Luminescence and Preventive Effect of Two New Cd(II) Compounds on Scar Formation after Cosmetic Surgery
Abstract
Two coordination polymers involving Cd(II), i.e., [Cd2(IDPA)2(bpe) (H2O)2]n·2n(H2O) (1) (H2IDPA is 5-(1-oxoisoindolin-2-yl)isophthalic acid, bpe is 1,2-bis(4-pyridyl)ethane, 4-bpmh is N, N-bis-pyridin-4-ylmethylene-hydrazine), and [Cd(IDPA)(4-bpmh)0.5(H2O)2]n·n(H2O) (2) are prepared under solvothermal conditions via tuning the auxiliary ligand from bpe to 4-bpmh. Moreover, the as-prepared complexes 1–2 reveal much stronger luminescence in contrast to that of the free organic ligands. Their preventive effect and mechanism on scar formation after cosmetic surgery was explored. First, the two complexes’ suppression of the viability of the human immortal keratinocyte line (HaCaT) cell line was examined via exploiting cell counting kit-8 (CCK-8) detection. The vascular endothelial growth factor (VEGF) signal pathway activation in HaCaT cells was detected by real-time reverse transcription-polymerase chain reaction (RT-PCR) assay.
1. Introduction
The healing of postoperative incisions is a complicated pathophysiological process, and multiple factors such as gene expression regulation and secretion regulation of various growth factors will affect the incision healing and cause scar formation [1]. To prevent and treat postoperative scar formation, it is necessary not only to understand the skin anatomy and incision healing mechanism but also to select the appropriate suture materials to minimize postoperative scar formation through professional wound suture techniques and appropriate physical and chemical intervention methods [2, 3]. There are many physical and chemical intervention methods for the prevention and treatment of postoperative scar formation, but the mechanism is not very clear [4]. It is of great significance to conduct in-depth study of postoperative scar prevention and treatment.
In the past few decades, the research on metal-organic frameworks (MOFs) has aroused widespread attention on account of the fascinating topological skeletons of MOFs, simultaneously due to their underlying applications as functional materials in various areas of magnetism, luminescence, catalysis, H2 storage ect [5–8]. These promising application properties of MOFs constantly inspire us to design and construct new functional MOFs. Although achieving controlled syntheses of MOFs containing desired architectures and performances is still one of the biggest challenges for us, some effective methods for the syntheses of MOFs, the like mixed-ligand approach, pillar-layer method, post-synthetic modification as well as second building subunit method, have been successfully developed after lots of hard works by chemists [9–12], and numerous reports have demonstrated the effectiveness of these synthetic strategies for the construction of MOFs with predictable structures [13–15]. Given the importance of luminescent MOFs for the detection of pollutants, considerable efforts have been devoted to the construction of luminescent MOFs [16–18]. According to the reports, one good choice to prepare the luminescent MOFs is using a large π-conjugated organic ligand with multicoordination sites, and the large π-conjugated fluorophore of the organic ligand is helpful to enhance the luminescence of MOFs [19, 20]. H2IDPA containing large π-conjugated fluorophore has been widely used by Ye and his co-workers to construct lots of MOFs with intense luminescence at room temperature [21, 22]. In view its good coordination affinity and capability to d10 metal ions, in this work, we selected it in combination with N-donor ligands utilized as organic building backbones for assembling with the Cd(II) ions under the conditions of solvothermal. Through altering auxiliary ligands, we created two fresh compounds involving Cd(II), i.e., [Cd2(IDPA)2(bpe)(H2O)2]n·2n(H2O) (1) and [Cd(IDPA)(4-bpmh)0.5(H2O)2]n·n(H2O) (2). In addition, we also systematically investigated the fluorescent performances together with the thermal stabilities of the above compounds. Furthermore, the preventive effect and mechanism of the compound on scar formation after cosmetic surgery was determined.
2. Experimental Methods
2.1. Materials and Instrumentation
Cd(NO3)2·4H2O (99.9%, Ar) was purchased from Sigma-Aldrich reagent company (Beijing, China), all the organic ligands used in this study were provided by Jinan Henghua Technology Co., Ltd; the solvent DMF was supplied by Tianjin Kangkede Chemical reagent company, the pure water used in this study was obtained from Hangzhou Wahaha Group Co. Ltd. (Hangzhou, China). The pH of the pure water was 6.83 ± 0.05, and its electrical conductivity was 1.82 ± 0.20 μS/cm. For exploring the elements of N, C together with H, the Vario EL III analyzer was exploited. Varian 800 The PANalytical X/Pert Pro was utilized to investigate PXRD at 0.05° step size with 1.54056 Å·Cu/Kα radiation. The thermogravimetric analyses for as-generated CPs were implemented via the term analyzer of NETSCHZ STA-449C with an increasing rate of 10°C/min under the nitrogen flow at a temperature between 30 and 800. The Edinburg FLS920 TCSPC could be implemented for analyzing luminescent performances of the complexes at RT.
Synthesis of [Cd2(IDPA)2(bpe) (H2O)2]n·2n(H2O) (1) and [Cd(IDPA) (4-bpmh)0.5(H2O)2]n·n(H2O) (2).
The mixture formed by 0.1 mmol of Cd(NO3)2·4H2O, 0.1 mmol H2L, 0.1 mmol bpe, 1 mL DMF, and 2 mL H2O was placed into a small glass vial (20 mL) and heated under a temperature of 110°C for three days. After cooling the above mixture to RT gradually, the colorless massive crystals of compound 1 were gathered with a yield of 48 percent by Cd(NO3)2·4H2O. Anal. Calcd. (%) for C44H36N4O14Cd2 : N, 5.24, C, 49.37 and H, 3.37. Found (%): N, 5.21, C, 49.34 and H, 3.41. IR (KBr pellet) (υ/cm−1): 3382 (s), 2926 (m), 2362 (m), 1942 (w), 1826 (w), 1609 (s), 1547 (s), 1400 (s), 1312 (s), 1186 (m), 1111 (m), 1053 (w), 1012 (m), 901 (w), 859 (m), 778 (m), 745 (m), 705 (w), 674 (w), 587 (w), 487 (m).
The mixture synthesized via 0.1 mmol of Cd(NO3)2·4H2O, 0.2 mmol of 4-bpmh, 0.1 mmol H2L, 1 mL DMF, and 2 mL H2O was placed into a small glass vial (20 mL) and heated under a temperature of 110°C for three days. After cooling the above mixture to RT gradually, the colorless massive crystals of compound 2 were gathered with the yield of 38 percent in the light of Cd(NO3)2·4H2O. Anal. Calcd. (%) for C22H21N3O8Cd : N, 7.40, C, 46.49, and H, 3.70. Found (%): N, 7.38, C, 46.52 and H, 3.72. IR (KBr pellet) (υ/cm−1): 3385 (m), 2360 (w), 1944 (w), 1829 (w), 1610 (m), 1583 (m), 1534 (m), 1446 (m), 1398 (w), 1315 (w), 1186 (w), 1142 (w), 1109 (w), 1051 (w), 1014 (w), 922 (w), 905 (w), 862 (w), 810 (w), 779 (m), 746 (w), 709 (w), 670 (w), 635 (w), 592 (w), 526 (w), 477 (w), 426 (w).
2.2. X-Ray Crystallography
For the above complexes, their crystal architectures were measured by Mercury CCD that was controlled through the computer via exploiting 0.71073 Å graphite mono-chromated Mo–Kα radiation at RT. Moreover, the complexes’ architectures could be solved via applying direct approaches by ShelXS and subsequently optimized through full-matrix least-squares strategies through the software of SHELXL-2014 according to F2 [23]. The compounds’ crystallographic data along with architectural optimizations are exhibited in Table 1. Table S1 reflects the chosen bond angles (º) together with the lengths (Å) of as-created complexes. Table S2 exhibits the specific H-bond parameters for them.
| Sample | 1 | 2 |
|---|---|---|
| Formula | C44H36N4O14Cd2 | C22H21N3O8Cd |
| Fw | 1069.57 | 567.84 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | Pn | P21/c |
| a (Å) | 14.0662 (12) | 10.6556 (6) |
| b (Å) | 10.1504 (4) | 17.4949 (10) |
| c (Å) | 15.6798 (12) | 11.4139 (8) |
| α (°) | 90 | 90 |
| β (°) | 113.993 (4) | 96.883 (6) |
| γ (°) | 90 | 90 |
| Volume (Å3) | 2045.3 (3) | 2112.4 (2) |
| Z | 2 | 4 |
| Density (calculated) | 1.737 | 1.782 |
| Abs. Coeff. (mm−1) | 1.117 | 1.091 |
| Total reflections | 15136 | 10380 |
| Unique reflections | 8692 | 4459 |
| Goodness of fit on F2 | 1.026 | 1.199 |
| Final R indices [I > 2sigma (I2)] | R a = 0.0203, wR2b = 0.0492 | R = 0.0803, wR2 = 0.2393 |
| R (all data) | R = 0.0211, wR2 = 0.0496 | R = 0.0866, wR2 = 0.2440 |
- a R 1 = Σ||Fo|–|Fc||/Σ|Fo|. bwR2 = |Σw(|Fo|2–|Fc|2)|/Σ|w(Fo)2|1/2, where w = 1/[2(Fo2) + (aP)2 + bP]. .
2.3. CCK-8 Determination
The CCK-8 detection was performed for examining the complexes’ biological activity, and their inhibitory effect against the HaCaT cell’s viability was tested. This study was finished following the instructions along with a slight change. Shortly, the HaCaT cells in the phage of logical growth were gathered and inoculated into plates (96 well). The cells were cultivated in 37°C incubators with 5% CO2 humidified surrounding for half a day, the compound was subsequently added into wells for the treatment at 1, 2, 4, 8, 10, 20, 40, 80 μM concentration for two days. After that, CCK-8 reagents (10 μl) were utilized to incubate the cells for four hours, and the values of absorbance (optical density, OD) of each well were tested through a microplate spectrophotometer system (Molecular Devices, Sunnyvale, CA, USA) at 450 nm. This study was carried out in triplicate, and the results were described with mean ± standard deviation.
2.4. Real Time RT-PCR
The real-time RT-PCR was accomplished for the measurement of the complexes’ reduction effect against the VEGF signal pathway activation in HaCaT cells. The experiment was carried out fully based on the protocols after some changes. Briefly, the HaCaT cells in the stage of logical growth were gathered and then inoculated into plates (96 well). The cells were cultivated in 37°C incubators with a 5% CO2 humidified surrounding for twelve hours, next, the wells were added with a compound for performing the treatment. Subsequently, the cells were gathered and the extraction of overall RNA in tissue was conducted through TRIZOL reagent. After testing its quality and quantity, it was next reverse transcript into cDNA. Eventually, the real-time RT-PCR was carried out for determining the VEGF signal pathway relative expression in HaCaT cells, using the GAPDH as an internal control.
3. Results and Discussion
3.1. Crystal Structure of Compound 1
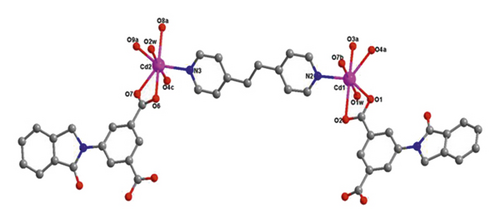
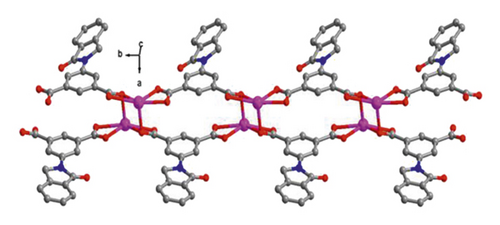
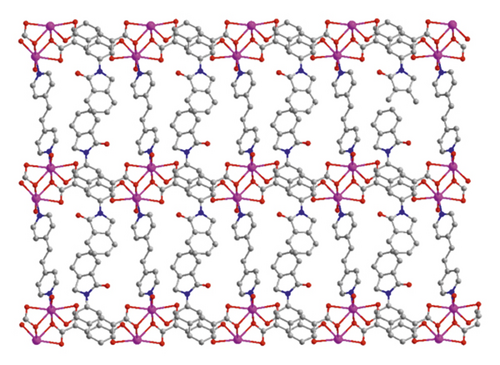
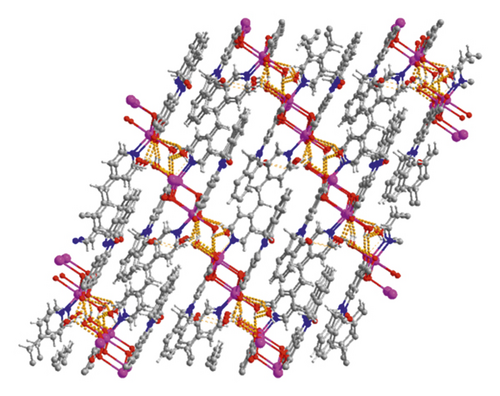
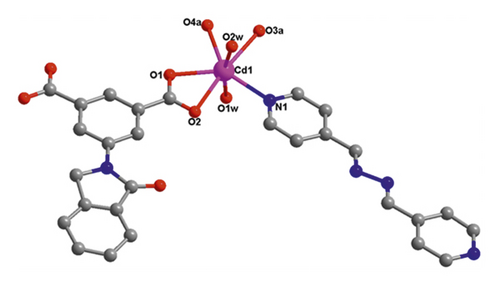
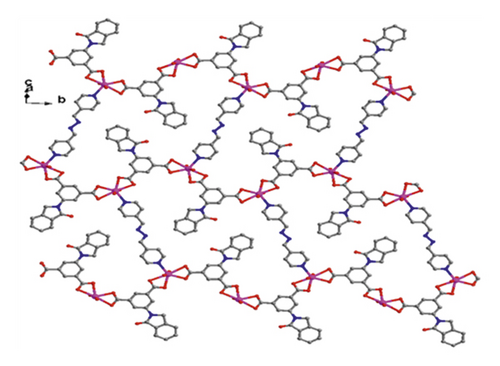
X-ray crystallographic investigations exhibited that 1 belongs to the monoclinic space group of Pn, revealing a 2-dimensional extended layered architecture. Each fundamental unit of compound 1 includes two isolated Cd(II) ions, two IDPA2−, a bpe, two free, and two coordinated molecules of H2O. Based on Figure 1(a), Cd1 ion, and Cd2 ion are 7-coordinated and exhibit slightly twisted pentagonal bipyramidal structures, they are defined through 5 carboxylic acid oxygen atoms offered by three IDPA2−, a pyridyl N atoms belong to a bpe ligand and a terminal H2O ligand. The bond separations of Cd–O/N varying from 2.242 (3) to 2.572 (3) Å are all by the reported compounds based on Cd(II) [24]. The IDPA2− in complex 1 utilities as a μ3-bridge with its two carboxylic acid groups in diverse coordination manners: bridging-chelating together with chelating modes (Figure S1(a)). Two consecutive Cd(II) ions are bridged through 2 carboxylic acid groups provided by IDPA2− into a dinuclear subunit of [Cd2(COO)2] with Cd⋯Cd separation of 3.78 Å, and thus, the above subunits are deeply bridged via IDPA2− ligands to produce a 1-dimensional chain motif extending along the crystallographic direction b (Figure 1(b)). Through the coordination of bpe to the Cd(II) ions, these 1D chains are connected , generating a 2D layer structure (Figure 2(c)). By considering the dinuclear subunits and organic ligands as 4-connected nodes and linear connectors, for example, 2-dimensional layer can be reduced into a 4-bridged SQL tetragonal plane net (Figure S2(a)). Notably, the H atoms of the coordinated H2O molecules can be accepted suitably by the carboxylic acid oxygen atoms belonging to another neighboring 2-dimensional layer, creating weak intermolecular H-bonding interactions that are revealed in Table S2. Hence, these weak H-bonding interactions deeply glued these 2-dimensional layers into a 3-dimensional supramolecular skeleton (Figure 2(d))
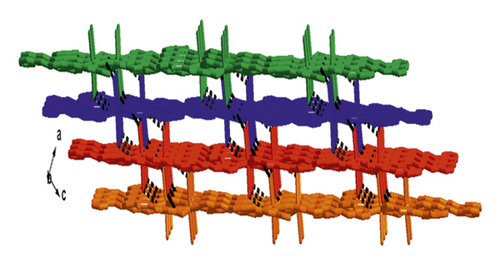

3.2. Crystal Structure of Compound 2
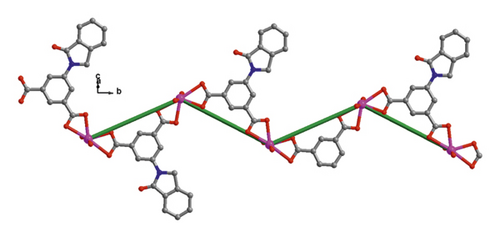
When bpe was substituted via another longer bis(pyridyl)-based ligand of 4-bpmh, compound 2 was successfully obtained that also crystallizing the monoclinic space group of P21/c. And its structured unit contains a Cd(II) ion, an IDPA2−, 0.5 4-bpmh ligands, and a free and two coordinated molecules of H2O. According to Figure 2(a), Cd1 is 7-coordinated via 4 carboxylic acid oxygen atoms belonging to two IDPA2−, two coordinated H2O molecules, a pyridyl N atom coming from a 4-bpmh, and the Cd1 ion coordination structure can be expressed as slightly twisted pentagonal bipyramid. The Cd–O/N separations vary from 2.299 (6) to 2.556 (6) Å, which are comparable with the lengths of reported compounds based on Cd(II) [20]. The IDPA2− in compound 2 employs a μ2-bridge with the two carboxylic acid groups of IDPA2− ligand in the uniform chelating manner (Figure S1(b)). The Cd(II) ions are bridged μ2-bridges of IDPA2− ligands, providing a 1-dimensional zigzag chain architecture along crystallographic axis b (Figure 1(b)), and in the above chain, the length of Cd⋯Cd isolated through IDPA2− is 9.68 Å. These neighboring 1-dimensional chains are associated together by the coordination of the 4-bpmh ligands, creating a 2-dimensional layer along plane bc (Figure 2(c)). By regarding the organic ligands and Cd(II) ions as 3-bridged nodes and linear connectors, this 2D layer of 2 represents a 3-connected hcb net (Figure S2(b)). Although both compounds 1 and 2 show 2D layered structures, the different coordination patterns of IDPA2− ligand result in their different structural features and topological nets. In 2, H-bonding interactions are also present between the carboxylic acid oxygen atoms coming from one layer and the coordination H2O molecules of another layer, and the specific H-bond parameters are reflected in Table S1. Therefore, the 3-connected hcb-type 2D layers are finally associated together via the intermolecular H-bonds, producing a 3-dimensional supramolecular skeleton (Figure 2(d)).
3.3. Powder X-Ray Diffraction (PXRD) Patterns and Thermogravimetric Analyses (TGA)
The PXRD analyses outcomes on polycrystalline samples of 1–2 are shown in Figure S3, from which we can see that there is great accordance of peak positions between the experimental and simulated patterns, indicating excellent homogeneity and purity of as-prepared bulk samples.
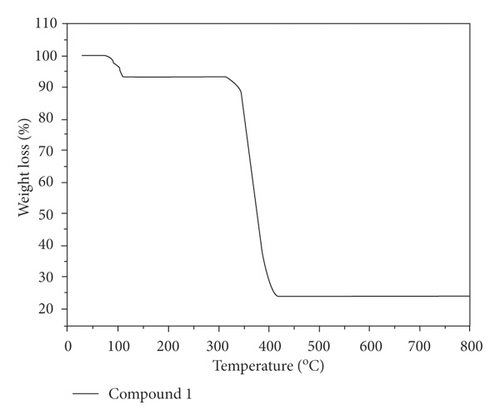
Moreover, we also explored the compounds’ thermal behaviors under nitrogen flow between 30 and 800°C, and the study outcomes are revealed in Figure 3. In the compound 1’s curve of TGA, 6.70% of weight loss existed at a temperature of 75–110°C and this is on account of the loss of the free together with the coordinated molecules of water (with 6.74% calculated value), and then when the temperature reaches 312°C, the 1’s weight can be stable, and thereafter, significant weight loss occurred from 312 to 418°C, which is associated with the organic ligand decomposition. For compound 2, the loss of the lattice and coordinated H2O molecules appeared from 75–122°C with an observed weight loss of 9.56% (calcd: 9.51%), and the collapse of the framework occurred in the range of 307–441°C because of the organic ligand decomposition.
3.4. Photoluminescent Properties of 1–2
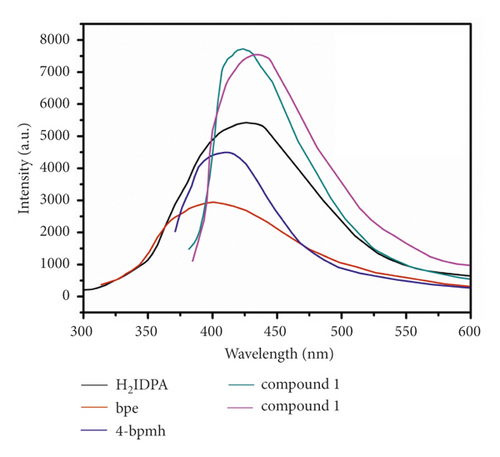
The transition metal compounds based on d10 generally exhibited outstanding luminescent performances for the potential application of luminescence sensing [25, 26]. Hence, in this work, the compounds’ luminescent spectra were detected at RT, and with the aim of further under the luminescence nature, the luminescent spectra of associated free ligands were also gathered under identical conditions. Based on Figure 4, the 4-bpmh, bpe, and H2IDPA emission spectra have maximum emission peaks at about 411 nm (λex = 330 nm), 400 nm (λex = 320 nm), 426 nm (λex = 345 nm), respectively, and these emissions may be caused by π∗to n or π orbital transitions [23]. The compounds’ emission spectra reveal the emission bands centered at 423 nm (λex = 340 nm) and 434 nm (λex = 340 nm). In comparison with N-donor ligands, the compounds’ emission spectra show obvious redshifts, and compared to the carboxylate ligand of H2IDPA, complex 1’s emission spectrum has a 3 nm blue-shift and compound 2’s emission spectrum has an 8 nm red-shift. Taking into consideration of the d10 configuration for Cd(II) and Zn(II) ions, the compounds’ luminescence emissions may be on account of ligand-to-ligand charge transfer or intraligand emission or the mixture of ligand-to-ligand charge transfer and intraligand emission [15]. In contrast to free ligands, much stronger luminescence emissions of the complexes principally resulted from the coordination of ligand to the metal ions that can significantly upregulate the rigidity of the organic ligand.
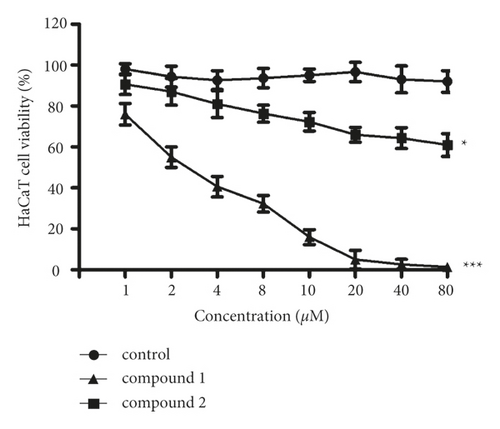
After creating compounds, their inhibitory activity against the viability of the HaCaT cells was tested. Hence, the CCK-8 detection was implemented in this paper. Based on Figure 5, it can be found that the 1 could markedly downregulate the HaCaT cell’s viability. Nonetheless, 2 only exhibited a little effect on the HaCaT cell’s viability. This result suggested the complex 1 treatment activity was much stronger in contrast to 2.
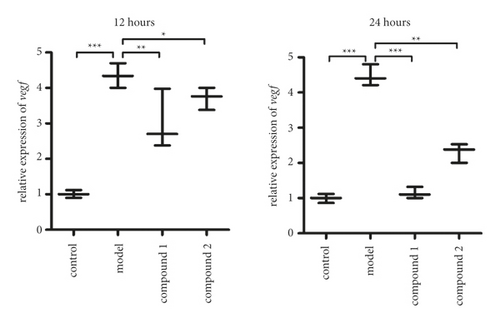
As previously described, complex 1 revealed superb inhibitory activity on the HaCaT cells viability. As the important role of the VEGF signal pathway in the viability of the HaCaT cells , the real time RT-PCR was deeply performed to test the VEGF signal pathway activation in the HaCaT cell. The results in Figure 6 exhibit that HaCaT cells have higher VEGF signal pathway activation level than control group. After treating via the compound 1, the activation of VEGF signal pathway in HaCaT cells was markedly downregulated, which is much superior to 2.
4. Conclusions
In the current paper, we prepared two compounds based on Cd(II) that were prepared under solvothermal conditions. The structural variations of 1–2 were achieved via regulating the N-donor auxiliary ligands: 4-connected SQL-type 2D net for 1 and 3-connected hcb-type 2D net for 2. Finally, these 2D layers are glued together via the intermolecular hydrogen bonds, affording 3D supramolecular frameworks. The compounds’ strong luminescence emissions reveal that IDPA2− is an outstanding organic building backbone for building luminescent hybrid materials. The outcomes of CCK-8 detection suggested that 1 was superior to complex 2 in decreasing the viability of the HaCaT cells. The VEGF signal pathway activation in HaCaT cells was also suppressed via complex 1, instead of 2. Eventually, with the previous experiments, compound 1 was more outstanding than 2 in treating scar formation after cosmetic surgery.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The research was supported by Quanzhou Municipal Science & Technology Project (2020C036R) and Natural Science Foundation of Fujian Province (2019J01595).