IR νmax/cm−1: 3023 (sp3 C-H) 1599, 1517, 860 (Ar C=C-H), 565–450 (Ar-P-C); 1H NMR (500 MHz, DMSO-d6, room temperature): δH 7.53–7.49 (6H, m, HAr), 7.49–7.28 (9H, m, HAr) 7.26–699 (2H, m, HAr), 4.98–4.95 (2H, d, J = 15.0 Hz), 3.89 (9H, s, Ar-OCH3).
2.2. Biological Activity
2.2.1. Source of Bacterial Strains
Gram-negatives, including Escherichia coli (ATCC 25922) and Pseudomonas aeruginosa (ATCC 27853), Gram-positives, including Staphylococcus aureus (ATCC 25923) and Enterococcus faecalis (ATCC 29212), and Mycobacterium smegmatis mc2 155, an acid-fast mycobacterium, were used for the antimicrobial study. The test organisms were all obtained from the Cell Culture Laboratory, Department of Pharmacology, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi, Ghana.
2.2.2. Culture Media and Reference Antibiotics
Nutrient Agar and Nutrient Broth were purchased from Oxoid Limited (Basingstoke, United Kingdom). Middlebrooks 7H10 Agar and Middlebrooks 7H9 Broth were purchased from Becton Dickinson (BD™) Chemicals, USA, and Ciprofloxacin (>98% HPLC) and Rifampicin (>97% HPLC) were obtained from Sigma-Aldrich™ (Michigan, USA).
The antimicrobial assay used was adapted from [14].
2.2.3. Determination of Minimum Inhibitory Concentration (MIC)
The minimum inhibitory concentrations (MICs) for each compound against the Gram-positive, S. aureus (ATCC 25923) and E. faecalis (ATCC 29212); Gram-negative, P. aeruginosa (ATCC 27853) and E. coli (ATCC 25922)); and Mycobacterium smegmatis mc2155 isolates were determined using the high-throughput spot culture growth inhibition (HT-SPOTi) assay. Stock solution of the compounds P-MEOXY (1), 2ET-TOL (2), 2ET-FLU (3), RABYL-TOL (4), PPh3Br (5), and P-Z (6) (50 mg/mL) were prepared in DMSO followed by a twofold serial dilution in a 96-well half-skirted plate to give a concentration range of (50–0.05 mg/mL). Subsequently, 2 µL of each dilution was dispensed into the corresponding wells of the 96-well plate followed by the addition of 200 μL of nutrient agar/Middlebrooks 7H10 agar with shaking. The final concentration range in the agar ranged between 500 and −0.5 μg/mL. The wells were then spotted with 2 µL of cell suspension (∼1 × 106 CFU/mL) using a multichannel pipette. Column 12 of the 96-well plate with agar and cells only were used as positive control. The plates were wrapped in aluminum foil and incubated at 37°C for 24 h. The MIC was defined as the lowest compound concentration that completely inhibited the growth of the microorganisms.
2.2.4. Modulation of Antibiotic Resistance Studies
2.2.5. Biofilm Inhibition Studies
Check the font size and reduce it a little for the formula so that it is a bit uniformed.
2.3. Statistical Analysis
The results obtained from the study were analyzed using GraphPad Prism 6.0 software (Version 8.01, GraphPad Software Inc., USA). The analysis of variance (ANOVA) was followed by the Neuman-Keuls posttest. All the values are expressed as mean ± standard error of the mean (SEM) from triplicate experiments.
3. Results and Discussions
3.1. Chemistry
The synthesis centered on nonclicked and hybrid (clicked) compounds bearing lipophilic groups (Figure 2) due to evidence from literature about the relationship between the lipophilic character of compounds and their biological activities. Optimization of the structural scaffold possibly enhances the biological activity with different electronic donating/withdrawing groups such as methyl, methoxy, and fluoro on the phosphonium head. In the first attempt to synthesize the nonclicked compounds (1, 2, and 5), a reaction was carried out between benzyl bromide and various triphenylphosphines in toluene under reflux conditions. The reaction afforded precipitates that were crushed out of the solution and isolated by suction filtration under vacuum as white solids. In the synthesis of the clicked compounds (2, 3, and 6), the products did not require further purification and were characterized by spectroscopy (NMR, FT-IR, HRESIMS). The synthesis of the clicked compounds involved the first step of generating p-nitrobenzyl azide from p-nitrobenzyl bromide in water: acetone (1 : 4) mixture at room temperature for 6 hrs. This was followed by the copper-catalyzed 1, 3-dipolar cycloaddition in an organoaqueous solvent mixture at room temperature for 8 hrs. The reaction was monitored by thin layer chromatography (TLC). The product was worked up using diethyl ether in a solvent-solvent extraction and the combined organic portions were concentrated in vacuo using a rotary evaporator to obtain a beige solid which was further purified by silica column chromatography in a gradient elution fashion to afford a white solid hereafter called the clicked compound. After confirming the structure of the clicked compound by spectroscopic characterization, the clicked compound was reacted with phosphorous tribromide to convert the hydroxy group to a good leaving group, bromine. This made the brominated product susceptible to nucleophilic substitution bimolecular reaction (SN2) reaction with respective triphenylphosphines to introduce the substituted phosphonium heads to form the hybrid compounds. They were characterized by spectroscopy (NMR (1H, 13C/DEPT-135, and 31P), FT-IR, HRESIMS). The synthetic scheme is represented in Figure 1 and the supplementary information is in Figures SM1–SM25.

The synthesis of the library of phosphonium salts (1–6) was performed according to methods in literature appended to the various procedures. The compounds were obtained with good yields and high purity confirmed by melting point and spectroscopic methods. The treatment of nitro benzyl bromides with various triphenylphosphine derivatives afforded compounds 1, 4, and 5. via a nucleophilic substitution bimolecular reaction. Copper-catalyzed azide-alkyne 1, 3-dipolar cycloaddition click reaction was used to attach the 1,2,3-triazole intermediate to the phosphonium salts as the hybrids (2, 3, and 6) after creating the brominated functionality which provided the pathway for the final step via nucleophilic substitution bimolecular (SN2) mechanism. The structures of the synthesized compounds 3–6 were established on the basis of IR, NMR (1H, 13C, and DEPT-135), and LC-MS spectroscopic data, and thereafter, IR and 1H NMR were used to confirm the nonhybrids (compounds 1–3). For instance, the FT-IR spectrum for detecting the functional groups of compound 2, one of the most bioactive compounds, displayed an olefinic hydroxyl absorption band at 3032 cm−1 in the aliphatic C-H region and was confirmed by the presence of the N=N in the triazole and carbon-phosphorous band at 1455 cm−1 and 450–560 cm−1, respectively. From the 1H NMR spectrum, an envelope of 17 protons resonating downfield from 7.31 ppm to 8.20 ppm which encompasses the lone triazole proton at 7.57 ppm in the aromatic region. The signals at 5.70 ppm and 5.68 ppm represent a singlet for the methylene carbon tethering the triazole nitrogen (N1) and methylene attached to the phosphorous (ipso carbon), respectively. The high-intensity peak observed in the upfield region at 2.40 ppm is characterized by the presence of the 9 methyl protons on the phosphonium head. The13C NMR-DEPT 135 spectrum showed a cluster of 120–135 ppm which is characteristic of aromatic carbons. The methylene carbon between the nitro aromatic group and the triazole nitrogen 1 is characterized by a chemical shift of 50 ppm while two peaks (29 and 30 ppm) designated as the methylene carbon attached to phosphorous linking the triazole carbon 4 and the phosphonium head. The confirmation of the phosphorous from the 31 P NMR was observed as a singlet at 22 ppm. The high-resolution accurate mass provided extra supporting data for the molecular ion peak m/z for (M-HBr)+C36H32N4O2P+ as 597.2374. However, the structures of compounds 4, 5, and 6 were confirmed with 1H NMR spectroscopy (Figures SM11–SM15).
3.2. Susceptibility of Compounds to Test Organisms in Minimum Inhibitory Concentration (MIC) Determinations
The potency of the test compounds to inhibit bacterial growth was quantitatively assessed by the MIC values obtained experimentally (Table 1). Five different pathogens were used, of which two were Gram-negative bacteria, two were Gram-positive bacteria, and an acid-fast bacterium. Four of the test organisms showed susceptibility to the test compounds while Enterococcus faecalis showed complete resistance (no susceptibility) to all the six test agents. Among the pathogens tested, Mycobacterium smegmatis was the most resistant, with little susceptibility to the test compounds used. However, Pseudomonas aeruginosa possessed the least resistance, followed by Staphylococcus aureus, then by Escherichia coli, and Klebsiella pneumoniae. Compound 2 showed the most effective antimicrobial activity as it inhibited the growth of Pseudomonas aeruginosa at 0.0125 µg/mL and Staphylococcus aureus at 31.25 µg/mL. It also showed moderate antimicrobial activity against Escherichia coli (250 µg/mL). Compounds 4 and 6 exhibited comparable antimicrobial activity against Pseudomonas aeruginosa with an MIC of 1.95 µg/mL. Staphylococcus aureus strain was more susceptible to compound 3 at MIC 62.5 µg/mL. The compounds did not show any antimycobacterial activity against Mycobacterium smegmatis with the exception of compound 4 at 500 µg/mL. The MICs of the compounds from the HT-SPOTi assay are shown in Table 1.
| Compound | Minimum inhibition concentration (µg/mL) | ||||
|---|---|---|---|---|---|
| P. aeruginosa | E. coli | S. aureus | E. faecalis | M. smegmatis | |
| P-MEOXY (1) | 3.9 | >500 | 500 | >500 | >500 |
| 2ET-TOL (2) | 0.0125 | 250 | 31.25 | >500 | >500 |
| 2ET-FLU (3) | 7.8 | >500 | 62.5 | >500 | >500 |
| RABYL-TOL (4) | 1.95 | 500 | 250 | >500 | 500 |
| PPh3 (5) | 31.25 | >500 | 250 | >500 | >500 |
| PZ (6) | 1.95 | >500 | 500 | >500 | >500 |
| Amoxicillin | 3.9 | 1.95 | 1.95 | 500 | NT |
| Ciprofloxacin | 0.008 | 0.078 | 0.3 | 10 | NT |
| Rifampicin | NT | NT | NT | NT | 1.95 |
- NT: not tested for the microorganisms used for the antimicrobial activity.
3.3. Resistant Modulatory Studies
Combination therapy is employed in some microbial infections as a standard option or plans to achieve treatment goals. Resistance modulation investigations offer an advantage in the study of the modulating effect of potential lead compounds on the activities of already available antimicrobials [15]. The nature of the therapeutic effects of potential modulating agents in preventing antimicrobial resistance needs to be investigated through the establishment of the fractional inhibitory concentration index (FICI) to make an intended combination therapy effective in achieving the required treatment outcomes [16].
Antimicrobial resistance modulatory activity study was carried out using the combination assay model for the three most active test compounds P-MEOXY (1), 2ET-TOL (2), and RABYL-TOL (4) against the two most susceptible pathogens, Staphylococcus aureus and Pseudomonas aeruginosa, to investigate their synergistic or antagonistic effects, based on the results obtained from the susceptibility studies.
From the FICI data obtained, compounds 2ET-TOL (2) and RABYL-TOL (4) successfully modulated the activities of amoxicillin against some of the test organisms. It was observed that the combination of compound 4 with amoxicillin exhibited a synergistic effect against Pseudomonas aeruginosa but showed no interaction against Staphylococcus aureus. However, the combination of compound 2 with amoxicillin exhibited an antagonist effect against Staphylococcus aureus and showed no interaction against Pseudomonas aeruginosa. No interaction against Pseudomonas aeruginosa was observed with a combination of compound 1 with amoxicillin (Table 2).
| Compounds | FICI | |
|---|---|---|
| P. aeruginosa | S. aureus | |
| Amoxicillin + P-MEOXY (1) | 1 (NI) | — |
| Amoxicillin + 2ET-TOL (2) | 0.75 (NI) | 6 (AT) |
| Amoxicillin + RABYL-TOL (4) | 0.25 (S) | 2.125 (NI) |
- The synergistic effect is expressed as the fractional inhibition concentration index (FICI), calculated from the MIC of the various compound/antimicrobial combinations. The FIC index interactions were defined as follows: FICI ≤0.5, (S) synergy >0.5 to ≤4.0 (NI) no interaction, and >4.0, (AT) antagonistic.
3.4. Biofilm Inhibition Studies
Microorganisms develop resistance against antimicrobial agents through several mechanisms including the formation of biofilms [17]. Bacteria biofilms constitute about 65% of microbial infections, and those existing in them develop resistance to antimicrobial agents a thousand folds than bacteria existing in planktonic forms [18]. Potential antimicrobial agents should possess strong biofilm inhibition or reduction effects to be considered good candidates in antimicrobial-resistant drug development [19, 20].
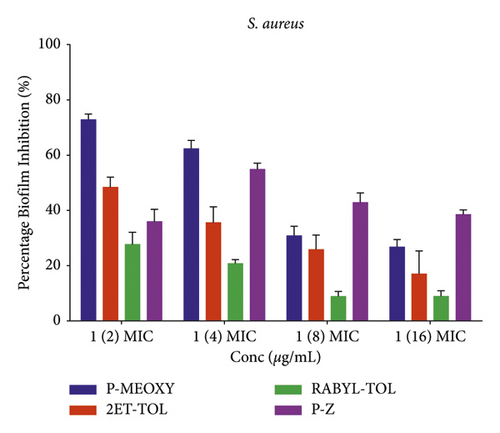
The test compounds showed variable effects on the inhibition of biofilms in the strains of organisms used at subinhibitory concentrations with P. aeruginosa biofilm being more susceptible to the compounds compared to S. aureus, from the biofilm inhibition studies (Figure 3). The biofilm inhibitory activity against P. aeruginosa was observed to be in a concentration-dependent manner for P-MEOXY (1), P-Z (6), RABYL-TOL (4), and concentration-independent manner for 2ET-TOL (2) indicating different susceptibility patterns of P. aeruginosa biofilms to the compounds. The concentration-dependent effect of RABYL-TOL revealed higher activity with lower concentrations (1/16 MIC >1/8 MIC >1/4 MIC >1/2 MIC) (Figure 3(a)). The good activity was observed against P. aeruginosa biofilms with P-MEOXY (at 1/2, 1/4, 1/8 MICs), 2ET-TOL (at all concentrations), and P-Z (at 1/2, 1/4 MICs) (Figure 3(a)). However, all the test compounds exhibited a concentration depending on biofilm inhibition against S. aureus, except P-Z which showed a concentration-independent effect. P-MEOXY showed good biofilm inhibition at 1/2 MIC, and 1/4 MIC against S. aureus biofilm (Figure 3(b)).

3.5. Structure-Activity Relationship of the Phosphonium Salts
Compound 1 (P-MEOXY) which is a hybrid with the methoxy groups at the para position on the triphenyl ring tethered to the triazole and its counterpart compound 2 (2ET-TOL) which has methyl groups at the same positions exhibited impressive activity against Gram-negative Pseudomonas aeruginosa followed by it. However, compound 1 performed poorly against the Gram-positive organisms and Mycobacterium tuberculosis. This could be explained by the positive inductive effect of the methoxy and methyl groups which dispersed the positive charge on the phosphonium head making the quaternary group not so essential for activity against Gram-negatives. However, of the hybrids, it was observed that the 2ET-FLU had the least activity generally but performed better than compound 1 against Staphylococcus aureus. This could suggest that the negative inductive effect due to the electron-withdrawing nature of the fluorine group created a more positive center on the phosphonium head which was essential for better activity against Staphylococcus aureus. With the nonhybrids, the compounds 4 (RABYL-TOL) and 6 (PZ) which had substituted phosphonium heads with methyl and methoxy groups, respectively, generally exhibited better activity than PPh3 which possessed an unsubstituted phosphonium head. This suggests that the electronic effect on the quaternary phosphonium head played a crucial role in influencing the antibacterial activity of the compounds.
4. Conclusion
A library of six (6) phosphonium salts including three hybrids was synthesized through the SN2 reaction between the triphenylphosphine derivatives and aromatic benzyl bromides. Click chemistry was however used to synthesize the 1,2,3-triazole intermediates. Compound 2 showed the most effective antimicrobial activity as it inhibited the growth of Pseudomonas aeruginosa at 0.0125 µg/mL and Staphylococcus aureus at 31.25 µg/mL. The compounds did not show any antimycobacterial activity against Mycobacterium smegmatis with the exception of compound 4 at 500 µg/mL. From the FICI data, compounds 2ET-TOL (2) and RABYL-TOL (4) successfully modulated the activities of amoxicillin against some of the test organisms. All the test compounds exhibited a concentration-dependent biofilm inhibition against S. aureus, except P-Z (compound 6) which showed a concentration-independent effect, and good activity was observed against P. aeruginosa biofilms with P-MEOXY (compound 1) (at 1/2, 1/4, 1/8 MICs), 2ET-TOL at all concentrations, and P-Z (compound 6) (at 1/2, 1/4 MICs). The antibacterial activities of the library of the phosphonium salts provide strong proof as scaffolds for future synthetic optimization.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this study.
Authors’ Contributions
Cedric D. K. Amengor conceptualized the study; Cedric D. K. Amengor synthesised and characterised the compounds, Francis Ofosu-Koranteng characterised the compounds by HRESIMS. Benjamin K. Harley and Emmanuel Orman contributed to the interpretation of the spectroscopic data; Cynthia Amaning-Danquah performed the antimicrobial assays; Cedric D. K. Amengor prepared original draft; Emmanuel Bentil Asare Adusei Joseph Adu, Francis K. Kekessie, Cedric D. K. Amengor, Cynthia Amaning-Danquah, Paul K. Peprah, and Yussif Saaka reviewed and edited the manuscript.
Acknowledgments
The authors are very much grateful to all staff and technicians of the Department of Pharmaceutical School of Pharmacy (UHAS), Ghana for their support. The authors also express our gratitude to the Institute for Health Research, UHAS, for their unflinching support in driving research at the university. The authors are also grateful to Mr. Edward Ntim Gasu at the Central Laboratory Complex, KNUST, for the assistance in IR and NMR spectroscopy. This research received no external funding. Funding was provided by the institutions and collaborators involved.
Open Research
Data Availability
The data used to support the findings of this study are available at the Department of Pharmaceutical Chemistry, School of Pharmacy, the University of Health and Allied Sciences, Ho.