Hydrogel-Crosslinked Microneedles Based on Microwave-Assisted Drying Method
Abstract
We present a method and several applications for the synthesis of hydrogel-crosslinked microneedle arrays utilizing microwave-assisted drying, ensuring a significant reduction in reaction preparation time while maintaining quality. We demonstrate the feasibility of drying hydrogels using microwaves and thus extend to crosslinked microneedle fabrication. Crosslinking was performed using 1,4-butanediol diglycidyl ether (BDDE) as a crosslinking agent. Infrared spectra of the microneedle arrays were measured with attenuated total reflection-Fourier transform infrared (ATR-FTIR). The surface morphology of the microneedle arrays was observed with scanning electron microscopy (SEM). The microneedle arrays were evaluated in terms of mechanical strength, swelling kinetics, rheological properties, degradation rate, and glucose iontophoresis. The results show that this method can shorten the reaction preparation time by 5 hours, and the prepared crosslinked microneedle array has better crosslinking efficiency, swelling effect, and greater mechanical strength than traditional methods.
1. Introduction
When an organism is not suitable for oral administration, the most common nonoral administration method is injection administration. However, injection administration introduces several issues, including accidents caused by operator error, pain from injections, and local skin necrosis [1]. Therefore, the concept of microneedle was proposed to solve this problem. Microneedles are tiny needles with drug delivery capabilities, commonly composed of a micron-scale hollow or solid microneedle arrays, with lengths ranging from several hundred micrometers to millimeters, allowing the needles to penetrate the skin surface without touching the pain nerves [2], resulting in fewer adverse reactions in subjects [3]. Therefore, it has various applications in medicine and other fields [4–8].
Crosslinked hydrogels are chemically or physically crosslinked to form interconnected hydrophilic polymers capable of absorbing large amounts of water [9], commonly with a three-dimensional structure [10]. These materials, which are ideal for microneedle manufacturing, can be synthesized using different crosslinking methods, with the main method being the use of relatively high-temperature heat treatment steps for prolonged periods. The synthesized hydrogel-crosslinked microneedles can perform minimally invasive sampling and detect macromolecules such as glucose and vancomycin in the skin efficiently and quantitatively [11]. The introduction of hydrogel-crosslinked swollen microneedles for wrinkle removal is a huge breakthrough. The drug can be used as an ion channel for iontophoresis after being delivered to the dermis, and some biological macromolecules can be sent into the body to achieve transdermal penetration, which is the effect of drug administration [12]. In electronic applications, hydrogel-crosslinked microneedles can be electrochemically constructed due to their good ion transportability and can be used to make biosensors [13] for macromolecular detection [14, 15]. The corresponding external circuit is built for the biosensor and mounted on the microcontroller for embedded development. The in situ blood glucose and uric acid monitoring microneedle bracelet and the accurate real-time detection of human health are hoped to be achieved in the future [16, 17]. Meanwhile, the osmotic-driven hydrogel microneedles can extract microliters of blood glucose concentrations through skin interstitial fluid in a few minutes, significantly improving the detection efficiency [18]. The emergence of the microwave-assisted crosslinking method will be the key point for the mass production of hydrogel-crosslinked microneedles, providing an excellent drive for this process development. Therefore, it has a promising future in body fluid detection and as a drug carrier.
The formed hydrogel-crosslinked microneedles are easy to absorb moisture and swell due to their hydrophilicity. When the microneedle is inserted into the skin, the microneedle rapidly expands after absorbing the liquid in the interstitial space of the skin, thereby forming the channel we need for substance transport, which can deliver the drug attached to the microneedle or the drug reservoir into the human body, to achieve the purpose of painless and efficient drug delivery. In addition, this channel can also be used as a channel for biosensors to detect macromolecules, laying the foundation for the detection of body fluids.
Sodium hyaluronate can be crosslinked under alkaline conditions, connecting its linear or branched molecular chains to form a network-like polymer structure. A vacuum drying oven is commonly used for the microneedle crosslinking drying process. However, the vacuum drying oven’s efficiency is low, at 12 hours [19]. On the contrary, microwave drying combines low energy consumption with a simple synthetic procedure that allows limited side reactions [20], and it can be used to synthesize various polymers, including hydrogel preparations, via microwave radiation. The microwave dryer can adjust different microwave powers and work directly on water molecules through microwave irradiation. Water molecules change their original molecular structure after absorbing microwaves due to their large dielectric constant [21]. The electric field’s speed is polar motion, resulting in frequent collisions that generate a lot of friction heat, causing each part of the material to gain heat energy at the same time and heat up. Previous studies by Larraneta et al. demonstrated that the microwave method can promote the crosslinking of polymethyl vinyl ether [22]. In addition, microwave synthesis has a high reaction rate and yield in a relatively short period, making microwave-assisted a highly effective method for producing and experimenting with sodium hyaluronate-crosslinked microneedles in the future.
The BDDE used in this experiment is the HA filler crosslinking agent widely used in the market. Its crosslinking ability mainly depends on the reactivity of the epoxide groups existing at both ends of the molecule; its stability and biodegradability make it an industry standard. Furthermore, BDDE is much less toxic than other ether crosslinking chemistries such as divinyl sulfone and 2,7,8-diepoxyoctane, which has been experimentally confirmed.
In this study, the effects of various microwave power on the drying rate and other properties of the microneedles were investigated. After obtaining the optimum power from this, comparing the degree of crosslinking, mechanical strength, and iontophoresis with the finished product made in the vacuum drying oven, it is found that microwave drying has significant advantages. For example, the hydrogels prepared by microwave irradiation are not only more porous but also have more uniform and deeper pores, which can easily form a continuous and highly crosslinked porous structure. At the same time, the reaction time required for synthesizing hydrogels can be reduced from several hours to a few minutes, which significantly increases the yield of hydrogels and has a higher swelling rate [23–25],
2. Materials and Methods
2.1. Materials
Medical-level sodium hyaluronate dry powder (SH), 1,4-butanediol diglycidyl ether (BDDE), glucose, sodium dihydrogen phosphate (NaH2PO4), and disodium phosphate (Na2HPO4) are the chemical reagents that were purchased from Shanghai McLean Biochemical Technology Co., Ltd. The Hyal1 enzyme is provided by Sigma-Aldrich. Reference to various literature methods was made through multiple sets of comparative experiments to explore their synthesis method.
2.2. Methods
2.2.1. Preparation of Sodium Hyaluronate Solution
The sodium hyaluronate solution was synthesized by the following procedure: adding 1.4 g of sodium hyaluronate powder with a molecular weight of 1.62 × 106 Da, 0.5 g of sodium hyaluronate powder with a molecular weight of 0.44 × 106 Da, and 0.3 g of sodium hyaluronate powder with a molecular weight of 4 × 105 Da in 100 mL of deionized water and stirring it. The solution was completely dissolved by stirring with a magnetic stirrer. The above-dissolved solution was placed in a vacuum machine and evacuated until the internal pressure reached -0.1 MPa and repeated several times until there were no obvious bubbles in the solution. This solution was used to study the drying of sodium hyaluronate in microwaves.
2.2.2. Crosslinking Agent (BDDE) Proportioning Preliminary Experiment
To obtain the material ratio with the best crosslinking effect, the gradient ratio of BDDE and sodium hyaluronate powder was set from 1 : 2 to 1 : 20. The prepared solution was used to prepare microneedles by the method discussed in Section 2.2.3, and the swelling rate kinetic test was carried out, as shown in Figure S1. We found that the swelling ratio was the largest when the ratio of BDDE and sodium hyaluronate powder was 1 : 4.
2.2.3. Preparation of Crosslinked Sodium Hyaluronate Solution
The same operation discussed in Section 2.2.1, but add BDDE crosslinking agent with a ratio of 1 : 4 to sodium hyaluronate [23] before stirring and perform the subsequent steps discussed in Section 2.2.1 after the dropwise addition.
2.2.4. Microneedles Prepared by Traditional Oven Drying Method
This method has been authorized by Xiamen Weizhen Pharmaceutical Technology Co., Ltd., and we used this method to prepare crosslinked microneedles. Pour the sodium hyaluronate solution prepared in Section 2.2.1 into a silica gel mold of about 4 cm × 3 cm to prepare non-crosslinked microneedles. After injecting the solution, the silicone mold should be evacuated to ensure the integrity of the tip and the flatness of the microneedle array. The silicone mold containing the solution was left to stand for 10 minutes, placed in an oven at 70°C, and heated for 5 hours. The preparation method of crosslinked microneedles is similar to that of non-crosslinked one. The difference is that the solution prepared in Section 2.2.3 is used, heated in an oven at 70°C until completely dry, and the drying time is about 7 hours. This method dries well but takes a long time. Therefore, the crosslinked microneedles prepared under this condition were used as the control group for the subsequent experiments.
2.2.5. Microneedles Prepared by Only Microwave Drying
The relationship between DF and time under different power microwave drying can be obtained from the expression, and the slope of the curve indicates the effect of different drying methods on the drying degree of non-crosslinked microneedles. We will give the discussion results in Section 3.1.
2.2.6. Microwave-Assisted Time Test of Crosslinking Microneedles
The preliminary work for microwave preparation is similar to that in Section 2.2.4. Similarly, use BDDE mixed with sodium hyaluronate in a ratio of 1 : 4. Placing a piece of equal-sized microneedle array in a custom-made microwave drier can freely adjust and control the microwave heating power at a low level, which not only allows for rapid drying but also avoids severe degradation of the material. The optimal power for the reaction is 400 W (from the experiment in Section 2.2.5) which is determined by the drying rate at different powers. Then, combining microwave drying with traditional oven drying methods by applying different microwave times, comparing the three possible methods, oven-microwave (O-M), microwave-oven (M-O), and oven-microwave-oven (O-M-O), the optimal method for microwave-assisted preparation of crosslinked microneedles was obtained. Figure 1 shows the specific process of preparing microneedles by the microwave crosslinking method: first oven drying for 1 hour, then microwave drying for 30 minutes, and finally returning to oven drying for 30 minutes. It overcomes not only the shortcoming of a long drying time using an oven but also the shortcoming of poor flatness when only using microwave drying. We will demonstrate the feasibility of this approach in Figure 1.
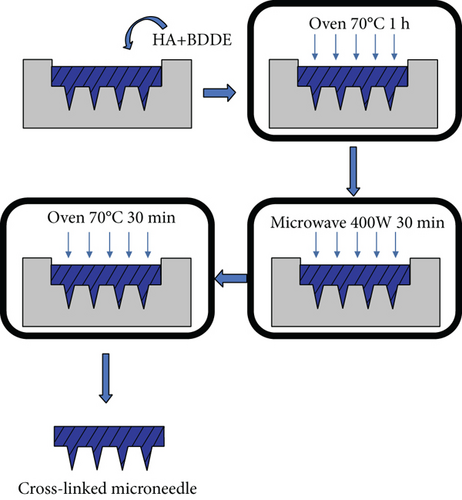
2.2.7. Synthesis of Hydrogel Gelation Kinetics
We use rheological properties to represent gelation kinetics. To study the rheological properties of hydrogels, we swelled the hydrogel microneedles prepared in Sections 2.2.4 and 2.2.5 and measured the loss modulus and storage modulus. The storage modulus is also called the elastic modulus, which reflects the elasticity of hydrogel. Loss modulus, also known as viscosity modulus, reflects the viscosity of hydrogel. The state of the prepared hydrogel was studied by comparing the storage modulus and the loss modulus. The samples were characterized by rheological measurements using a strain-controlled rheometer (MCR102) in parallel plate mode with an irradiation time of 25 min. The dynamic oscillation mode is used to measure the storage modulus and loss modulus. Repeat the experiment several times to obtain the average value. Meanwhile, the microneedle arrays and hydrogels prepared in the above experiments were scanned by SEM.
2.2.8. Mechanical Strength Test
The mechanical strength of the hydrogel microneedles was evaluated by the HLD vertical hand push-pull test bench. Place the hydrogel microneedles obtained by different drying methods, with or without a crosslinking agent, and with or without microwave-assisted drying on the test table, and turn the manual rocker to make the vertical distance between the probe and the tip of the hydrogel microneedle array. Make it as small as possible and the display number is 0 N, and at the same time clear the number of the digital display scale at this time. Then rotate the rocker at a constant speed, so that the probe continuously exerts downward pressure until the hydrogel microneedle breaks when the compression reaches a critical value, which is the tip braking force, which is the maximum peak value of the force-distance curve.
2.2.9. Infrared Measurements
Attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy was used to evaluate the crosslinking degree of BDDE and SH. The FTIR model iS50 (Thermo Fisher Scientific, USA) with a resolution of 0.09 cm−1 and a sample scan range of 400–4000 cm−1, averaging 64 scans, was used to obtain the spectra.
The hydroxyl groups of SH and BDDE undergo nucleophilic reactions to generate ether bonds [26] (Figure 2). The degree of crosslinking of sodium hyaluronate was determined using the peak area of the ether bond near 1150 cm−1.
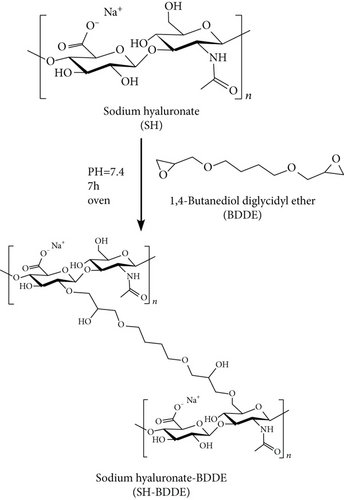
2.2.10. Swelling Kinetics
2.2.11. In Vitro Degradation Study
2.2.12. Study on Iontophoresis in Hydrogel-Crosslinked Diffusion Cells
The effect of crosslinked microneedle array iontophoresis using microwave (30 min at 400 W) and oven (70°C for 7 h) was investigated using iontophoresis in a horizontal Franz diffusion cell. The hydrogel microneedles can be used as ion channels for macromolecular transport using iontophoresis [27]. The crosslinked microneedles were preequilibrated in PBS at pH 7.4 for at least 15 min after piercing the agarose gel film. The agarose gel is prepared from 0.4 g agarose and 50 mL 0.5∗TBE dilution buffer to simulate human skin. Put 20 mL PBS and 20 mL of 1250 mmol/L glucose solution in the sampling and storage chambers of the diffusion cell, respectively. Use cycloacrylic adhesive to secure it to the reservoir chamber of the diffusion cell, with the tip side facing the sampling chamber. In the sampling and reservoir chambers, a 2 mm diameter platinum wire electrode is placed 2 mm from the skin. Figure 3 shows the placement device. A voltage of 30 V is applied to the electrodes, and the positive electrode is connected to the storage chamber, whereas the negative electrode is connected to the sampling chamber. The glucose concentration in the reservoir is the same for both experiments. The concentration is obtained by testing the glucose concentration of the sampling chamber at 20 min intervals. Then, the obtained results are curve-fitted.
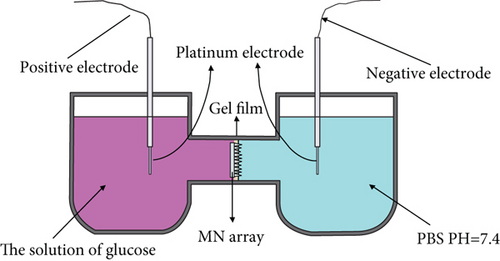
3. Results and Discussion
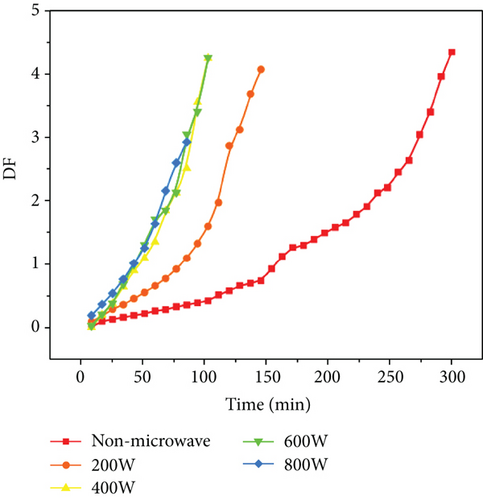
3.1. Comparison of Traditional Oven Drying and Microwave-Assisted Preparation of Non-Crosslinked Microneedles
First, we compare traditional oven drying and only microwave drying. The results are shown in Figure 4. We can find that the traditional oven drying takes 5 h to complete the drying process. However, the microwave drying time in the figure is completed within 2.5 h. Thus, the microwave drying rate is higher than the oven drying rate. The temperature of the upper surface of the microneedle array increased faster as microwave power increased, and the overflow rate of water molecules also increased, accelerating the evaporation of water, and subsequently decreasing the drying time. The curve shows that when the power is 200 W, the drying rate is much lower than other higher microwave powers. But higher power does not mean that the moisture can evaporate quickly, and the drying rate changes only slightly when the microwave power is between 400 and 800 W. Moreover, in the period of 50 to 100 min, we discovered that a higher microwave power may not result in a faster drying rate and that a suitable power is the best solution for rapid drying of microneedles. Therefore, we considered the effect and energy savings, and the optimum microwave drying power was determined to be 400 W.
The synthesized microneedles were subjected to a surface flatness test under the same observation conditions, and the results are shown in Figure S2. It is not difficult to discover that the surface flatness of microwave drying is poor. Further, as the power increases, the number of bubbles on the surface of the microneedle gradually increases, as does the size of the bubbles which does not satisfy the requirements of apparent flatness and beauty.
The reason for the analysis is that the working mechanism of microwave drying and oven drying is different. This difference causes water vapor to be trapped in the microneedle array during microwave drying, and then solidifies during crosslinking, resulting in a large number of air bubbles. The purpose of oven drying is to heat the air inside the box and pass hot air at a high flow rate into it, transferring heat from the inside to the outside via heat conduction and the acceleration of the surface air flow rate. This process will cause the surface of the microneedle array to cure first, but few bubbles will form because the evaporation rate of water is faster than the curing rate of the array surface. Microwaves can only affect polar molecules in the microwave drying system. In the sodium hyaluronate solution in this experiment, water is a polar molecule. Under the influence of microwaves, the water will be gradually heated and volatilized, resulting in a drying effect. It is exceedingly difficult to obtain an appropriate microwave power throughout the experiment, assuming that sufficient drying time is required, so that the diffusion and evaporation rate of water molecules inside the array is larger than or equal to the curing speed of the array surface. Therefore, during the experiment, we will discover that in pure microwave under dry conditions, a surprising number of solid-state bubbles will form on the surface due to the rapid evaporation of water. The polar molecules will be affected by the alternating electromagnetic field when the dielectric material is in an alternating electromagnetic field [20, 28],. Because polar molecules’ charge distribution is nonuniform and asymmetric, their positive and negative charge centers do not overlap, rotate under the influence of the electromagnetic field, and collide with molecules in random thermal motion within the material, converting microwave energy into heat energy that can be used to heat the object.
Under traditional oven drying conditions, heating drying takes 5 hours, while microwave drying takes only 1.5 hour to complete under 400 W. Therefore, we can know that by combining these two methods, the final heating time must be much less than 5 h. To speed up the testing progress, we reduced the total experimental time to 2 h through multiple sets of preexperiments. At the same time, we insert the microwave heating step into the front, middle, and back sections of the oven drying and repeat the experiment many times. The three groups are named oven-microwave (O-M), microwave-oven (M-O), and oven-microwave-oven (O-M-O). Four-time intervals of 25, 30, 35, and 40 min were set for each group of microwave heating sections for comparison. During the testing process, we found that the microneedle arrays in the O-M group were not completely dry at 20 min, showing a viscous colloidal state.
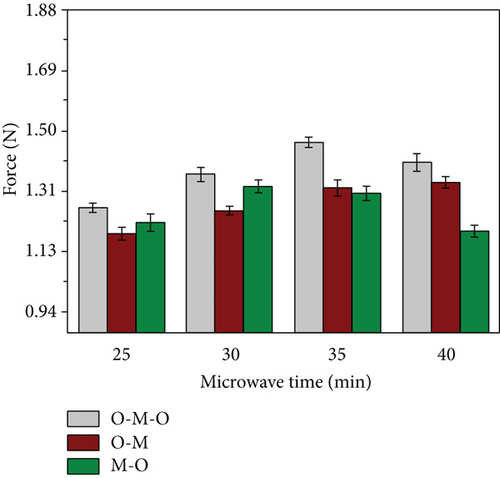
The formation of the microneedle array gradually stabilized as the microwave drying time increased. However, few air bubbles were still generated. In the M-O group, the solid bubbles generated by water evaporation gradually increased as the microwave time increases, and the original appearance cannot be restored by oven drying, and fabrication stability was extremely low. In the O-M-O group, we determined a two-stage heat drying for half an hour, and the finished products of the four groups of experiments are all outstanding, with the finished products with microwave times of 30 and 35 min being the best (Figure 5); they have a more superior stable generation interval than the other two groups. Afterward, the tip’s breaking force was investigated under various conditions (Figure 6). The O-M-O group was discovered to be superior to the other groups. Therefore, we can preliminarily determine the microwave-assisted drying method as oven-microwave-oven (O-M-O). This will be an important procedure for drying hydrogel-crosslinked microneedles in the subsequent experiments.
3.2. Infrared Test Results of Hydrogel-Crosslinked Microneedles
The degree of crosslinking of sodium hyaluronate directly affects the material properties, which is a key factor for iontophoresis in crosslinked hydrogels. Therefore, the degree of crosslinking was investigated using infrared spectroscopy. Since the hydroxyl group of SH and BDDE undergo nucleophilic reaction to generate an ether bond, the analysis of the degree of crosslinked hydrogel using infrared spectroscopy mainly depends on two groups: the hydroxyl (O-H) and ether bond (R–O–R′) [26, 29].
The hydroxyl stretching vibration produces an absorption peak at 3200-3650 cm-1 and the ether bond (fatty ether) produces an absorption peak at 1100-1300 cm-1. The two groups were quantified by comparing the intensity of the peaks under different crosslinking conditions. The absorption peak at approximately 3400 cm-1 in Figure 7(a) shows that the uncrosslinked O-H peak is sharper, while both methods of crosslinking result in a slightly shifted peak towards the lower wavenumbers and lower intensity broad peak. This is because the peaks are sharply shaped at higher wavenumbers when the O-H is in the free state, and the formation of hydrogen bonds causes the peaks to broaden and shift to lower wavenumbers. This indicates that the free O-H is reduced after crosslinking and that more hydrogen bonds are formed. About 1100 cm−1 can be seen in the uncrosslinked group and the oven-crosslinked group of ether bonding peak strength is not very different, but the microwave-crosslinking group involved in the peak strength is almost twice the former; the role of the oven showed a clear advantage.
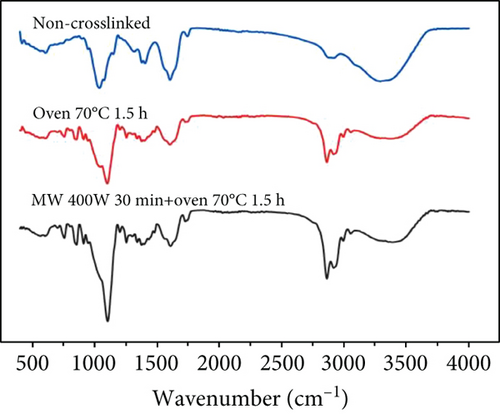
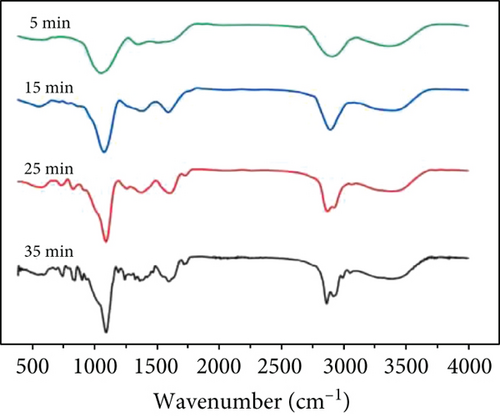
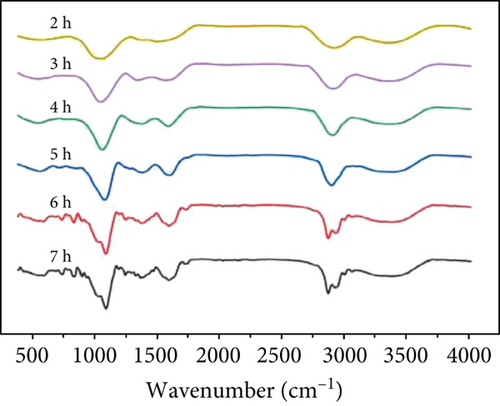
At the same time, microwave crosslinking achieved better results in a shorter period the conventional oven crosslinking, as can be observed in Figures 7(b) and 7(c).
3.3. Test Results of Swelling Kinetics of Hydrogel-Crosslinked Microneedles
The properties of hydrogel materials are closely related to the degree of crosslinking, particularly their swelling kinetics. Through this experiment, our synthesized hydrogel-crosslinked microneedles can achieve the maximum swelling rate within a minute. Figures 8(a) and 8(b) show that under the same drying time conditions, the hydrogel-crosslinked microneedles without microwave assistance (a) obtained by swelling the pattern part are mushy. This is because the crosslinked microneedle array is placed in the water and some of the arrays were dissolved during the swelling process due to insufficient crosslinking during the drying process, while the pattern obtained by the swelling of the microwave-assisted hydrogel microneedles (b) was relatively complete. This proves that microwave-assisted drying can promote crosslinking.


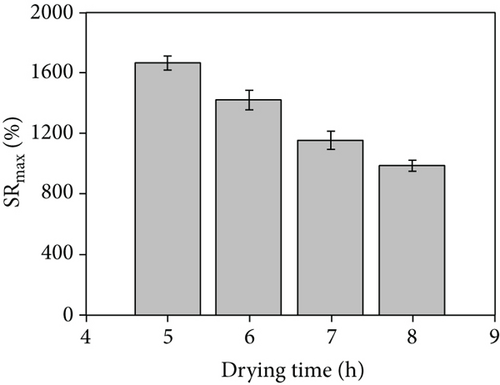
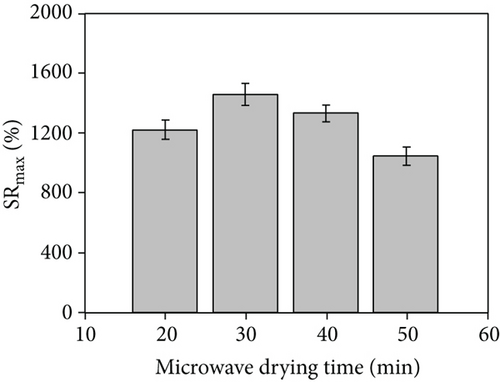
The drying time significantly influence the maximum swelling rate. As shown in Figure 8(c), under the traditional oven drying conditions, the drying time is longer, and with the increase in drying time, the maximum swelling rate of the hydrogel-crosslinked microneedles decreases; that is, the maximum water absorption rate decreases, which is due to excessive crosslinking, the crosslink density increases and the structure is tighter, thereby reducing the entry of water [22, 30]. Figure 8(d) shows the histogram obtained after microwave assistance. Although the maximum swelling rate is slightly reduced compared with the traditional method, the crosslinking time is significantly shortened, and the histogram shows an upward trend at 20 min. This is because the microwave-assisted crosslinking time is extremely short and the sodium hyaluronate solution absorbs insufficient energy, resulting in insufficient crosslinking of the hydrogel microneedles and partial dissolution during the swelling process, lowering the swelling rate.
Figures 9(a)–9(d) are the electron microscope images of the hydrogel-crosslinked microneedles prepared by microwave-assisted drying. It shows that the microneedles begin to deform after being subjected to pressure, and the greater the applied force, the more the microneedles bend until they break at the maximum bearing capacity.
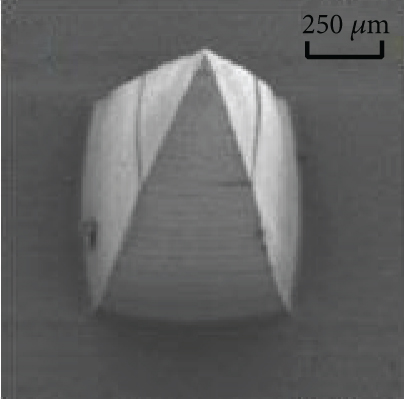
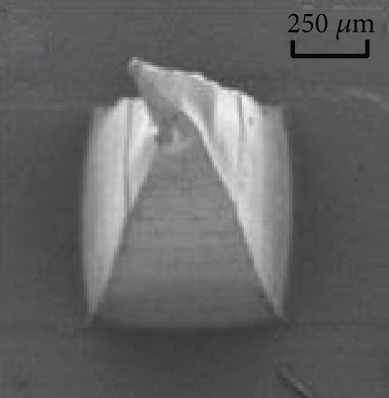
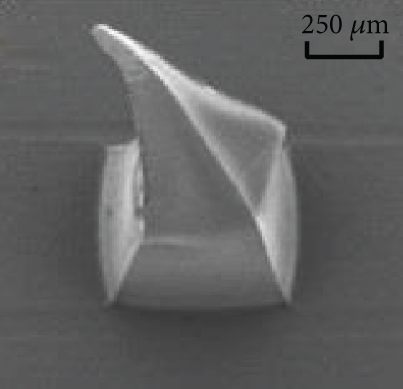
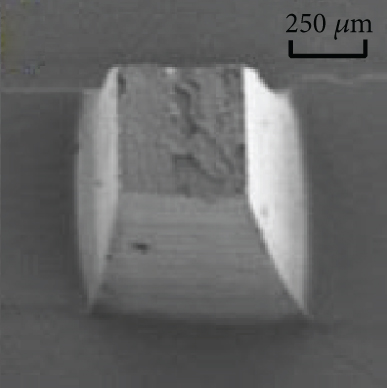
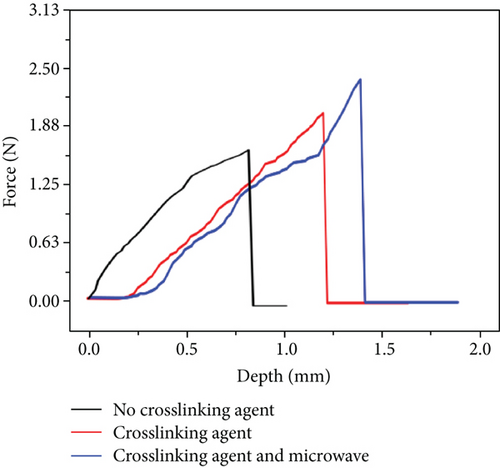
Crosslinking also affects the mechanical strength of the hydrogel microneedles. Figure 9(e) shows the curves between the depth and pressure under each condition, including no crosslinking agent, crosslinking agent, crosslinking agent, and microwave. There is a significant difference in the tip braking force between non-crosslinked and hydrogel-crosslinked microneedles, indicating that the use of crosslinking agents greatly improves the mechanical strength of hydrogel microneedles, which is due to the crosslinking reaction between sodium hyaluronate and crosslinker, which makes the surface structure more compact [31]. In addition, the mechanical strength of the hydrogel-crosslinked microneedles after microwave-assisted drying was improved again, which once again proved that microwave-assisted drying can promote crosslinking.
3.4. Results of Gelation Kinetic about Sodium Hyaluronate Hydrogel
The viscoelastic behavior of the hydrogel is shown in Figure 10. We define gel 1 refers to the hydrogel prepared by the microwave-assisted crosslinking method and gel 2 refers to the hydrogel prepared by the traditional oven drying method. With the increase in the rate, the storage modulus of the hydrogel increased. This indicates that the hydrogel has a relative network structure [32], which could be confirmed from the SEM image of sodium hyaluronate hydrogel (Figure S3), making the main chain less vulnerable to shear stress. At the same time, it can be found that the modulus change trend of the two groups of gel is the same, and the storage modulus and loss modulus of gel 1 are much higher than those of gel 2, so we can judge that properties of gel 1 will be better than those of gel 2. The storage modulus (Figure 10(a)) of all hydrogels was 1 × 102 − 2 × 103 Pa, slightly lower than the loss modulus of 10-250 Pa (Figure 10(b)), and the frequency range is 10−1–102 rad/s. The results show that with the increase of the angular frequency, the storage modulus changes little and the two curves of the loss modulus intersect. When the frequency is small, the viscosity of gel 1 is greater. When the frequency continues to rise, gel 2 exceeds gel 1, and there is no sign of damage in the measured angular frequency range. On the whole, the viscoelastic properties of sodium hyaluronate hydrogel in this range are very stable, and the difference between storage modulus and loss modulus is small, which conforms to the viscoelastic characteristics of the gel. In addition, the loss modulus of the whole material is larger, showing viscous behavior. The research of Zhang et al. shows that the properties of the viscous material are better for drug delivery [33]. This is more helpful for subsequent experiments.
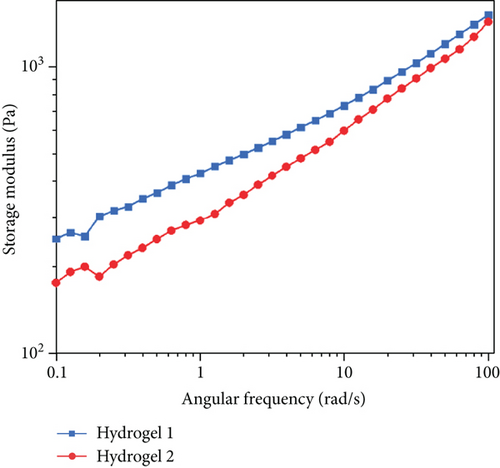
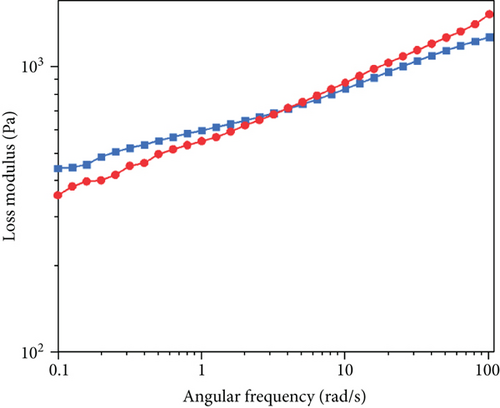
3.5. In Vitro Decomposition and Iontophoresis Test Results of Hydrogel-Crosslinked Microneedles
To evaluate the effects of pH and enzymes on the water-erosion susceptibility of the complexes, the changes in sample weight when microneedle arrays fabricated by two different methods (i.e., traditional oven drying and microwave-assisted methods) were compared in an aqueous environment. We refer to the research of Gamarra et al. that the degradation studies about hyaluronic acid with organ phosphonium compounds [34]. Each method is divided into three groups of experiments: the first two groups set the pH value at 5.5 and 7.4, meanwhile the third group is set to pH = 7.4, and the Hyal1 enzyme is added. As shown in Figure 11(a), the results clearly show that the two different methods lose only 21.6% and 19.7% of the initial weight during the entire degradation process at pH = 5.5, while at pH = 7.4, the traditional oven drying method and the microwave-assisted method lose weight decreased by 10.9% and 9.8%, respectively. In the absence of enzymes, the rate of decomposition is much slower at physiological pH than at acidic pH. At two pH values, the microneedle arrays prepared by the traditional oven drying method decomposed faster than the microwave-assisted method. This is very reasonable because, in the production process, the microwave-assisted method uses more energy for the chemical reaction per unit time, which can promote the crosslinking reaction as shown in Figure 3 to proceed in the positive direction, making the reaction more complete. Therefore, in the microneedles obtained by the traditional oven drying method, there is more SH that has not completed the crosslinking reaction, and this part is directly melted into the water after entering the water, which greatly reduces the quality. At the same time, we can also find that after the addition of the Hyal1 enzyme, the quality has dropped significantly, which shows that the effect of the enzyme on the degradation is very significant. It also proved that the new method has stronger enzyme resistance.


An iontophoresis test on glucose was conducted to determine the effect of the crosslinking method on osmosis. Figure 11(b) shows the permeation kinetics of these two types of microneedle arrays on thin films. The fitting curves of glucose permeation of the two microneedles showed the same trend. In addition, the results of microwave crosslinked microneedle arrays after 120 min were greater than those of conventional heat-baked microneedle arrays. In both cases, the amount of glucose permeated after 120 min was approximately 78% of the amount of glucose in the reservoir (78%–75% for MW-prepared MN and 70%–63% for conventional MN). This result is expected because as the swelling rate increases, more glucose is absorbed into the hydrogel, resulting in more glucose diffusing out per unit time during diffusion. Concurrently, the electroosmotic flow of the solvent and the electrophoresis of the charged molecule are involved in iontophoretic transport [27], which causes the glucose to increase its permeation speed into the sampling chamber under the influence of the electric field. In the MW group with a better swelling rate, more glucose is transferred through iontophoresis per unit time. This conclusion can also be found in Figure 11(b). It can be seen in Figure 11(b) that the glucose permeation slope of microwave-assisted crosslinking microneedles is higher than that of oven drying crosslinking, indicating that the ion introduction rate of microwave-assisted crosslinking microneedles array is higher than that of traditional oven drying crosslinking microneedles array. These two studies show that the sampling results of the two curves are equivalent. Murthy et al. showed that using an ETS technique, the skin can transport a significant amount of glucose under ion osmosis [35]. Microwave hydrogel-crosslinked microneedles can achieve the effect of other technologies on agarose gel film using iontophoresis, and it is expected that the penetration efficiency will improve when applied to the human skin in the future. Therefore, this technology is expected to be extended to transdermal iontophoresis in the future.
4. Conclusions
In this study, we demonstrate the feasibility of microwave-accelerated drying of hydrogels, as well as the advantages of the microwave-assisted crosslinking process over conventional thermal heating in synthesizing hydrogel microneedle arrays. The crosslinking time was reduced from 7 to 2 h. Therefore, microwave irradiation significantly improves the microneedle fabrication efficiency, and microwave heat treatment has better mechanical strength, higher crosslinking efficiency, swelling rate, and iontophoresis rate than conventional oven heating. Therefore, the results of this study are helpful for the improvement of the microneedle crosslinking process in the future, and it can be considered to install a microwave generating device in the oven to produce new processing equipment.
Disclosure
Dongyu Chen and Yu Zhang should be considered as co-first authors.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
Dongyu Chen and Yu Zhang contributed equally to this work.
Acknowledgments
This work was financially supported by the Fundamental Research Funds for the National Natural Science Foundation of China (61801411) and the Science and Technology Program of Fujian Province (2021Y01010295).
Open Research
Data Availability
The data used to support the findings of this study are included within the article and supplementary materials.




