Improving the Extraction of Active Ingredients from Medicinal Plants by XynA Modification
Abstract
Active ingredients of medicinal plants have unique pharmacological and clinical effects. However, conventional extraction technology has many disadvantages, such as long-time and low-efficiency. XynA-assisted extraction may overcome such problems, since the plant cell wall is mainly composed of cellulose. Based on the three-dimensional protein structure, we found the C-terminal domain and N-terminal domain twisted together and resulted in more flexibility. We carried out a series of truncations, with XynA_ΔN36 getting more yields of active ingredients. As shown by HPLC analysis, the efficiencies for extraction of salvianic acid A and berberine from Salvia miltiorrhiza and Phellodendron chinense were increased by approximately 38.14% and 35.20%, respectively, compared with the conventional extraction protocol. The yields of the two compounds reached 2.84 ± 0.05 mg g−1 and 3.52 ± 0.14 mg g−1, respectively. Above all, XynA_ΔN36 can be applied to the extraction of salvianic acid A and berberine, and this study provides a novel enzyme for the extraction technology, which aids rational utilization and quality control of medicinal plants.
1. Introduction
Medicinal plants play important roles in the treatment of clinical diseases. For example, they may benefit patients infected by 2019-nCoV and patients with Alzheimer's disease [1, 2]. Over the past few decades, the pharmacological effects of active ingredients from medicinal plants have attracted increasing international attention. Currently, research on methods for extraction of active ingredients has become an active field [3, 4]. Many research teams aimed to optimize the processes of traditional extraction and established green, economical, and low-consumption methods for rapid analyses of complex compounds in medicinal plants [5, 6]. Compared to traditional organic reagent extraction, enzyme-assisted extraction could stabilize the chemical structure of active ingredients in a wild way. Besides, enzyme-assisted extraction could avoid the organic solvent effect towards the pharmacodynamics study.
The first step for extracting effective components from medicinal plants is to break the cell wall and allow the target to enter the medium quickly and efficiently. Plant cell walls consist of dense structures composed of cellulose, hemicellulose, lignin, and pectin [7]. Hemicellulose is a heteropolymer mainly composed of xylan, which is a complex polysaccharide for which the structure depends on the plant species and tissue sources [8]. Recently, cellulase, protease, and xylase all are commonly used enzymes [9, 10]. Selecting appropriate enzymes to pretreat herbs can effectively destroy the cell wall structure by decomposition and accelerate the dissolution of active ingredients from cells. Besides, this kind of enzyme-assisted extraction method can reduce resistance to solvent extraction and shorten the extraction time.
Xylanases mainly participate in the hydrolysis of xylan linked by β-1,4-glycosidic bonds. The glycoside hydrolase 10 (GH10) and glycoside hydrolase 11 (GH11) families are the two largest xylanase families [11]. Recent studies have pointed out that GH10 family enzymes can be applied in many fields, such as food, herb extraction, and animal feed [12–14]. Bacillus sp. KW1 XynA is a member of the GH10 family, and it has both xylanase and cellulase activity simultaneously, which is rare in this family [15]. It participates in the hydrolysis of polysaccharides, such as mannan, galactosan, cellohexaose, and p-nitrobenzene-cellobioside. Furthermore, biotechnological application of XynA has been realized. For example, it can be used synergistically after cellulase to hydrolyse corn stover. In addition, it has broad working ranges for pH or temperature. However, there are no reports on the effects of the enzyme for extraction of medicinal plants. Recently, the structure of XynA was analysed by our team, and there were significant differences compared to other GH10 family members [16]. Based on the structure, the protein could be reconstructed to improve application of XynA for extraction of active ingredients.
Salvia miltiorrhiza and Phellodendron chinense are both perennial medicinal plants and are cultivated widely. Many different pharmacological activities have been reported, such as antitumor, antibacterial, and anti-inflammatory activities [17, 18]. Salvianic acid A and berberine are the main active ingredients of Radix Salvia miltiorrhizae and Cortex Phellodendron chinensis, respectively, in the Chinese Pharmacopoeia. Berberine has been used to treat intestinal infections and bacillary dysentery [19]. Due to high levels of cellulose in Radix Salvia miltiorrhizae and Cortex Phellodendron chinensis, which prevents the active ingredient outside cell, they were chosen to study the application of XynA on extraction. Also, combined with the protein structure, we could design a better enzyme.
Our present study was carried out to investigate and improve the application of XynA for medicinal plants and increase the yields for extraction of salvianic acid A and berberine from Salvia miltiorrhiza Bge. And Phellodendron chinense Schneid, respectively, with enzyme engineering. Then, HPLC methods for analysing salvianic acid A and berberine are established. The different constructs based on the protein structure are also discussed. Especially, we focused on the domains which did not fit density well. The effects of XynA truncations on the extraction yield of salvianic acid A and berberine are analysed in detail.
2. Experiments
2.1. Materials
E. coli DH5α and BL21-CodonPlus (DE3) competent cells were both provided by TransGen Biotech (Beijing, China). Radix Salvia miltiorrhizae and Cortex Phellodendron chinensis were obtained from Guangdong Qingyuan Traditional Chinese Medicine Co., Ltd. and Puning Baicao Traditional Chinese Medicine Co., Ltd., respectively. They both were dried and placed into a crusher through a 50-mesh sieve. Standard salvianic acid A and berberine with purity ≥98% were purchased from Shanghai Haling Biotechnology Co., Ltd. HPLC-grade solvents (methanol and acetonitrile) were purchased from Oceanpak Alexative Chemical., Ltd. Analytical grade reagents (formic acid and phosphoric acid) were purchased from Tianjin Kemiou Chemical Reagent Co., Ltd. Trichoderma reesei cellulase (cellulase, a commercial enzyme) was obtained from Shengdong Biotech, Inc. All other reagents and solvents were commercially available and of analytical grade.
2.2. Enzyme Design and Expression
The truncations XynA_ΔC (residues 1–326), XynA_ΔN (residues 68–408), XynA_ΔN36 (residues 37–408), and XynA_ΔNC (residues 68–326) were PCR-amplified from the XynA gene (GenBank ID QCO69162) and cloned into the pET-28a(+) expression vector with NdeI and XhoI sites. After sequencing, the plasmids were transformed into E. coli BL21-CodonPlus (DE3) competent cells. Protein expression and analysis were performed as previously reported [20]. In brief, the E. coli BL21-CodonPlus (DE3) cells were cultivated in the Luria-Bertani medium containing 30 μg/mL kanamycin and 32 μg/mL chloramphenicol, and protein expression was initiated by 0.2 mM isopropyl-β-d-thiogalactoside at 25°C for 12 h. After initiation, the E. coli cells were collected and lysed. After centrifugation at 20,913 g for 1 h (4°C), the supernatant was heated in a water bath at 65°C for 1 h and then centrifuged again [15]. Next, the target protein was purified by using a Ni-NTA column with an imidazole gradient ranging from 10 mM to 250 mM. Furthermore, the fraction containing target protein was purified by using a Hitrap QHP column (1 mL) with a NaCl gradient ranging from 50 mM to 1000 mM. Finally, the purified protein was also analysed by 12% SDS-PAGE, and appropriate fractions were pooled, dialyzed into a protein buffer (280 mM NaCl, 20 mM Tris-HCl, pH 8.0) and concentrated to 3.0 mg/mL. The purified protein was stored at −80°C.
2.3. Size-Exclusion Chromatography Analysis
Gel filtration analysis of XynA and XynA_ΔN36 was performed using AKTA pure 25 M1 (GE Healthcare, United States), as previously reported [21]. XynA and XynA_ΔN36 (500 μg) were centrifuged at 20,913 g (4°C) for 10 min. Subsequently, the two proteins were successively injected into a fast protein liquid chromatography system. The elution buffer contained 20 mM Tris-HCl pH 8.0, 150 mM NaCl, and 1 mM dithiothreitol.
2.4. Enzyme-Assisted Extraction of Salvianic Acid A and Berberine
Salvianic acid A and berberine were extracted from Radix Salvia miltiorrhizae and Cortex Phellodendron chinensis, which were both filtered through a 50-mesh sieve, with the assistance of enzymes and analysed quantitatively using HPLC. Cellulase, XynA, and XynA_ΔN36 were used to accelerate the extraction of both salvianic acid A and berberine, respectively. First, a moderate volume of extraction solution (containing 50 mM Na2HPO4-NaH2PO4, pH 6.0) was added to a conical flask containing medicinal plants (50 mL, 3 g for Salvia miltiorrhiza and 20 mL, 1 g for Phellodendron chinense). Then, the enzyme was added to the herb solution, and the mixture was incubated at 60°C for 12 h [15]. After that, the mixture was decocted two times, 1 h for each. The extract was filtered immediately, and the eluate was collected. Next, 50% ethanol was added to the filtrate, and the precipitate was removed by centrifugation at 1,707 g for 20 min. Finally, the filtrate was subjected to rotational evaporation to obtain the crude extract.
3. HPLC Analysis
3.1. Sample Preparation
Salvianic acid A and berberine standards (1.0 mg) were weighed and then dissolved in ultrapure water using a 5 mL volumetric flask to prepare a standard solution with a concentration of 0.2 mg/mL.
The 0.5 g crude extract was also dissolved in ultrapure water using a 5 mL volumetric flask. Subsequently, the resulting sample solution was filtered through a 0.22 μm microporous membrane and placed in a brown sample vial.
3.2. HPLC Conditions
HPLC analysis was performed by using a YMC-Pack ODS-A C18 column (250∗4.6 mm, particle size 5 μm), and 10 μL was injected into the system of Agilent 1260 series (Agilent Technologies, United States). The column oven temperature was set to 25°C. All mobile phases were filtered with a 0.22 μm membrane filter before use. Each sample solution was injected three times.
3.3. For Salvianic Acid a
Water (containing 0.5% formic acid, mobile phase A) and methanol (mobile phase B) were used as the mobile phases. Samples were analysed using the following stepwise gradient programme: mobile B 25% to 85% from 0 to 20.0 min and mobile B 85% to 25% from 20.0 min to 25.0 min. The detection wavelength was 281 nm. The flow rate was 0.3 mL/min.
3.4. For Berberine
Water (containing 0.1% phosphoric acid, mobile phase A) and acetonitrile (mobile phase B) were used as the mobile phases. Samples were analysed using the following stepwise gradient programme: mobile B 50% to 60% from 0 to 10.0 min; mobile B 60% to 80% from 10.0 min to 15.0 min; mobile B 80% to 100% from 15.0 min to 20.0 min; and mobile B 100% to 50% from 20.0 to 25.0 min. The detection wavelength was 265 nm. The flow rate was 1.0 mL/min.
3.5. Data Analysis
All experiments were performed in triplicate, and the data are expressed as mean ± standard deviation (SD). Statistical differences were performed using Student's t-test by using SPSS version 19.0 (IBM Corporation, Armonk, NY, USA).
4. Results and Discussion
4.1. Effect of Enzyme-Assisted Extraction on the Extraction Yield
To improve the efficiency for extraction of active ingredients from medicinal plants, we established an enzyme-assisted extraction system. First, XynA protein was overexpressed in E. coli cells and purified. Subsequently, because the active ingredients mostly come from roots and stems, roots of Salvia miltiorrhiza and bark of Phellodendron chinense were selected. The incubation condition of enzyme was optimized as previously reported [20]. After coincubation with XynA, the medicinal plants were extracted, and the active ingredient-rich crude extract was dried through rotational evaporation. Finally, salvianic acid A and berberine were quantitatively analysed by HPLC.
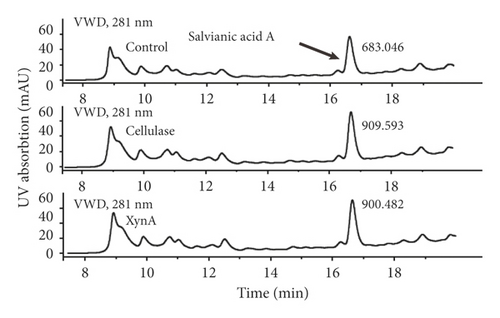
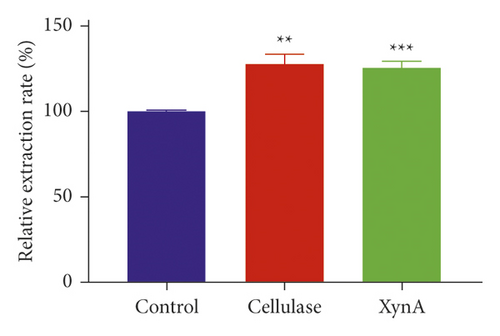
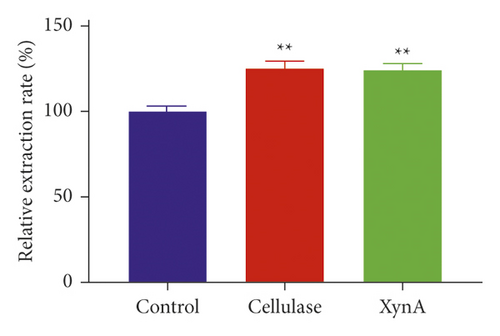
Cellulase, a commercial enzyme, was used as a positive control, and we used an extraction without any enzyme as the negative control at the same incubation condition with the XynA group. First, the application of XynA for extraction of plant roots was investigated. The retention time of salvianic acid A through HPLC was 16.50 min, as shown in Figure 1(a). Similarly, the extraction of plant stems was carried out, and the retention time of berberine was 10.10 min, as shown in Figure 1(c). The results for extraction of salvianic acid A and berberine with different enzymes were compared with those for traditional methods, and this is shown in Figure 1(b) and Figure 1(d). The y-axis indicates relative extraction rate. Enzyme-assisted extraction using cellulase and XynA results in a higher extraction efficiency, typically 20% higher than that of the negative control, although there were no significant differences between cellulase and XynA. In addition, we tried to extract quercetin from Ginkgo biloba leaves; however, there was no significant increase in extraction yield after incubation with enzymes, either cellulase or XynA, compared with conventional solvent extraction. This result may be due to different compositions of cell walls from different plant tissues [22]. For example, the amount of cellulose in different plants may range from 25% to 53%, and hemicellulose may range from 11.0% to 50.0% [23]. Above all, the extraction of herb leaves needs further study.

4.2. Specificity of XynA Protein Structure
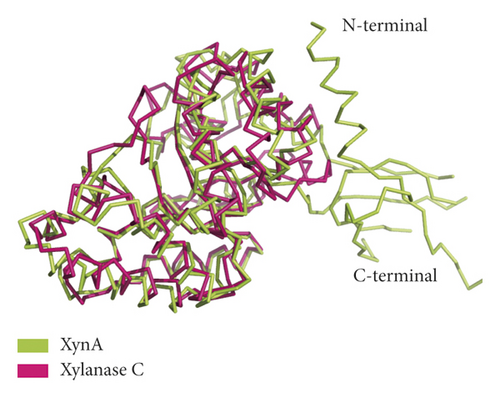
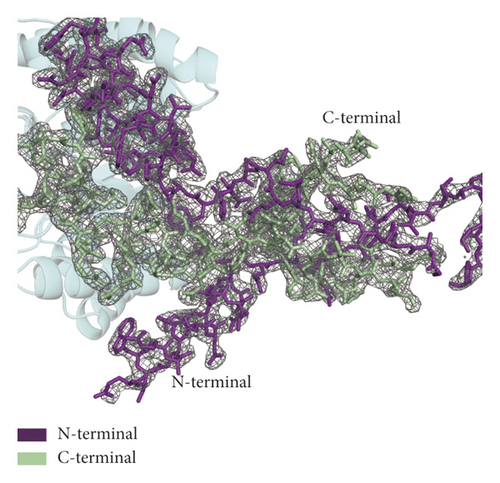
Xylanase C also belongs to the GH10 family and is a hydrolase from Aspergillus niger CBS 513.88. Compared with the GH10 family member xylanase C (PDB ID 4XUY), XynA (PDB ID 7D88) has a unique three-dimensional structure, as shown in Figure 2(a). The RMSD value is 1.468 Å over 194 Cα atoms between the two structures, indicating that the two structures have high homology. XynA is larger than xylanase C. Furthermore, we observed the density maps around C-terminal and N-terminal domains and found that there was overlay between the two maps, especially for β 1–2 at the N-terminal domain and the β 12–14 region at the C-terminal domain, as shown in Figure 2(b). Due to the flexibility of these overlapping regions, the phenomenon may affect the stability of the protein and the binding ability with its substrate.
4.3. Enzyme Engineering and Protein Expression
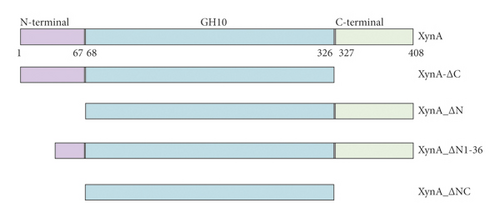
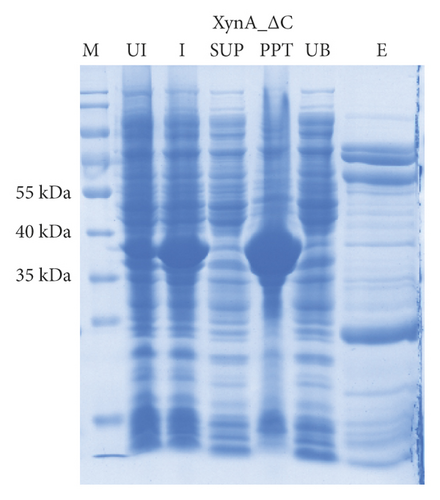
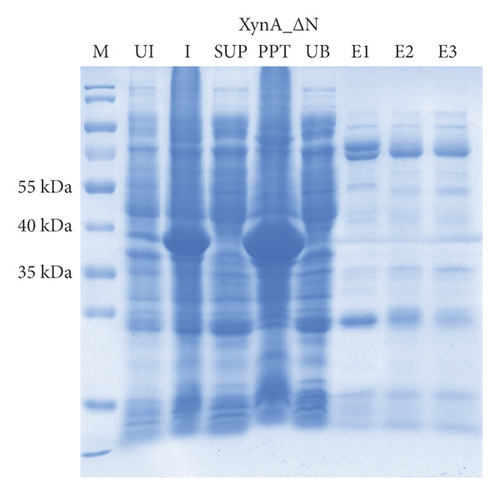
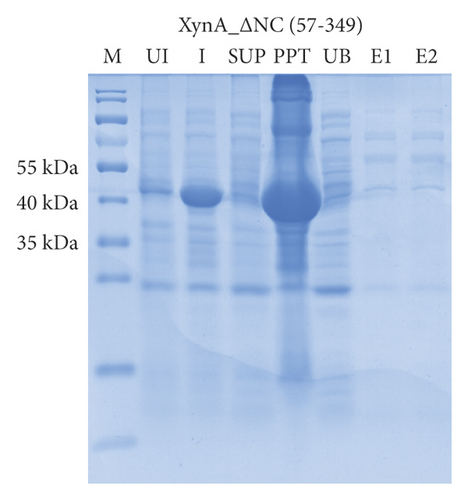
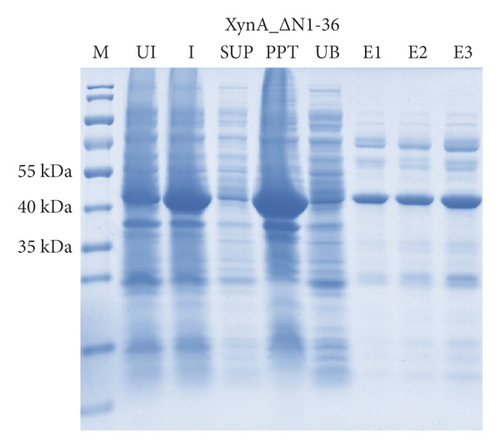
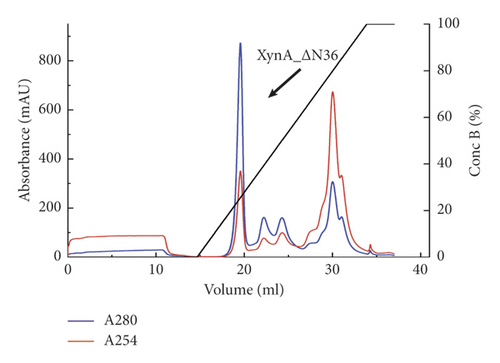
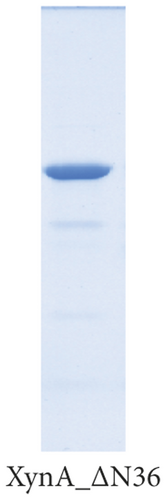
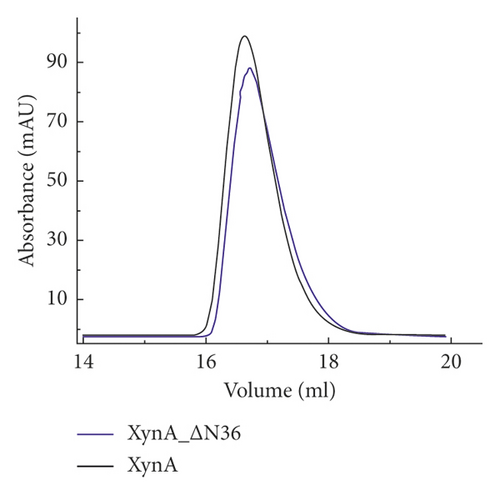
As previously reported, the xylanase activity and thermostability of a member of GH10 family Xyn II increased significantly after residues at the end regions were deleted [24]. It is hypothesized that truncations of the C-terminal or N-terminal domain may improve enzyme activity, resulting in an increase in the efficiency for extraction of active ingredients. A series of attempts to truncate the C-terminal or N-terminal domain were carried out, as shown in Figure 3(a). Most proteins, such as XynA_ΔC, XynA_ΔN, and XynA_ΔNC (57–349) were insoluble and found in inclusion bodies despite the use of multiple strains, as shown in Figure 3(b)–3(d). However, XynA_ΔN36 behaved well, as shown in Figure 3(e). Furthermore, the XynA_ΔN36 protein was purified using anion exchange chromatography, the target was eluted at 20% of the maximum ionic strength as shown in Figure 4(a), and the purification of protein was achieved to approximately 95% purity, which is applicable for extraction experiments, as shown in Figure 4(b). Subsequently, we analysed the protein conformation using size-exclusion chromatography, and the two proteins were eluted nearly at 16 ml, as shown in Figure 4(c). Compared with the XynA protein, XynA_ΔN36 eluted slightly slower because of the deletion of 36 residues at the N-terminal domain [25]. However, there was a single peak for both proteins, indicating that the deletions did not change the protein conformation.

4.4. Effect of Enzyme Truncation-Assisted Extraction on the Extraction Yield
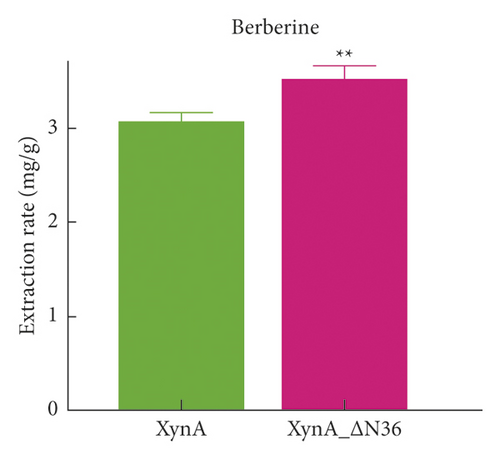
To evaluate the extraction efficiency of XynA_ΔN36, the standards salvianic acid A and berberine were used to quantitatively analyse the extraction. The calibration curve for salvianic acid A showed good linearity in the range of 0–2 μg, and the calibration curve was y = 0.0004x − 0.0089, with r2 = 0.999, where y indicates the peak area and x indicates the amount of salvianic acid A. Similarly, for berberine, the calibration curve is y = 0.0001x − 0.009, with r2 = 0.999, and the linearity range was 0–1 μg. The extraction efficiencies, which indicates the contents of active ingredients for salvianic acid A and berberine were used as evaluation indices. The extraction rates of salvianic acid A were 2.58 ± 0.07 mg g−1 and 2.84 ± 0.05 mg g−1 for XynA and XynA_ΔN36, respectively, as shown in Figure 4(c). Moreover, the XynA_ΔN36 extraction rate of berberine (3.52 ± 0.14 mg g−1) was higher than the XynA extraction rate (3.08 ± 0.08 mg g−1) (Figure 4(d)), indicating that the deletion of the N-terminal domain improved the extraction efficiency of XynA.
5. Conclusion
XynA-assisted extraction was found to significantly enhance the extraction of active ingredients in medicinal plants, and this method was successfully applied to the extraction of salvianic acid A and berberine. Due to the specificity of the protein structure, enormous efforts were carried out to reform the enzyme, and fortunately, the extraction yields of salvianic acid A and berberine using XynA_ΔN36 were 2.84 ± 0.05 mg g−1 and 3.52 ± 0.14 mg g−1, respectively; these represent increases of 38.14% and 35.20% compared with the conventional solvent extraction. As XynA is a novel enzyme of the GH10 family, its application should attract more attention. In particular, this method could make the extraction of medicinal plants more environmentally friendly and aids the quality control of medicinal plants through the active ingredient contents. On the other hand, the mechanism of XynA-assisted extraction should be further studied.
Abbreviation
-
- GH10:
-
- Glycoside hydrolase 10
-
- Ni-NTA:
-
- Nickel-nitrilotriacetic acid
-
- PDB:
-
- Protein data bank
-
- RMSD:
-
- Root-mean-square deviation
-
- SDS-PAGE:
-
- Sodium dodecyl sulfate polyacrylamide gel electrophoresis.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors’ Contributions
Lin Zhang curated the data and wrote the original draft. Wei Xie designed the methodology and performed the investigation. Xiaojun Teng performed the software analysis and formal analysis. Kui Wang provided the resources and conducted visualization. Wei Xie conceptualized the study, reviewed and edited the manuscript. Caiyan Wang supervised and administered the project. Lin Zhang and Wei Xie contributed equally.
Acknowledgments
This work was funded by the Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (Caiyan Wang, 2019), China Postdoctoral Foundation (no. 2021M700962), and National Key Research and Development Program of China (grant no. 2017YFE0119900).
Open Research
Data Availability
No data were used to support this study.