CO2 Electroreduction in Organic Aprotic Solvents: A Mini Review
Abstract
An annual increase of CO2 concentrations in the atmosphere causes global environmental problems, addressed by systematic research to develop effective technologies for capturing and utilizing carbon dioxide. Electrochemical catalytic reduction is one of the effective directions of CO2 conversion into valuable chemicals and fuels. The electrochemical conversion of CO2 at catalytically active electrodes in aqueous solutions is the most studied. However, the problems of low selectivity for target products and hydrogen evolution are unresolved. Literature sources on CO2 reduction at catalytically active cathodes in nonaqueous mediums, particularly in organic aprotic solvents, are analyzed in this article. Two directions of cathodic reduction of CO2 are considered—nonaqueous organic aprotic solvents and organic aprotic solvents containing water. The current interpretation of the cathodic conversion mechanism of carbon (IV) oxide into CO and organic products and the main factors influencing the rate of CO2 reduction, Faradaic efficiency of conversion products, and the ratio of direct cathodic reduction of CO2 are given. The influence of the nature of organic aprotic solvent is analyzed, including the topography of the catalytically active cathode, values of cathode potential, and temperature. Emphasis is placed on the role of water impurities in reducing CO2 electroreduction overpotentials and the formation of new CO2 conversion products, including formate and H2.
1. Introduction
Since 1958, when systematic direct measurements of CO2 in the atmosphere began, there has been a steady annual increase in its concentration. In 1958, its content was ∼315 ppm and in 2021, ∼415 ppm [1]. The tendency to further increase CO2 concentration in the atmosphere causes such negative planetary consequences as the greenhouse effect and increasing acidity of ocean and sea waters. To alleviate such global problems, research has been intensively conducted in the last decade to develop effective technologies for capturing and disposing of atmospheric CO2. The most efficient direction is converting this gas into valuable chemicals and fuels. The best known and most studied are the following four methods (Figure 1): thermocatalytic [2, 3], photocatalytic [4–6], enzymatic [7, 8], and electrochemical catalytic reduction [9–14].
Electrochemical reduction of CO2 is marked by vast possibilities in terms of the range of conversion products, including CO, CH4, C2H4, CH3OH, CH3COOH, HCOOH, and (COOH)2. This fact and the increased use of renewable energy sources give grounds to consider the up-and-coming electrochemical methods, particularly in “green” technologies of valuable substances (Figure 2).
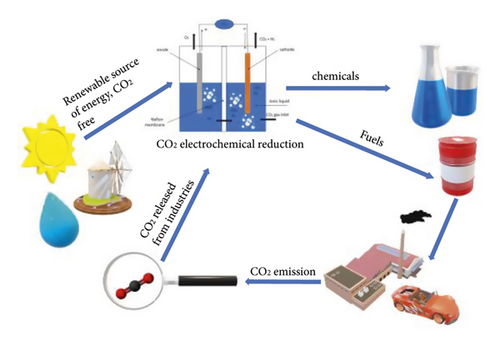
Electrochemical CO2 conversion at catalytically active cathodes is investigated in aqueous and nonaqueous solutions. They significantly differ in the limiting electrode potentials and the values of the cathode current densities, the course of electrochemical processes and reduction products, and the selectivity of the latter (Figure 3). Nonaqueous include ionic liquids, methanol solutions, organic aprotic solvents, and salt melts.
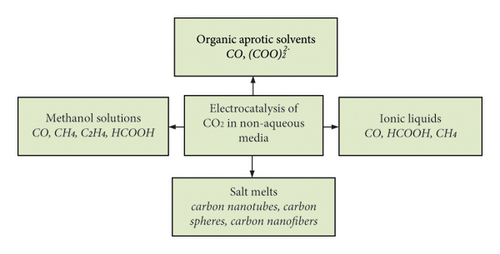
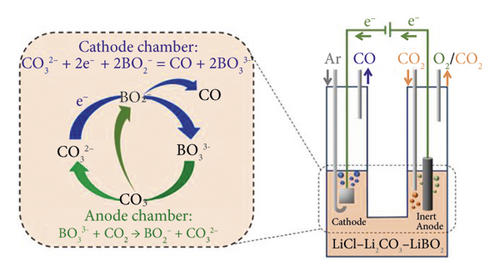
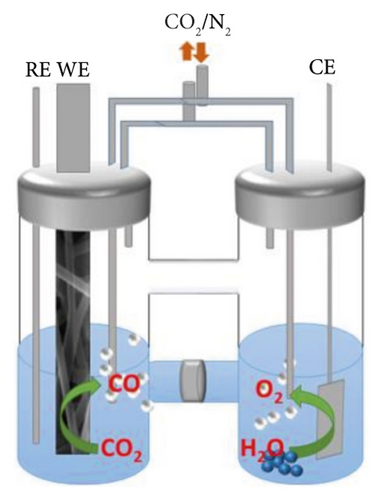
Electrochemical reduction of CO2 in nonaqueous media or solutions with low water content makes it possible to eliminate or reduce these negative factors of aqueous solutions. In addition, CO2 has a high solubility in organic solutions. Among them, the most studied are ionic liquids [19–26], methanol solutions [27–29], and organic aprotic solvents [30–40]. In recent years, there has also been engagement in the CO2 electrochemical reduction process in molten salts [41–46]. The following valuable nanomaterials can be obtained in such an environment: carbon nanofibers [43], carbon nanotubes (CNTs), carbon spheres (CSs), and honeycomb carbon [44]. In addition, the simplicity of the design of the diaphragm-free electrolyzer (Figure 4), high conversion speed (icathode = 100 mA × cm−2), and high Faradaic efficiency (80–90%) of products [42] make this method promising. However, high-cost ionic liquids and decomposition at a very negative potential of CO2 [37] limit their practical application. Although CO2 conversion in salt melts is unique in carbon nanomaterials synthesis, high temperature (≥500°С) is an energy-holding factor.
The relatively low cost of organic aprotic solvents, wide “electrochemical windows,” and high solubility make them a promising medium in the electrochemical conversion of CO2. Such a medium is also effective for studying the catalytic activity of cathodes in a wide range of electrode potentials. The purpose of this review is to summarize the scientific literature on the mechanism of cathodic conversion of carbon (IV) oxide into CO and organic products, the impact of water impurities, cathode material and topography of its surface, and the main factors of electrolysis (electrode potential, electrolyte composition, and temperature), list of products, and their Faradaic efficiency. This paper also aims to focus on the problematic issues of electrochemical conversion of CO2 in organic aprotic solvents and ways to solve them.
2. Features of Electrochemical Recovery of CO2 in Organic Aprotic Solvents
Specifics of CO2 conversion at catalytically active electrodes in organic aprotic solvents are primarily due to (1) deficiency or absence of protons in nonaqueous solution and (2) high solubility of carbon (IV) oxide. These factors cause new electrochemical and chemical processes, changes in the spectrum of CO2 conversion products, and even the formation of new compounds and significant acceleration of cathode processes.
2.1. Cathodic Reduction of CO2 in Nonaqueous Organic Aprotic Solvents
The proportion of reactions (13)–(15), their rate, i.e., the value of icathode, Faradaic efficiency of conversion products, and, accordingly, the ratio of products of direct cathodic reduction of CO2, i.e., oxalate and CO, depend on many factors. Among them, the main ones are as follows: the nature of the organic aprotic solvent; nature and topography of the catalytically active cathode; the value of the cathode potential; temperature.
2.1.1. Influence of the Nature of the Organic Aprotic Solvent
AN, DMF, DMSO, and PC (Table 1) media are most often used for cathodic CO2 reduction, which, in addition to high electrochemical resistance, are characterized by high values of Donor Number and CO2 solubility [27]. DN (kJ·mol-1): AN—59.0; DMF—111.4; DMSO—124.8; PC—63.2. CO2 Solubility (mmol·L-1) is an order of magnitude greater than the value of this value in water: AN—314 ± 6; DMF—194 ± 14; DMSO—131 ± 7; PC—134 ± 9; Н2О—34.5 ± 4.46.
| Electrode | Electrolyte | E, V | i cathode, mA⋅cm−2 | CO2 conversion products | Faradaic efficiency, % | References |
|---|---|---|---|---|---|---|
| Сu on basal Pt(hkl) single crystal faces | TBAPF6 in AN or PC | −1.8 |
|
CO | − | [31] |
| Au foil | TBAP in PC | −2.8 | 16.0 | CO | 84.9 | [32] |
|
|
|
|
Zinc oxalate |
|
[33] |
|
|
−3.0 | 2.8 | CO | 91.8 | [49] |
| Bulk gallium | TBACl, TBABr, TBAI and TBAP in DMSO, DMF, NMP, PC and AN |
|
|
CO |
|
[50] |
| Cathode: stainless steel | TBAP in AN |
|
2.7…19.6 | Zinc oxalate | 62.4 | [51] |
| Anode: sacrificial zinc | 5.0…15.0 | 73.9 | [52] | |||
|
TBAP in AN |
|
4.0…12.0 | Zinc oxalate | 96.8 | [53] |
| Cathode: MoO2 microparticles on Pb supporting substrate | TBAPF6 in AN | −2.4 | 25.0 | CO oxalic acid |
|
[54] |
Molecules of the aprotic solvent, due to the indivisible electron pair, act as a Lewis base, causing the donor-acceptor interaction with the carbon atom of the low-polar molecule CO2 L:⟶CO2 and the cathode surface L: ⟶cathode surface.
(1) Influence of Donor Number. The donor-acceptor interaction L: ⟶CO2 causes inhibition of electron transfer, which affects the ratio of oxalate and CO as products of cathodic reduction of CO2; namely, with a decreasing electron-donor capability, increased oxalate formation is observed [27]. Therefore, AN, which has the lowest DN value, is often used as a medium for oxalate obtaining with a high value of Faradaic efficiency—∼80% [33], ∼62% [51], ∼74% [52], and ∼97% [53]. However, this dependence [oxalate]: [CO] on DN of aprotic solvent should be considered as a trend because the course of electrochemical reactions (13) and (15) and, accordingly, the value of FE are influenced by the values of cathode potential, cathode nature, and temperature. The electron-donor capability of the aprotic solvent also determines the adsorption of organic molecules on the cathode surface [55–57]. Reactions (13) and (15) take place on the latter, the course of which depends on the adsorption-desorption of CO2 and CO molecules, CO2 radicals, and CO32− anions. Therefore, due to the predominant content of molecules of an aprotic solvent, the value of their DN should affect the ratio [oxalate]: [CO].
(2) Influence of CO2 Solubility. Solubility of CO2 is one of the factors of mass transport, concentration cathodic polarization, and, accordingly, the reaction rates (13) and (15). It is the highest CO2 solubility in AN that mainly determines the highest values of cathode currents among organic aprotic solvents, for example, at Ga cathode, E = −3.0 V in AN icathode = 2.3 mA·cm −2 (CO2 solubility ∼314 mmol·L−1), in DMF—0.7 (∼111.4), and DMSO—1.4 (∼194) [50]. A similar pattern is observed at other catalytically active cathodes. Thus, E = −3.0 V on a boron-doped diamond working electrode in AN, DMF, and PC icathode = ∼5.5, ∼2.3, and ∼1.2 mA⋅cm −2, respectively [58]; on Au cathode—∼24, ∼15, and ∼7 [59].
2.1.2. Nature and Topography of Catalytically Active Cathode
Electrochemical reduction of CO2 involves the adsorption of carbon (IV) oxide, solvent molecules, ions, intermediates, and end products on the cathode surface. The nature of the latter significantly affects the equilibrium processes of adsorption↔desorption due to inappropriate adsorption energies of key reaction intermediates [60]. The result is the dependence of the rate of certain electrochemical reactions and, accordingly, the product selectivities and Faradaic efficiencies of the CO2 conversion process on the catalytic activity of the cathode surface. Therefore, systematic studies of cathode production ⟶ surface structure ⟶ catalytic activity ⟶ product selectivities and Faradaic efficiencies are a priority in the direction of electrochemical conversion of carbon (IV) oxide.
The influence of the nature of the cathode surface, namely, its electrocatalytic action on the rate of carbon dioxide reduction, selectivity of product selectivities, and Faradaic efficiencies, is most studied for aqueous solutions [9, 11, 14, 16, 27, 61], which allowed to form scientific principles of CO2 conversion. The peculiarity of the environment of anhydrous organic aprotic solvents determines the course of fundamentally different electrode processes and, accordingly, reduction products. Thus, electron-donor molecules of organic solvent (L) are adsorbed on the electrode surface in parallel with CO2 molecules (Figure 5). However, due to the high electrochemical stability of organic molecules, their destruction does not occur even at E = −3.0 V, while in aqueous solutions, there are reactions (11) and (12). This significantly affects product selectivities and Faradaic efficiencies. Adsorption of R4N+ cations is also possible on the cathode surface, as conductive additives, such as R4NClO4, R4NPF6, and R4NCl (Table 1), are present in aprotic solvents, which are also characterized by high electrochemical resistance [58]. Thus, the adsorption of organic solvent molecules and tetraalkylammonium cations causes only cathodic polarization. Accordingly, the nature of the cathode surface affects the adsorption energies L, R4N+, and CO2 (Figure 5), as well as intermediate (·CO2) and final (CO) products.
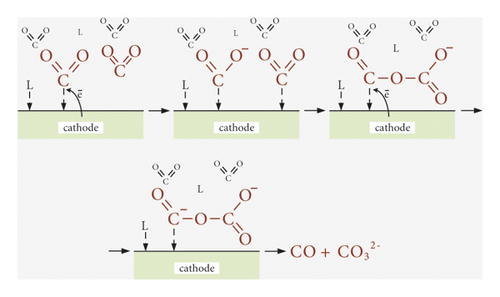
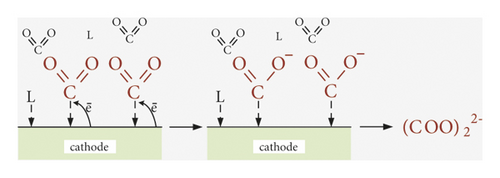
Depending on the nature of the cathode surface in organic aprotic solvents, the reduction of CO2 with the formation of oxalate or CO is possible. Thus, according to scheme (Figure 5(a)) on copper [31], gold [32, 49], and gallium electrodes [50], CO is formed, according to scheme (Figure 5(b)) on stainless steel [51, 52] and lead [33, 53] electrodes—oxalate with high values of Faradaic efficiency (Table 1). On the electrode based on MoO2 [31], the parallel reduction is possible according to schemes (a) and (b) with the formation of two products. However, the amount of literature is still little to discuss the system dependencies of CO2 conversion products on the nature of the cathode surface.
The use of a lead gas diffusion electrode [33] makes it possible to achieve values of cathode current densities much higher than on those using a lead plate [53] due to the large specific surface area. However, the product of CO2 conversion is not affected by the topography of the cathode. Even higher efficiency is observed using Cu nanoparticles embedded in N-doped carbon (Cu@NC) arrays (Figure 6).
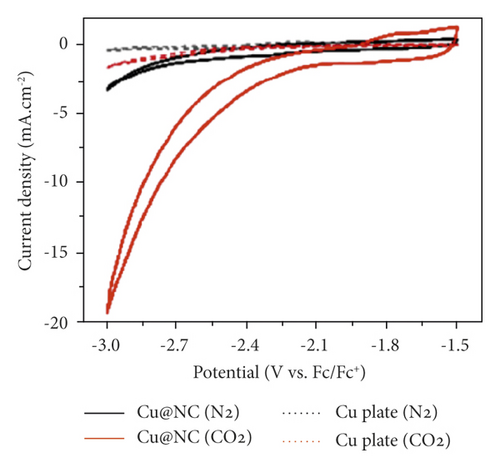
In nonaqueous media, the effect of nanostructured cathodes on the rate of CO2 reduction and selectivity of the obtained products is little studied. However, positive results of the use of nanometals in aqueous solutions [63, 64] can also be expected in the environment of organic aprotic solvents. The highest efficiency is manifested in the size of metal nanoparticles of several nanometers and especially nanoclusters [65, 66], the synthesis of which is characterized by simplicity in the technological aspect [67].
2.1.3. Cathodic Potential Values and Temperature
The formation of the ⋅CO2(−) radical by the cathodic reaction (13) takes place in the environment of organic aprotic solvents at low values of electrode potentials. Thus, in DMF, E0CO2/⋅CO2 = −2.21 V according to [68] and −1.97 V according to [58]. Since, in such a nonaqueous medium, ⋅CO2(−) is the starting electrochemical stage of CO2 reduction (Figure 5), the quantitative formation of conversion products begins at approximately −2.0 V [32, 33, 58–60, 69] on electrodes of different nature. A typical Е−icathode dependence is shown in Figure 6. The value of Еcathode is limited by the electrochemical stability of organic aprotic solvents, and in AN, DMF, and PC solutions, it takes values not lower than −3.5…−4.0 V.
The environment of some organic aprotic solvents makes it possible to carry out the electrochemical reduction of CO2 in a wide temperature range due to the low values of their melting point and high boiling point. Thus, for AN, DMF, and PC, these values are −46 and 82°C, −61 and 155°C, and −49 and 242°C, respectively. The authors [48] showed that on the inert (Mercury) cathode in the DMF medium, there is an increase in CO yield and, accordingly, a decrease in oxalate yield with decreasing temperature. Thus, at a CO2 concentration of 152 mmol·dm −3, the distribution of products is as follows: oxalate, 67%; CO, 25% and oxalate, 11%; CO, 89% at temperatures of 25 and −20°C, respectively.
2.2. Cathodic Reduction of CO2 in Organic Aprotic Solvents Containing Water
| Electrode | Electrolyte | E, V | icathode, mA⋅cm−2 | CO2 conversion products (Faradaic efficiency, %) | References |
|---|---|---|---|---|---|
|
|
−2.4 |
|
CO | [34] |
|
0.1 M TBAP in dry AN with <10 ppm H2O in humid AN | −1.2 (CV) |
|
|
[35] |
|
|
−1.8 |
|
|
[36] |
|
0.1М TBAPF6 in DMF + 0.5 vol% H2O | −2.5 (CV) | 20.0 (CV) | CO (90%) | [37] |
|
|
−2.3 | 6.72 | CO (83%), H2 (15%) | [38] |
| Cathode: MoO2 microparticles on Pb supporting substrate |
|
|
25.0 (const) | CO formate | [54] |
|
|
−3.0 |
|
CO (84.3%), H2 (15%) | [59] |
| Cu@NC (СuNPs, ∅ 4 nm) | 0.1 M TBAPF6 in DMF + 0.5% H2O |
|
|
Formate (64%), CO (20%), H2 (13%) | [62] |
|
|
−1.56 | 0.5 | CO (65%), formate (27%) | [70] |
| Ag2S, deposited on Ag electrode (two-compartment electrolysis cell) | TBAP in PC + ≤ 6.8 wt% H2O | −2.35 | 9.85 | CO (92%) | [71] |
| Pt plate | 0.1 M TEAP in AN + 4 mM H2O | −3.2 (CV) | 5 (const) |
|
[72] |
| Au microelectrode |
|
−1.8 |
|
CO | [73] |
With the water increase in the solution composition, cathode currents increase significantly [34, 36, 38, 59, 60, 73]. Thus, for E = −2.5 V in anhydrous acetonitrile solution, icathode = 0.4 mA·cm −2, while for the water content of 0.01, 0.25, and 2 mol·L−1, it is 0.75, 0.95, and 1.3 mol·L−1, respectively [34]. This is also identical to the decrease in cathode potentials by icathode = const (Figure 7). Low water concentration in the solution composition practically does not reduce the high solubility of CO2 provided by organic aprotic solvents. The low content of H2O also does not significantly affect the course of parallel cathodic reactions (11) and (12) with the electrochemical release of H2. This is due to these organic solvents high electron-donor properties, which promote the formation of stable associates (L:⋅⋅⋅Н−О−Н) or (L:⋅⋅⋅Н+), weakening the electrochemical activity of water molecules. The water in the solution composition will be limited to 6.8 vol% because, at higher concentrations, the electroconductive organic component of the nonaqueous solution begins to precipitate [59]. In addition, high water concentrations increase the proportion of reactions (11) and (12).
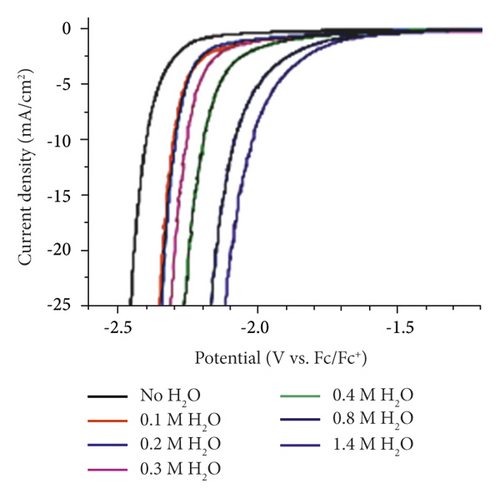
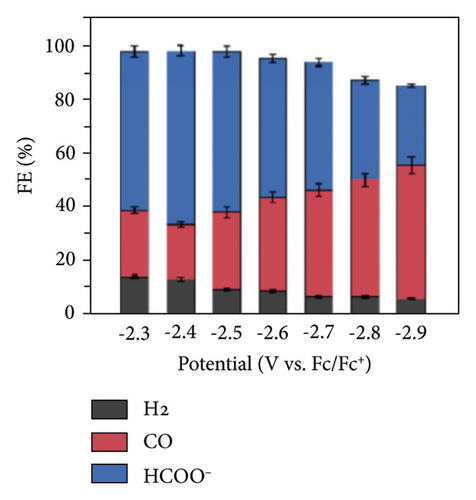
The water content in the aprotic solvent as a source of protons is an important factor influencing the course of reactions (11, 12, 16, 17) and, accordingly, the distribution of the products of cathodic reduction of CO2 (Figure 8(a)). Moreover, it is natural to increase the share of H2 with increasing concentrations of H2O in the solution. Therefore, it is limited to 1-2%. An important factor influencing the distribution of CO2 conversion products is the value of the cathode potential (Figure 8(b)).

The mechanism of the effect of water in aprotic solvent − H2O solutions on CO2 reduction products is limited to cathodic reactions involving proton-donor components, in particular (16)–(18). However, as shown in [37], the water factor also depends on the nature of the catalytically active cathode. For E = −3.0 V in 0.1 M TBAP nonaqueous PC solution using Ag, Zn, and Au cathode icathode = ∼4, ∼8, and ∼9 mA × cm−2, respectively. In solutions containing 6.8 wt % H2O, icathode = ∼5, ∼14, and ∼15 mA × cm−2. Therefore, the effect of water on the growth of icathode is much higher on Zn and Au cathode than Ag, which is obviously due to the different interaction of water molecules with the electrode surface. This issue is practically not covered in the literature. Still, its importance for understanding the mechanism of CO2 reduction in aprotic solvent-H2O solutions is confirmed by recent work, which considers, for example, microkinetic models of interaction of solution components with the cathode surface depending on its nature [74] and influences the nature of the electrode for the distribution of products in anhydrous and water-containing aprotic solvents (Figure 10).
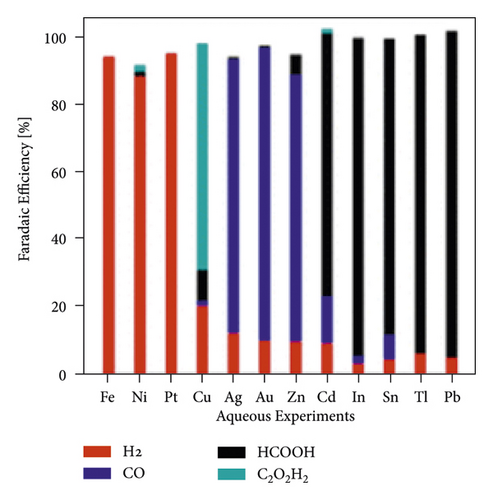
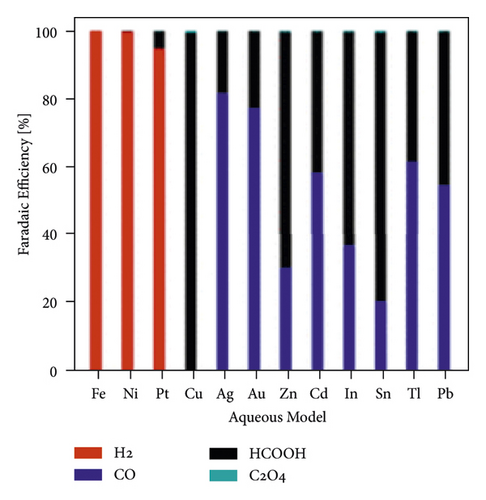
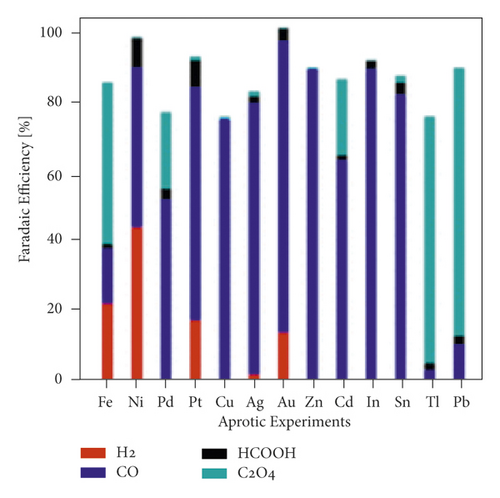
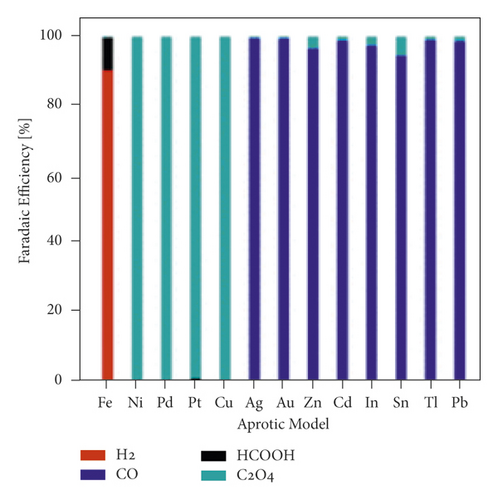
In [75], a systematic analysis of the nature influence of the metal cathode on the selectivity and Faradaic efficiencies of CO2 reduction reaction products in aqueous solutions and nonaqueous aprotic solvents is presented. However, due to the growing interest in aprotic solvent−H2O solutions, studies aimed at establishing the dependence of the distribution of CO2 conversion products on the nature of the cathode are relevant.
3. Conclusions
The capture and conversion of CO2 are one of the areas aiming to solve a complex environmental problem. The most studied methods of converting this gas into valuable chemicals and fuels are thermocatalytic, enzymatic, photocatalytic, and electrochemical catalytic reduction. The latter best meets the criteria of “green” technologies and cost-effectiveness and is characterized by various conversion products: CO, CH4, C2H4, CH3OH, CH3COOH, HCOOH, (COOH)2, etc. However, several unresolved problems of electrochemical reduction of CO2 in aqueous solutions, primarily low speed, low selectivity for target products, and Faradaic efficiency, constrain its widespread industrial application.
One of the ways to solve the problems caused by aqueous solutions is the electrochemical conversion of CO2 in a nonaqueous medium, mainly organic aprotic solvents. High electrochemical stability of the latter and high solubility of carbon (IV) oxide allows conducting electrochemical processes at cathode potentials −2.5… −3.5 V, providing a high conversion rate and selectivity. Two directions of cathodic reduction of CO2 are studied—nonaqueous organic aprotic solvents and organic aprotic solvents containing water.
The mechanism of CO2 reduction in nonaqueous aprotic solvents involves the formation of only two products—CO and oxalate. The values of the cathode current density, Faradaic efficiency of conversion products, and, accordingly, the ratio of direct CO2 reduction products depend on the following main factors: the nature of the organic aprotic solvent; nature and topography of the catalytically active cathode; values of cathode potential and temperature. The most effective among aprotic solvents is acetonitrile, which has the highest solubility of CO2 and provides the highest rate of oxalate and CO with Faradaic efficiency up to 90%. The nature of the cathode significantly affects the mechanism of CO2 reduction, which causes the formation of oxalate or CO: it produces mainly CO on copper, gold, and gallium electrodes; it produces oxalate with high Faradaic efficiency on stainless steel and lead electrodes. A highly developed and nanostructured cathode surface contributes to higher values of cathode current density but has virtually no effect on product selectivity. As the values of the cathode potentials increase, starting from −1.8…−2.0V, the cathode currents increase, which makes this parameter one of the main ones to ensure a high rate of CO2 conversion.
In the environment of organic aprotic solvents with low water content, the cathode overpotentials of CO2 electroreduction are significantly reduced, which makes it possible to carry out the process at high cathode currents. At the same time, the presence of water causes the formation of additional products—formate and H2. The water content in the aprotic solvent is a factor influencing the course of cathodic reactions and, accordingly, the distribution of CO2 reduction products. To date, the interdependence between the nature of the organic solvent, water content, the nature of the cathode, and the value of the cathode potential is little studied, which constrains the practical application of CO2 electroreduction in aprotic solvent-H2O.
Abbreviations
-
- AN:
-
- Acetonitrile
-
- DMF:
-
- N, N-Dimethylformamide
-
- DMSO:
-
- Dimethylsulfoxide
-
- PC:
-
- Propilencarbonate
-
- NMP:
-
- N-Methyl-2-pyrrodione
-
- IL:
-
- Ionic liquid
-
- L:
-
- Electron-donor molecules of organic aprotic solvent
-
- FE:
-
- Faradaic efficiency
-
- Е0:
-
- Standard electrode potential
-
- E:
-
- Electrode potential
-
- Еcathode:
-
- Cathode electrode potential
-
- icathode:
-
- Cathode current density
-
- MNCs:
-
- Metal nanoclusters
-
- MNPs:
-
- Metal nanoparticles
-
- NWs:
-
- Nanowires
-
- DN:
-
- Donor number
-
- FTO:
-
- Fluorine doped tin oxide
-
- TBAP:
-
- Tetrabutylammonium perchlorate
-
- TEAB:
-
- Tetraethylammonium tetrafluoroborate
-
- TBACl:
-
- Tetrabutylammonium chloride
-
- TBABr:
-
- Tetrabutylammonium bromide
-
- TBAI:
-
- Tetrabutylammonium iodide
-
- TBAPF6:
-
- Tetrabutylammonium hexafluorophosphate
-
- TEABF4:
-
- Tetraethylammonium tetrafluoroborate
-
- TEATfO:
-
- Tetraethylammonium trifluoromethanesulfonate.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
Acknowledgments
This work was carried out with the partial financial support of the National Research Foundation of Ukraine; Project registration no.: 2020.02/0309 (“Design of polyfunctional nanostructured mono- and bimetals with electrocatalytic and antimicrobial properties”).
Open Research
Data Availability
All related data used to support the findings of the study are mentioned within the article with references.