A Competent MWCNT-Grafted MnOx/Pt Nanoanode for the Direct Formic Acid Fuel Cells
Abstract
A novel “MnOx/Pt/MWCNT-GC” nanocatalyst is recommended for the electrooxidation of formic acid (EOFA), the principal anodic reaction in the direct formic acid fuel cells (DFAFCs). The sequential (layer-by-layer) protocol was employed to prepare the catalyst through the electrodeposition of Pt (nano-Pt) and manganese oxide (nano-MnOx) nanoparticles onto the surface of a glassy carbon (GC) electrode supported with multiwalled carbon nanotubes (MWCNTs). The nano-MnOx could successfully mediate the mechanism of EOFA by accelerating the charge transfer, “electronic effect”. On the other hand, MWCNTs could enhance the catalytic performance by changing the surface geometry that inhibited the adsorption of poisoning CO, which is a typical intermediate in the reaction mechanism of EOFA that is responsible for the potential deterioration of the catalytic performance of DFAFCs. Interestingly with this modification, a significant enhancement in the catalytic activity and stability toward EOFA was achieved. Several techniques will be employed to evaluate the catalyst’s morphology, composition, crystal structure, and activity and further to understand the role of each of the nano-MnOx and MWCNTs in the catalytic enhancement.
1. Introduction
The electrooxidation of formic acid (EOFA) has recently gained an incredible attention in the sector of the power generation for potential applications in the direct formic acid fuel cells (DFAFCs) [1, 2]. In fact, with the global movement to address the climate change and sustain affordable and clean electrical power sufficient enough to keep pace with the rapidly growing population and industrialization, it became mandatory to reduce the share of fossil fuels in the power schemes and replace it with alternative greener and renewable technologies [3–5]. The DFAFCs have presented a better scenario for employing small organic liquid fuels as formic acid (FA) instead of H2 for the power generation for several portable electrical devices. The common risky challenges associating the production, transport, saving, and operation of H2 have absolutely disappeared with FA while retaining a higher (if compared to 1.2 kWh kg−1 and 0.18 kWh L−1 of H2) gravimetric and volumetric energy density of 1.7 kWh kg−1 and 2.1 kWh L−1 with a little fuel crossover flux via Nafion® membranes [6, 7]. Moreover, DFAFCs offered a competitive theoretical open-circuit potential (1.48 V vs. RHE); excelling that (1.23 V vs. RHE) [8, 9]. Nonetheless, the kinetics (rate and mechanism) of EOFA still encounters the convenient and reliable commercialization of DFAFCs.
Normally, Pt and Pd catalysts are recommended for EOFA, but Pd suffers an inherent instability resulting perhaps from its dissolution in harsh acidic media [10]. On the other hand, Pt experiences severe poisoning with reaction intermediates as CO that is released spontaneously to block most of the Pt active sites. Research is oriented, parallel to minimizing the Pt loading, to overcome its poisoning and to improve its catalytic efficiency toward EOFA. This was attempted by modifying (doping/alloying) Pt with other metals (Pd, Ni, Co, Cu, Mn, etc.) and/or metal oxides (NiOx, CoOx, CuO, MnOx, etc.) in the presence of a proper substrate to structurally and electronically improve the surface Pt characteristics. In this regard, the modification of Pt with Pd and CeO2 on multiwall carbon nanotubes (MWCNTs) succeeded to improve the mass activity of the catalyst at least three times while maintaining a seven-fold enhancement in its stability if compared to the commercial PtRu−C catalyst [11]. The role of MWCNTs as a substrate has been investigated separately where a minute amount of MWCNTs could effectively improve the reaction kinetics (1.6 times that of a bare Pt/GC electrode) and mitigate the inherent poisoning of a Pt/GC catalyst toward methanol oxidation [12]. The surface functionalization of AuPd@ZrO2 nanoparticle-based anodes with MWCNTs inspired as well a remarkable enhancement in the catalyst’s activity toward EOFA which depended on the AuPd2 size, Pd surface coverage, PdOx content, and ZrOx stoichiometry [13]. Therein, nonstoichiometric ZrOx nanoparticles were bonded to MWCNTs through C-OOH groups, forming the Zr-O-C bonds while Pd was deposited in a ternary phase (Pd, AuPd, and Au) nanoparticles which formed with ZrOx an intermetallic Pd-O-Zr phase. A remarkable enhancement in the catalyst’s durability was attained with the MWCNT-functionalization that enriched the surface with plenty of oxygen containing functional groups which facilitated the desorption of poisoning CO species. An improvement in the electronic properties of the catalyst was also suggested. A similar behavior was reported by Maturost et al. but with CeO2 and PdPt bimetallic alloy on the surface of MWCNTs [11]. They also suggested a substantial improvement in the kinetics of EOFA and its mass transfer efficiency owing to the catalyst structure (Pd and/or Pt particle size and dispersibility) and its electronic properties that got optimized with the MWCNT-functionalization. Recently, a substrate functionalization with a minute amount of MWCNTs imparted a significant enhancement in the catalytic activity and durability of nano-Pt and NiOx/Pt nanocatalysts for methanol oxidation [12] and EOFA [1], respectively. This extended to tune the mechanism of EOFA exclusively in the desired (low-overpotential) dehydrogenation pathway with a complete suppression for the CO poisoning [1]. Other oxides as manganese oxide (MnOx) are of interest for fuel cell applications as Mn enjoys the existence in multiple stable oxidation states and has a vacant d-orbital that accommodates the electrons involved in the fuel oxidation and, hence, facilitate its reaction kinetics [14–17]. Herein, the excellent catalytic performance of a MnOx/Pt nanostructured anode on a MWCNT-grafted substrate toward EOFA is reported.
2. Experimental
2.1. Catalyst Fabrication
All the chemicals used in this investigation were of analytical grades and were used without prior purifications. A glassy carbon (GC, d = 3.0 mm) rod/substrate was used as the working electrode in the catalyst’s preparation and in the electrocatalytic measurements. Before using, the GC electrode was polished mechanically with No. 2000 emery paper before repeating polishing with aqueous slurries of successively finer alumina powder (down to 0.06 mm) on a polishing microcloth. Next, the GC electrode was rinsed thoroughly with second distilled water. A spiral Pt wire and an Ag/AgCl/NaCl (3 M) electrode were always used as the counter and reference electrodes, respectively.
To prepare the catalyst, the cleaned GC electrode together with the Pt wire and the Ag/AgCl/NaCl (3 M) reference electrode were all dipped in 0.1 M Na2SO4 solution containing 1 mM H2PtCl6 and a charge of 10 mC was passed at 0.1 V to deposit nano-Pt onto the GC surface. This electrode will be next abbreviated as the Pt/GC electrode. To deposit MnOx, the Pt/GC electrode served as the working electrode with the regular electrochemical setup and a charge of 16 mC was allowed to pass at 0.1 V from in 0.1 M Na2SO4 containing 1.0 mM Mn(CH3COO)2 solution. This electrode will also be abbreviated as the MnOx/Pt/GC electrode.
To inspect the role of the substrate grafting with MWCNTs, a GC substrate was functionalized with MWCNTs (multiwalled, internal diameter: 5-10 nm, outer diameter: 25 nm, length: 10-30 μm, specific surface area: >55 m2·g-1, purity: >99.9%) before the deposition of nano-Pt and nano-MnOx. To do this, 10 mg of MWCNTs was mixed with 1 mL 5% Nafion/ethanol solution under sonication for 1 h. Then, 10 μL of the obtained suspension was sprayed on the surface of a GC substrate and left to dry in air at room temperature for another 1 h before washing with double distilled water. The MWCNT-grafted GC electrode was further modified with nano-Pt and nano-MnOx (as mentioned previously), and the catalyst will be termed the MnOx/Pt/MWCNT/GC catalyst.
2.2. Electrochemical Measurements
The electrochemical measurements were carried out in a traditional three-electrode glass cell at room temperature (~25 ± 1°C) using a Bio-Logic SAS potentiostat (model SP-150) operated with EC-Lab software. The catalytic performance of the modified electrodes toward EOFA was investigated in 0.3 M FA solution (pH = 3.5).
2.3. Material Characterization
The morphology and elemental composition were evaluated using a field-emission scanning electron microscope (FE-SEM, Quattro S, Thermo Fisher Scientific USA) whose accelerating voltage extended from 200 V to 30 kV with a magnification range from 6 to 2500000x that equipped with an energy dispersive X-ray spectrometer (EDS, AMETEK USA Element Detector). The crystallographic information was obtained using a high-resolution X-ray diffractometer (XRD-PANalytical X’Pert Pro powder) operated with a Cu anode (wavelength 0.154 nm, maximum 2.2 kW, and 60 kV).
3. Results and Discussions
3.1. Electrochemical Characterization
Electrochemically, the characterizations of the as-prepared catalysts were obtained and useful information about the catalytic ingredients could be obtained. The cyclic voltammograms (CVs) of the Pt/GC, MnOx/Pt/GC, and MnOx/Pt/MWCNT-GC catalysts in 0.5 M NaOH at a potential scan rate of 100 mV s–1 are depicted in Figure 1. The typical characteristic behavior of a polycrystalline Pt surface in an alkaline medium was observed at the Pt/GC electrode (Figure 1(a)). The hydrogen adsorption/desorption (Hads/des) peaks were observed in the potential range from −0.5 to −0.9 V with the Pt oxidation (Pt→PtO) extending in the anodic potential biasing from −0.2 to 0.6 V. The reduction of PtO (PtO→Pt) was also obvious in the cathodic scan at ca. −0.35 V [14, 18, 19].
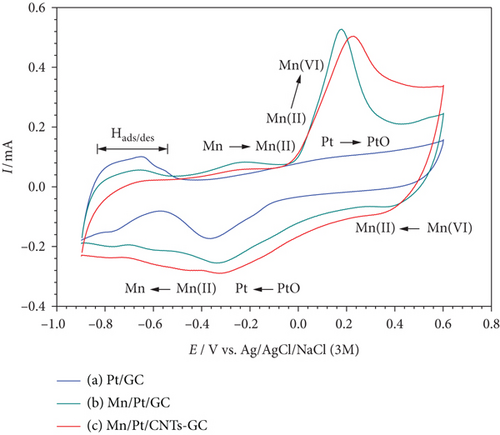
- (i)
Decreases in the charge associating the Hdes peaks were observed which were consistent with the consumption of the Pt surface in the deposition of nano-MnOx [20]. The decrease in this peak was larger for the MnOx/Pt/MWCNT-GC (Figure 1(c)) catalyst which presumably indicated the role of MWCNTs in the geometrical reorganization of nano-Pt. This reduction in the intensity of the Hdes peaks did not accompany similar decreases in the intensities of the PtO→Pt peaks as they interfered with the peaks corresponding to the Mn transformations
- (ii)
Two new anodic peaks were observed at ca. −0.25 and 0.19 V that were assigned, respectively, to the (Mn→Mn (II)) and (Mn (II) to Mn (IV)) oxidations [21]. Their corresponding cathodic peaks appeared, respectively, at ca. 0.4 and−0.27 V
- (iii)
An observable increase in the double layer capacitance which was attributed to the surface composition change [22]
| Electrode | ECSA (cm2) | θ (%) |
|---|---|---|
| Pt/GC | 0.65 | — |
| Mn/Pt/GC | 0.57 | 12 |
| Mn/Pt/CNT-GC | 0.60 | 10 |
3.2. Material Characterization
Material characterizations of the MnOx/Pt/MWCNT-GC electrode (the best one exhibited the highest catalytic activity and stability toward EOFA, refer to Section 3.3) have been extended to further determine its morphology, composition, and crystal structure. Figure 2(a) shows the FE-SEM image of the MnOx/Pt/MWCNT-GC electrode. It illustrated the electrodeposition of MnOx and Pt onto the MWCNT-modified GC surface as well-distributed spherical particles having an average size of ca. 85 nm. It is thought that modifying the GC surface with the MWCNTs was responsible for such homogenous loading of Pt and MnOx as previously observed for the deposition of Pt over the MWCNT-GC electrode [12, 22]. In this regard, we would highlight that the other two electrodes (Pt/GC and MnOx/Pt/GC) have been characterized in other previous study and unfortunately they did not exhibit such a homogenous texture like that of the proposed catalyst, MnOx/Pt/MWCNT-GC [14, 15].

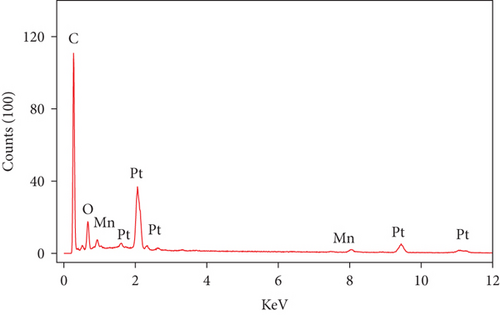
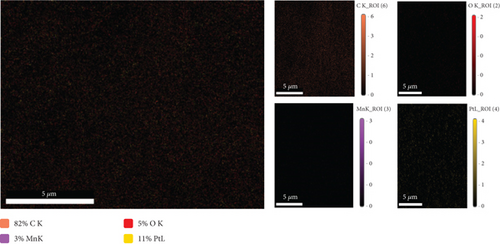
Compositionally, the EDS analysis of the MnOx/Pt/MWCNT-GC electrode (Figure 2(b)) confirmed the deposition of the different catalyst ingredients (C, O, Pt, and Mn) and assisted in calculation of their relative ratios (see Table 2). Figure 2(c) additionally provides the elemental mapping for the MnOx/Pt/MWCNT-GC electrode which further confirmed the homogeneous distribution of all catalyst ingredients.
| Element | Weight (%) | Atomic (%) | Error (%) |
|---|---|---|---|
| C K | 54.36 | 92.71 | 7.73 |
| O K | 1.99 | 2.55 | 32.97 |
| MnK | 0.56 | 0.21 | 25.43 |
| PtL | 43.08 | 4.52 | 7.65 |
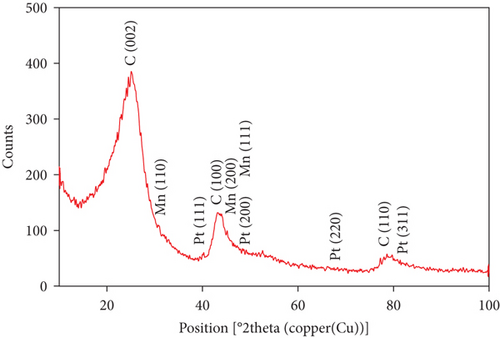
Furthermore, in order to investigate the crystal structure, XRD technique was utilized. Figure 2(d) shows the XRD pattern of the modified MnOx/Pt/MWCNT-GC electrode. Several diffraction peaks were identified at ca. 25°, 43°, and 79° corresponding, respectively, to the (0 0 2), (1 0 0), and (1 1 0) planes of hexagonal C structure [24]. Also the diffraction peaks identified at ca. 40°, 47°, 68°, and 82° belonged, respectively, to the (1 1 1), (2 0 0), (2 2 0), and (3 1 1) planes of the Pt face-centered cubic (fcc) lattice [25]. Moreover, the diffraction peaks appeared at ca. 30°, 45°, and 47° were corresponding, respectively, to the (1 1 0), (2 0 0), and (1 1 1) planes of the cubic β-Mn oxide structure [26].
3.3. Electrocatalysis of EOFA
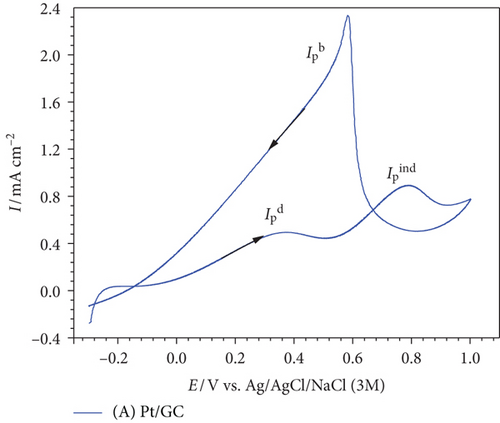
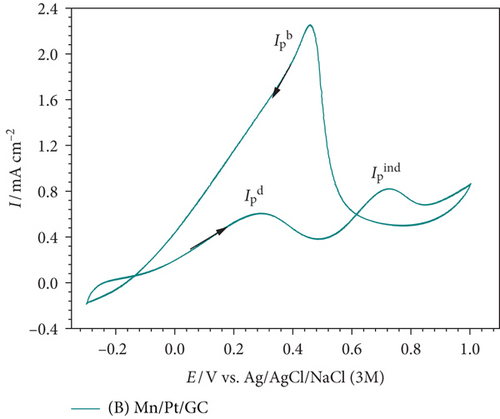
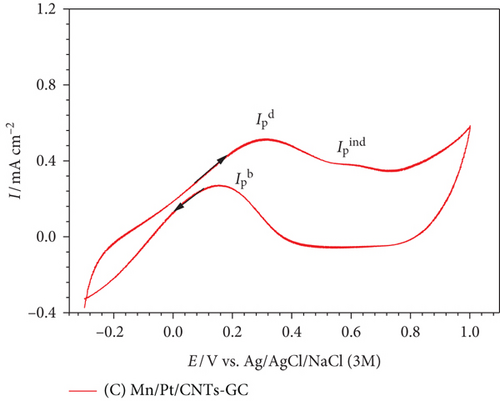
Now after most of the poisoning CO has been oxidized in the forward scan, EOFA could proceed in the backward cathodic scan mainly via the direct pathway (peak at ca. 0.5 V) with a high current density () [36].
After modifying the Pt/GC electrode firstly with nano-MnOx (MnOx/Pt/GC electrode) then with MWCNTs (MnOx/Pt/MWCNT-GC electrode), the degree of catalytic enhancement toward EOFA will be tracked using three parameters, , , and onset potential of EOFA (Eonset). A high value indicates the availability of excess active Pt sites free to participate in the direct EOFA at low potential. Meanwhile, a high value corresponds to a low level of CO poisoning, whereas a more negative Eonset value correlates to a less required overpotential for the reaction, thermodynamics enhancement [37]. At the Pt/GC electrode (Figure 3(a)), the values of were ca. 0.93, 0.24, and –0.005 V. Although nano-MnOx is not active toward EOFA [20, 38], the catalytic performance of the MnOx/Pt/GC electrode (Figure 3(b)) was higher than that of the Pt/GC electrode toward EOFA in terms of higher (3.13) and (0.50) and lower Eonset (–0.076 V). Fascinatingly after the modification with MWCNTs (which is also inactive toward EOFA [39, 40]) in the case of the MnOx/Pt/MWCNT-GC electrode (Figure 3(c)), the CO poisoning almost disappeared. This was reflected from the highest (18.6) and (1.2) and the lowest Eonset (–0.079 V) values. Table 3 provides a summary of the values obtained from Figure 3.
| Electrode | Eonset (V) @ 0.1 mAcm–2 | Rct (kΩ) | QCO (mC) | ||
|---|---|---|---|---|---|
| Pt/GC | 0.93 | 0.24 | –0.005 | 3.04 | 1.10 |
| Mn/Pt/GC | 3.13 | 0.50 | –0.076 | 0.75 | 1.09 |
| Mn/Pt/CNT-GC | 18.6 | 1.20 | –0.079 | 0.92 | 0.01 |
Furthermore, the catalytic stability of the modified electrodes was inspected. Figure 4 shows the chronoamperometric (i-t) curves obtained at the Pt/GC (Figure 4(a)), MnOx/Pt/GC (Figure 4(b)), and MnOx/Pt/MWCNT-GC (Figure 4(c)) electrodes in a 0.3 M aqueous solution of FA (pH = 3.5) at a potential of 0.25 V for 1800 s. A poor catalytic stability was observed at the Pt/GC electrode which owned a fast chronic decay in current density, in agreement with previous investigations [31, 32]. Unfortunately, this undesirable decay was kept almost the same for the MnOx/Pt/GC electrode (Figure 4(b)) but surprisingly slowed down to a great extent at the MnOx/Pt/MWCNT-GC electrode (Figure 4(c)). The maximum stability, in terms of the highest and steady-state current density, was obtained at the MnOx/Pt/MWCNT-GC electrode. Till now, modifications with nano-MnOx and MWCNTs were effective in maximizing the catalytic activity and the stability toward EOFA. But the question here is what is the role of each of nano-MnOx and MWCNTs in such observed enhancement? The next section will answer this question.

3.4. Mechanisms of Enhancement
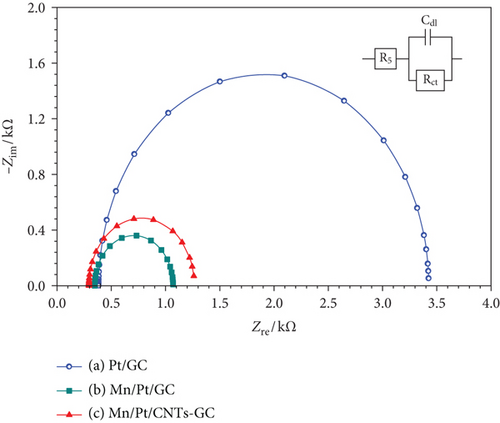
The electrochemical impedance spectroscopy (EIS) was employed to monitor the charge transfer resistance (Rct) of the proposed modified electrodes during EOFA. In principle, Rct that is equivalent to the polarization resistance of the electrochemical system is represented by the diameter of the extrapolated semicircle in the Nyquist diagram [41]. In this regard, the larger the diameter of the semicircle the higher Rct, and consequently, the slower kinetics of the reaction is [42]. Figure 5 shows the Nyquist plots obtained at the Pt/GC, MnOx/Pt/GC, and MnOx/Pt/MWCNT-GC electrodes in a 0.3 M aqueous solution of FA (pH = 3.5) at a potential of 0.2 V in the frequency range (10 mHz to 100 kHz). The fitting has been carried out by the EC-Lab software, and the equivalent circuit of this system was displayed in the inset of Figure 5, where Rct, Rs, and Cdl refer to the charge transfer resistance associating EOFA, the solution resistance, and the double layer capacitance, respectively. Figure 5 shows a lower Rct (0.75 and 0.92 kΩ), respectively, at the MnOx/Pt/GC and MnOx/Pt/MWCNT-GC electrodes compared with 3.04 kΩ obtained at the Pt/GC electrode (data are summarized in Table 3). This inferred about a facilitated charge transfer and improved catalytic activity of the MnOx/Pt/GC and MnOx/Pt/MWCNT-GC electrodes toward EOFA. It is important to mention here that the Rct values obtained at the MnOx/Pt/GC and MnOx/Pt/MWCNT-GC electrodes were so close which implies that the modification with MWCNTs did not participate in, rather the nano-MnOx was the catalyst component responsible for, such way of enhancement [20].
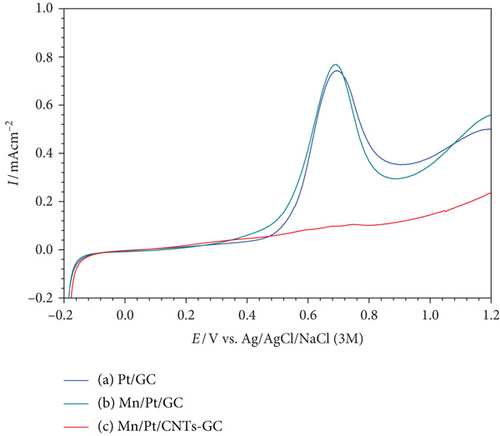
To precisely recognize the role of MWCNTs in the catalytic enhancement, CO was chemisorbed from 0.5 M formic acid at open-circuit potential on the Pt/GC, MnOx/Pt/GC, and MnOx/Pt/MWCNT-GC electrodes for 10 min. Then, this adsorbed CO layer was stripped electrochemically in 0.5 M Na2SO4 (pH = 3.5) as shown in Figure 6. At the Pt/GC electrode (Figure 6(a)), the surface active sites were blocked at lower potentials because of the adsorbed CO which was next oxidized at ca. 0.70 V. It is worthy to add here that the charge under the CO oxidation peak (QCO) reflects the amount of CO adsorbed at lower potentials and so can give a picture about the degree of surface poisoning. Two observations were clear after modifying the Pt/GC electrode with nano-MnOx (MnOx/Pt/GC electrode, Figure 6(b)); the first observation was that the QCO was almost the same for the Pt/GC and the MnOx/Pt/GC electrodes (1.10 and 1.09 mC, respectively, see Table 3) which suggested that the geometric enhancement, responsible for retarding the CO adsorption at the Pt surface, did not exist [43–45]. The second observation was starting the CO oxidation at the MnOx/Pt/GC electrode at lower potentials (ca. 170 mV negative shift) compared with the Pt/GC electrode. This behavior highlighted the effectiveness of the modification with nano-MnOx and also concluded that such enhancement arose mainly from the modification of the electronic properties of the Pt surface by weakening the Pt-CO bond and thus facilitating the oxidative removal of poisoning CO. [43–45]. This, fortunately, supported the mechanism of enhancement (faster reaction kinetics via a lower Rct) previously proposed from Figure 5. Yet, the role of MWCNTs was not detected, but it will be when looking for the CO stripping curve obtained at the MnOx/Pt/MWCNT-GC electrode (Figure 6(c)). Fascinatingly, at the MnOx/Pt/MWCNT-GC electrode (Figure 6(c)), the QCO almost disappeared (ca. 0.01 mC). This time, the geometric enhancement was behind such a huge decrease in the amount of adsorbed CO after the modification with MWCNTs. As reported previously, MWCNTs can facilitate the deposition of well-dispersed, nonagglomerated nano-Pt (as in our case, Figure 2(a)) that assisted in retarding the CO adsorption at the Pt surface [12, 22]. This is beside its high electronic conductivity, high corrosion resistance, and good structural, mechanical, and chemical stability [46].
4. Conclusion
The sequential electrodeposition of nano-Pt and nano-MnOx, respectively, at a GC electrode modified with MWCNTs has been carried out aiming to develop a novel anodic catalyst for EOFA. The electrocatalytic activity, stability, and mechanism of enhancement of the best modified electrode (MnOx/Pt/MWCNT-GC) were discussed and compared to the other modified electrodes (Pt/GC and MnOx/Pt/GC). values supported that the MnOx/Pt/MWCNT-GC electrode exhibited the highest electrocatalytic activity and stability toward EOFA. The mechanisms of enhancement by were thought to come mainly from both electronic (by the modification with nano-MnOx) and geometric (by the modification with MWCNTs) effects.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Acknowledgments
This research was supported by the British University in Egypt and Cairo University.
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.




