Nanoencapsulation of 4-Propylguaiacol in β-Cyclodextrin, Ethyl Cellulose, and Polyvinylpyrrolidone
Abstract
We previously identified 4-propylguaiacol to be a highly potent repellent against Rhipicephalus appendiculatus, which transmits East Coast Fever in cattle. So far, the major method that has been employed for tick control is the use of acaricides, which so far has posed a number of challenges. Encapsulation technology may offer a long-term solution to the existing problems by dispensing the repellent at a controlled rate. 4-Propylguaiacol was encapsulated in various nanoparticles, which included β-cyclodextrin, ethyl cellulose, and polyvinylpyrrolidone. The inclusion of 4-propyl guaiacol in the resulting complexes was confirmed by FT-IR, XRD, and SEM analysis. All the sharp peaks belonging to each of the encapsulating polymers were observed. However, some of the characteristic peaks of 4-propylguaiacol disappeared in the complex formed. The rates and duration of release of 4-propylguaiacol from 0.2 g of each inclusion complex were then compared at 38–40°C every 3 hours for 24 hrs. The observed rates of release for 4-propylguaiacol were 0.396 mg/hr., 0.632 mg/hr., and 0.648 mg/hr. Rate from β-cyclodextrin, ethyl cellulose, and PVP inclusion complexes, respectively. The release rate of 4-propylguaiacol in the β-cyclodextrin complex was more controlled than it was in ethyl cellulose and PVP complexes. This controlled release rate exhibited by the β-cyclodextrin complex in small doses for a relatively long time provides a potential tool for dispensing repellents on cattle to protect them from tick bites.
1. Introduction
Rhipicephalus appendiculatus is a hard tick found mainly in Africa. It is a vector of Theileria parva that causes East Coast Fever (ECF) in cattle [1]. It is estimated that about 1.1 million cattle suffer from ECF, causing losses of about US dollars 168 million (1). About 28 million cattle in the region are susceptible to the disease, and it kills at least 1 million cattle annually [1]. Ticks are generally controlled by the use of conventional acaricides. These acaricides, however, are too expensive for rural farmers. Residues of acaricides have been detected in meat and dairy products [2]. In addition, these acaricides were also found to cause contamination of the environment through cattle dung. To successfully manage the harmful ticks, a joint combination of strategies may need to be employed that controls ticks on individual hosts as well as in the host environment to avoid host reinfestation while grazing [3]. This puts great weight on exploring several strategies aimed at the control of ECF.
Encapsulation technology has found many applications in the food industry as a flavor carrier and as a treatment to impart some degree of protection against evaporation, reaction, or migration of substances in food [4, 5]. It can provide the necessary protection against oxidation for active compounds while increasing their solubility; it allows them to be used in the preparation of several fortified and functional foods [6]. The simplest means of encapsulation is to emulsify the ingredient in a solution containing the “wall” material, followed by freeze-drying or spray drying [7].
A primary criterion for the inclusion of a guest molecule within the host’s cavity is its size and other factors such as strict fit, van der Waals’ interactions, and hydrogen bonding, among others. In addition, the relative polarities of the characteristic groups decide which end of the guest molecule goes in the hydrophobic cavity, the preference being for the nonpolar end to be included inside the cavity [8]. Studies show that encapsulation has been shown to improve the bioavailability of compounds.
Beta-Cyclodextrin (β-CD) is a cyclic oligosaccharide having seven D (+) glucopyranose units. It is one of the most essential host compounds for guest molecules. A variety of guest compounds involving ionic species, organic molecules, and pharmaceutical drugs can be contained within the cavity of β-CD in aqueous solutions. The CD inclusion complex is highly stereo-specific, highly stable, and less toxic [9]. SeeFigure 1.

CDs form inclusion complexes with numerous guest molecules with suitable polarity because of their exceptional molecular structure, internal hydrophobic cavity, and external hydrophilic surface [9]. More interest in CDs extends to enhance the solubility, chemical stability, and bioavailability of poorly soluble drugs, especially in pharmaceuticals [10].
Ethyl cellulose (EC) is the most abundant natural biopolymer. It is a polymer of D-glucose structurally, in which the individual units are connected by β-glucose links from the anomeric carbon of one unit to the C-4 hydroxyl of the subsequent unit [9]. The cellulose backbone is made up of Anhydro glucose units, and the individual units can be substituted at positions 2, 3, and/or 6. See Figure 2.
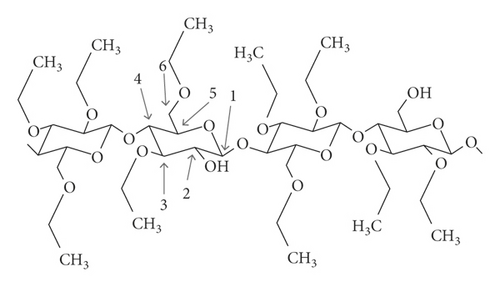
The degree of ethoxyl substitution ranges from 2.5 to 2.6 for standard grades of EC, and this corresponds to an ethoxyl content mean of 48.0–49.5% (w/w) [9]. EC and its derivatives have many important uses in the paper, fiber, and paint industries [11]. EC has been widely used across multiple microencapsulation methods and for several end-use applications. EC is a biocompatible and nonbiodegradable polymer which is broadly studied as an encapsulating material for pharmaceutical-controlled release [12]. The exploitation of EC in modified release applications usually involves applying a rate-modifying ethyl cellulose wall to a substrate, such as a microcapsule core, granule, powder, tablet, or bead [13].
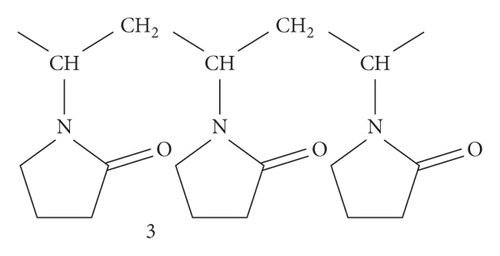
Polyvinylpyrrolidone (PVP) (3), ordinarily called polyvidone or povidone, is a water-soluble polymer prepared from the monomer N-vinylpyrrolidone. Preparation of PVP can be done by free-radical polymerization from its monomer N-vinylpyrrolidone with azobisisobutyronitrile as an initiator [14]. See Figure 3.
PVP has been found to encapsulate β-carotene through the electrospinning method when shielding β-carotene from degradation and to preserve its antioxidant properties [15]. The most commonly used polymers in the synthesis of metal nanoparticles include polyvinyl alcohol, polyethylene glycol, and PVP [16–18]. On the other hand, the increased interest on the usage of PVP as a nucleating/reducing/capping agent has been on the rise a lot owing to its good biodegradability, nontoxicity, excellent film-forming, ability and easy processability, mild reducing ability, superb stabilizing ability and outstanding solubility in several polar solvents [19]. PVP has been reported to reduce gold ions to Au atom and consequently encapsulate those metal NPs through O-atom of pyrrolidone ring [16, 17]. A charge transfer (CT) complex is formed by PVP with Pt NPs using nonbonding electron transfer from the O-atom to the electron-deficient NP [20]. PVP ligand interacts with Pd NPs through O-atom of the pyrrolidone ring [16]. Structural activity studies carried out previously on analogues of 4-methylguaiacol [21]. The objective of this study was to develop controlled release formulations of nanocapsules of 4-propylguaiacol in different polymers.
In this study, we encapsulated 4-propylguaiacol that will be further studied for the protection of cattle against ticks in a field evaluation. These kinetics studies on tick control are carried out for the first time. This research lays a solid background in the development of effective tick control intervention.
2. Materials and Methods
2.1. Reagent
4-Propylguaiacol (purity >99%), β-cyclodextrin (β-CD, Cat No. C4767, >97%), ethyl cellulose, 48.0–49.5% (w/w) ethoxyl basis, and polyvinylpyrrolidone (PVP K-30) were purchased from Sigma-Aldrich (USA).
2.2. Preparation of Nanocomplexes
An inclusion complex of 4-propylguaiacol was prepared as per the method described by Naefady [22]. Briefly, an aqueous slurry of nanoparticle matrix (β-CD, EC, and PVP) was prepared by dissolving 10 g in 150 mL of distilled water. Ten grams of 4-propylguaiacol were separately suspended in each of the slurries to form respective complexes. The complexes were sonicated for 4 h at 25°C to allow maximum encapsulation and filtered through a 0.45 mm polyvinylidene difluoride (PVDF) membrane. The residue was rinsed with distilled water and the filtrate was allowed to mix with the one collected earlier. The water-soluble filtrate was lyophilized in a freeze dryer to provide a dry 4-propylguaiacol β-CD inclusion complex. The inclusion complex powder obtained and the residue were used in the calculation of percentage yield/encapsulation. The complex was packed in a gas-tight container at 4°C until further experiments and analysis.
2.3. Spectroscopic Analysis
The inclusion complexes were analyzed using FT-IR, SEM, and XRD to ascertain encapsulation. FTIR-8300 (Shimadzu, USA); the KBr beam splitter with a spectral range of 4000–400 cm was used to study the shifting of the peaks of functional groups. SEM was used to study the surface morphology of the pure components and their complexes obtained by irradiation of microwaves were examined by means of a JEOL (JSM-700 1F Field Emission). X-ray powder diffraction patterns were recorded at room temperature using Expert Pro 2006, cathode in coppers, λ = 1,54 Å, V = 40 kV, I = 20 mA, with a scanning speed of 4θ per min over a 2θ range of 4°–60°.
2.4. Kinetic Release Studies
The rate of release of 4-propylguaiacol from the respective nanocomplexes was studied in the laboratory using a method described by Guan et al. [23]. Two hundred milligrams of each of the inclusion complex was packed in a plexi-wire gauze (mesh, 400) and kept in a room maintained at 38–40°C where the lost masses were recorded after 3, 6, 9, 12, 15, 18, and 24 h. The samples were each extracted with 10 mL of dichloromethane and analyzed using gas chromatography linked with the Flame Ionization Detector (GC-FID) for quantification. Analysis was done on a Shimadzu Gas Chromatograph system (Shimadzu, USA) with capillary column ZB-1 (30 m, ID 0.25 mm, and a film thickness of 0.50 μm). The carrier gas (nitrogen) was set at 5.0 mL·min−1. The oven temperature was programmed from 35 to 290°C. An initial temperature of 35°C was held for 1 min, increasing at 3°C·min−1, until it reached 290°C, then held for 3 min. The injection temperature was 270°C via split-less injection mode. Quantitative data were obtained by the electronic integration of the FID peak areas. This was done in triplicate.
3. Results and Discussion
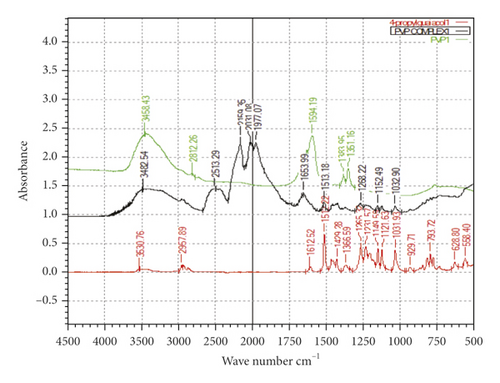
Microcapsules of 4-proply guaiacol, which was the most potent analogue of 4-methylguaiacol, were prepared with the polymers β-CD (1), ethyl cellulose (2), and PVP (3), respectively, in an effort to control the release rate. In order to confirm the inclusion of the compound within the polymer, FT-IR spectra were obtained. The FT-IR absorption spectra of 4-propylguaiacol, the host polymer β-CD, ethyl cellulose, PVP, and the three inclusion complexes (with and without each of the three complexes) are presented in Figures 4, 5, and 6, respectively.
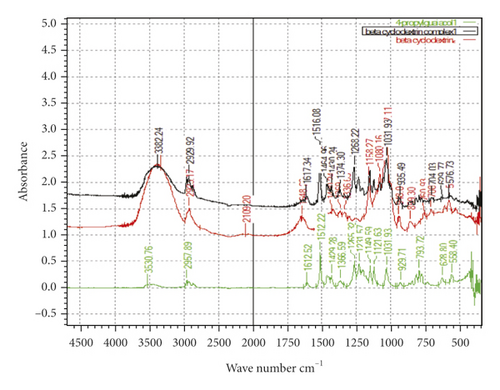
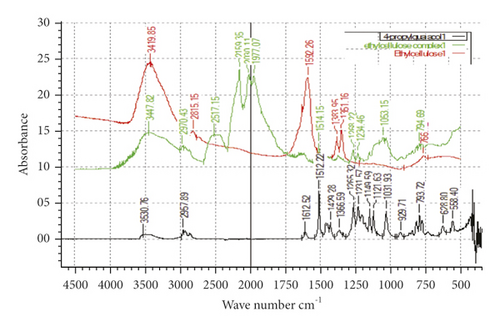
As expected, the FT-IR spectrum of the β-CD + 4-propylguaiacol inclusion complex showed a pattern similar to that of the β-CD. All the sharp peaks belonging to β-CD were observed [24]. However, some of the characteristic peaks of 4-propylguaiacol disappeared. Based on these results, it was concluded that the benzene ring of 4-propylguaicol (10) was contained inside the β-CD cavity by van der Waals forces and hydrophobic interactions [25]. In addition, it was possible that 4-propylguaicol could form hydrogen bonds in the β-CD cavity because of the presence of hydroxyl groups. Since 4-propylguaicol was included in β-CD, all the characteristic β-CD peaks were shifted to lower or higher wave numbers, like 3,339–3,382 cm−1, 2,923–2,929 cm−1, 1,648–1,617 cm−1, 1,080–1,031 cm−1, and 1,027–1,031 cm−1. Some of the peaks, such as 2,109, 935, and 576 cm−1, collapsed. The presence of 4-propylguaicol in the inclusion complex was confirmed. These data indicated the successful formation of the β-CD + 4-propylguaicolinclusion complex.
The FT-IR spectrum of 4-propylguaiacol exhibited a broad peak at 3,530.76 cm−1, indicating phenolic O-H stretching. The band at 2957 cm−1 corresponds to the methoxy-C-H stretch. Sharp absorption bands at 1,612 cm−1 corresponded to the C-C stretch. Another sharp, strong band at 1,512 cm−1 peak corresponded to the carbon-carbon aromatic ring stretch. Several bands were observed at 1265–1031 cm−1, which correspond to aromatic C-H in-plane bend. Other peaks were observed at 929–628 cm−1, which correspond to aromatic C-H out-of-plane bend. The FT-IR spectrum of β-CD showed a typical peak at 3,300−3,400 cm−1 owing to the O-H group stretching. A peak at 2,923 cm−1 due to C-H asymmetric/symmetric stretching was also observed. Additionally, a peak at 1,648 cm−1 represented the H-O-H water deformation bands present in β-CD. Peaks at 1,158 cm−1 and 1,027 cm−1indicated C-H overtone stretching and at 1,027 cm−1 C-H, C-O stretching. C-O-C vibration appeared at 1,158 cm−1.
Ethyl cellulose and PVP inclusion complexes exhibited stretching of O-H groups, aliphatic C-H stretching, polymeric O-H group stretching, and stretching of dimeric O-H groups. Other IR-related fingerprints were preserved in their respective IR wavelength region, which is an identified characteristic of the excipient utilized in the formulation.The complexes formed by ethyl cellulose and PVP seemed to absorb at the same frequencies. The new absorption peaks observed on ethyl cellulose appeared at 2513 cm−1, 2159 cm−1, 2030 cm−1, and 1977 cm−1. For the PVP complex, new absorption peaks appeared at 2513, cm−1 2159 cm−1, 2031 cm−1, and 1977 cm−1, respectively. There was a clear indication that inclusion had taken place. It is interesting to note that the ethyl cellulose and PVP inclusion complexes absorb the same IR region.
Microcapsules of 4-proplyguaiacol were analyzed using Powder X-ray diffraction (XRD) to confirm the inclusion of 4-propyl guaiacol in the polymers β-CD, EC, and PVP complexes. The X-ray diffraction patterns of β-CD, EC, and PVP with their respective inclusion complexes are presented in Figures 7, 8, and 9, respectively. The diffractograms also revealed the general crystallinity of the complexes. The β-CD complex was found to be crystalline as depicted by the presence of sharp peaks in the diffractogram shown in Figure 7. β-CD displayed peaks of 2θ = 6.4°, 9.4°, 10.8°, 12.7, 13.4°, 16.6°, 18.1°, 18.6°, and 19.0°. The inclusion complex displayed peaks at 2θ = 5.8°, 6.9°, 8.7°, 9.6°, 10.4°, 10.9°, 11.6°, 12.4°, 13.3°, 16.7°, 17.6°, 18.0°, 18.6°, 19.1°, 20.1°, 20.8°, and 21.9°.
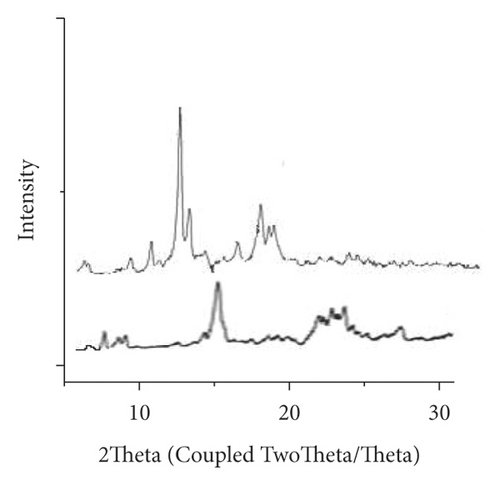

Some of the β-CD crystalline peaks collapsed in the patterns of β-CD + 4-propylguaiacol, especially the peak of 2θ = 12.7°. Several characteristic peaks shifted, for example, the peak 2θ = 6.4° to 2θ = 6.9°, confirming the successful inclusion complex formation.
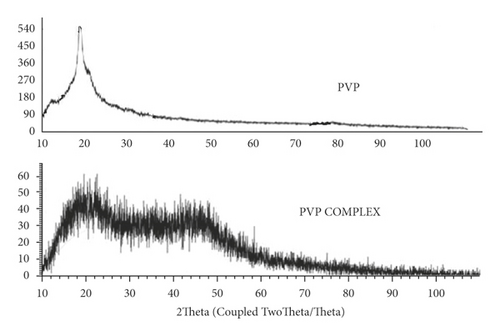
XRD diffractogram of EC (Figures 7 and 8) showed a peak at 2θ equals to 11.0° and 20.3°. XRD diffractogram of EC showed the semicrystalline nature of the polymer. This was contrary to observations [12], whereby EC polymer crystallinity was 51.8% and showed a peak at 2θ of 3.1°, 6.9°, 9.9°, and 18.6°. However, the XRD diffractogram of the EC/4-propyl guaiacol complex was amorphous; it did not have a distinct peak. Previous studies [26] on the characterization of thermal and physicochemical properties of biofield treated ethyl cellulose and methyl cellulose displayed no significant change in the XRD pattern of treated EC as compared to control (EC). The XRD diffractograms of treated EC and control showed polymers of semicrystalline nature. The XRD diffractogram of PVP (Figure 9) showed two peaks at 2θ equals to 11° and at 20°. In a previous study [27], it was reported that two typical peaks at 2θ = 10.31° and 19.41° were observed for pure PVP. It was observed that the resulting PVP complex was amorphous. This was an indication that there was an inclusion of 4-propylguaiaconl in the PVP polymer.
Previous studies [28] revealed that the crystallinity of CS/PVP decreases after crosslinking with glutaraldehyde. This could suggest that there was an interaction between these two components that led to new crystalline structures [29].
Scanning Electron Microscope (SEM) images obtained for β-cyclodextrin, β-cyclodextrin + 4-propylguaiacol complex, ethyl cellulose, ethyl cellulose + 4-propylguaiacol complex, PVP, and PVP + 4-propylguaiacol complex are shown in Figure 10.
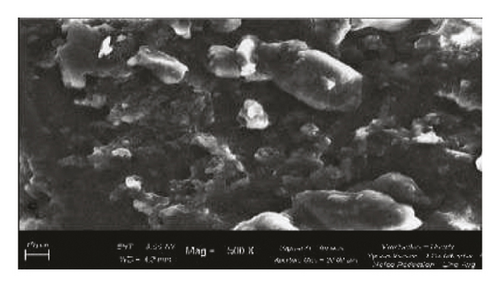

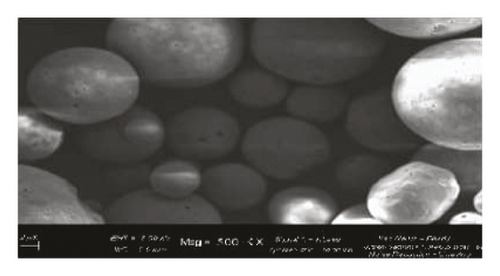
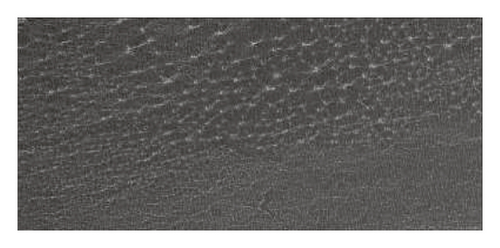

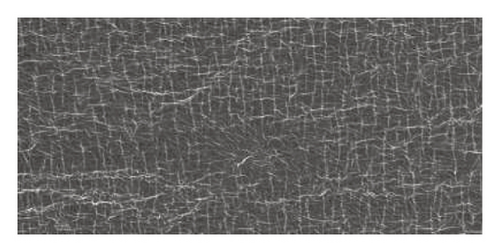
β-CD appeared as irregularly shaped crystals, confirming its crystallinity [9]. However, the 4-propylguaiacol: β-CD complex appeared more crystalline, suggesting that there was a change in morphology after complexation. From Figure 10, the SEM image of pure ethyl cellulose appears as spherical crystals, but the crystallinity was lost after complexation, as clearly indicated by the SEM image of ethyl cellulose + 4-propylguaiacol. This change in morphology was also demonstrated by PVC and its inclusion complex.
The principal criterion for the inclusion of a guest molecule in the host’s cavity is apparently its size and then other factors such as strict fit, hydrogen bonding, and van der Waals’ interactions, among other factors. Several studies carried out previously have shown compounds’ inclusion complexes forming with β-CD. For example, in cinnamic acids, the formation of a 1 : 1 complex of β-CD with trans-cinnamic acid [30, 31]. It is established from X-ray diffraction data that the guest molecules are entirely buried in the hydrophobic cavity of β-CD molecules [31]. In addition, [32] performed a comprehensive structural study of the inclusion compounds of caffeic and ferulic acids and disclosed that they enter into the β-CD with the phenol part within and the carboxyl part projecting outside. A 1 : 1 inclusion complex of benzoic acid with β-CD has been reported [33]. The formation of a 1 : 1 inclusion complex of β-CD with 9,10-anthraquinone in which nearly the entire guest molecule was encapsulated in the hydrophobic cavity was reported in [34]. The formation of a 1 : 1 inclusion complex for substituted 9,10-anthraquinones was also reported [35]. In the previous case, an axial and only partial inclusion of these guest molecules into β-CD was proposed.
Several studies have displayed the formation of 1 : 1 inclusion complexes of β-CD with flavonoids [36, 37], despite the fact that quantum mechanical studies in the case of quercetin suggested that a large portion of the flavonoid skeleton is included in the β-CD cavity and the stability of the complex is due to the intermolecular formation of one hydrogen bond [38]. FT-IR, XRD, and SEM studies have proposed the formation of a complex in which 4-propylguaicol is encapsulated in the β-CD cavity with methoxy and −OH hydrogen bonded to the secondary hydroxyl groups of the rim of the wider end of the β-cyclodextrin cavity. From these findings, it is clear that the β-cyclodextrin and 4-propylguaiacol complex may be a promising tool for a control release device. The polymer β-CD may release the encapsulated repellent at a slow rate, thereby providing longer protection for the cattle against the tick.
FT-IR, XRD, and SEM analysis carried out on the ethyl cellulose, PVP and their respective complexes with 4-propyl guaiacol showed that there were interactions between each polymer and 4-propylguaicol. For example, an XRD diffractogram of EC showed the semicrystalline nature of the polymer. A study carried out in [38] reported that EC is mainly amorphous with a poor crystalline part. The microcapsules obtained with the ethyl cellulose polymer were amorphous. This agrees with the studies by [39] where nifedipine + ethyl cellulose microspheres were amorphous.
From the FT-IR analysis, it is evident that there were structural changes in PVP after being loaded with 4-propyl guaiacol. Studies by [15] revealed that spectra from the analyses of FT-IR showed the presence of structural changes in PVP nanofibers after being loaded by β-carotene as a result of molecular interaction between β-carotene and PVP.
The rate of release of 4-propylguaiacol in inclusion complexes in β-cyclodextrin, ethyl cellulose, and PVP was evaluated, and graphs of concentration verse time are shown in Figure 11.
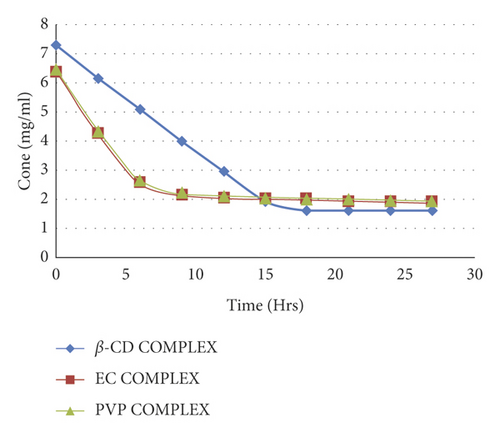
The observed rates of release were 0.396 mg/hr., 0.632 mg/hr., and 0.648 mg/hr. for β-cyclodextrin inclusion complex, ethyl cellulose complex, and PVP, respectively. The Β-cyclodextrin complex had a constant rate of release up to 15th hour, after which the release rate dropped to near zero. Both ethyl cellulose and PVP inclusion complexes had comparable release rates of 4-propyl guaiacol for 5 hours, which was higher than that of the β-cyclodextrin inclusion complex. It is reported that drug release from the polymer matrices is normally controlled by polymer erosion and/or drug diffusion through the polymer matrix or pores in the matrix [40]. For example, [39] prepared microspheres containing nifedipine and ethyl cellulose and found that.
Microspheres formulated using the polymer combination released nifedipine more slowly and more regularly in zero- and first-order kinetics.
The rate of release of 4-propylguaiacol from crystalline β-cyclodextrin complexes was lower than that of ethyl cellulose and PVP complexes which were amorphous in nature. This conforms to what has been reported that the behavior of drug release depends on the crystalline microstructure [41]. In addition, previous studies [42] also revealed that the release rate was higher in polyesters having a low degree of crystallinity due to higher macromolecular chain mobility.
4. Conclusions
4-Propylguaiacol was successfully encapsulated in β-cyclodextrin, ethyl cellulose, and PVP polymers to form respective inclusion complexes. The resulting complexes were confirmed using FT-IR, XRD, and SEM analyses. For inclusion, extreme changes in the shapes and morphologies of the particles were discovered using SEM analysis. Some interactions between 4-propylguaiacol and the polymers were also confirmed using FT-IR and XRD. The nanocapsules obtained with β-CD were crystalline, while those of EC and PVP were amorphous in nature. The release rate of 4-propyl + guaiacol in the β-cyclodextrin inclusion complex was slower than that in the ethyl cellulose and PVP/complexes. The phenomenon of crystallinity in the β-cyclodextrin inclusion complex and the slow rate at which the guest molecules are released identified this complex as most applicable to the controlled release experiment.
Additional Points
Highlights. Nanoencapsulation of 4-propylguaiacol was successful in all β-CD, EC, and PVP. β-CD nanocapsules were crystalline while those of EC and PVP were amorphous. Rate of release of 4-propylguaiacol was more controlled in β-CD nanocapsule than it was in EC and PVP.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
Acknowledgments
This present work was supported by funds from MOHEST/ADB in collaboration with Kenyatta University and the National Research Fund (NRF Kenya).
Open Research
Data Availability
The rate of release data used to support the findings of this study are available from the corresponding author upon request.