[Retracted] On Computation of Degree-Based Entropy of Planar Octahedron Networks
Abstract
Chemical graph theory is the combination of mathematical graph theory and chemistry. To analyze the biocompatibility of the compounds, topological indices are used in the research of QSAR/QSPR studies. The degree-based entropy is inspired by Shannon’s entropy. The connectivity pattern such as planar octahedron network is used to predict physiochemical activity. In this article, we present some degree-based entropies of planar octahedron network.
1. Introduction
All the graphs in this article are finite and undirected. A graph is set of points, where each pair of points (also known as vertex) are connected by an edge (also known as link or line). In network, vertices are called nodes, and in chemical graph, vertices are called atoms. In network, edges are called links or lines, while in chemical graph, they are called covalent bonds. The subbranch of chemical graph theory is topological indices. Many articles have been written on the topic of topological index. The representation of molecular graph by a drawing, a polynomial, a sequence of numbers, a matrix, or a derived number is called a topological index. As such, under graph isomorphism, these numeric numbers are unique. Most of the time, molecules and molecular compounds are nicely presented by molecular graph for better understanding.
Topological descriptors assume fundamental job in QSAR/QSPR studies in light of the fact that they convert a compound graph into a numerical number. We compare other physicochemical properties of carbon-based compounds (such as nanotubes, hydrocarbons, nanocones, and fullerenes). Due to these properties, topological descriptors have many applications in organic chemistry, biotechnology, and nanotechnology.
Cheminformatics is a branch of science that participates in mathematics, chemistry, and IT. In chemical graph theory, we consider molecular graph’s solution using the graph theory techniques which is the subdivision of mathematical chemistry. Molecules or atoms are represented by vertices in chemical graph theory, also the bonds between them by edges [1].
The definition of entropy was given by Shannon in 1948 [17]. In graph theory, the idea of graph entropy was given by Rashevsky in 1955 [18]. It has been used comprehensively to depict the design of graph-based systems in mathematical science [19]. The graph entropy is defined as the following:
Octahedron networks have its roots in physical world as natural crystals of diamond are octahedron; also, many metal ions have octahedron configuration. In physics, these networks can be used as circuits. The construction of planar octahedron network POH is based on silicate structure derived by Manuel and Rajasingh [20] and POH was derived by Simonraj and George [21] (for the complete construction of POH, see Figure 1, for triangular prism network TP, see Figure 2, and for hex planar octahedron network, see Figure 3; we refer the reader to read the article [22]).
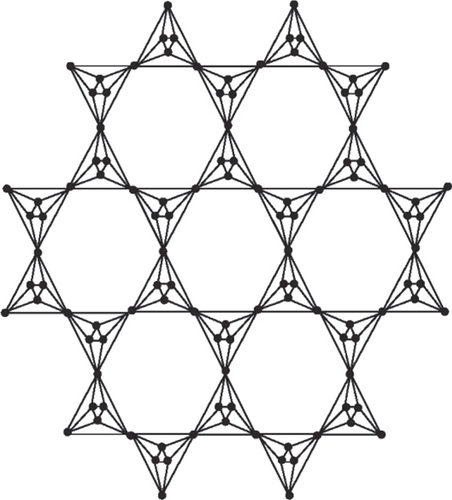
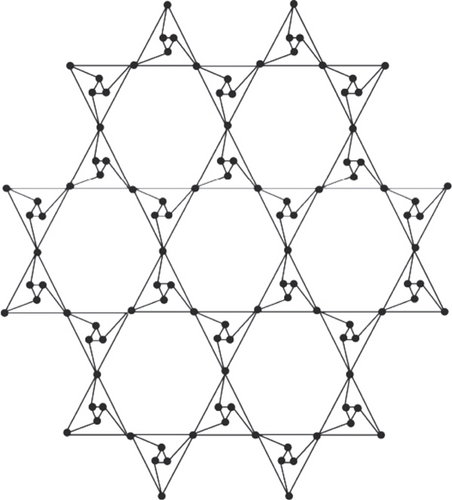
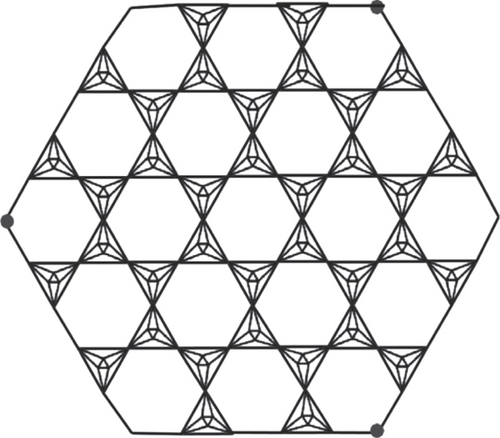
2. Main Results
Planar octahedron network and its derived forms are inorganic structures used in chemistry. Here, we research some degree-based entropies for these networks. These days, there is a broad examination movement on entropies (for further studies, see [23, 24]; for basic definitions and notations, we refer the reader to [25, 26]).
2.1. Results on Planar Octahedron Network
In this section, we will compute Randić, ABC, and GA entropies for planar octahedron network. The edge partition of POH(n) is written in Table 1.
| Number of edges | |
|---|---|
| (4, 4) | 18n2 + 12n |
| (4, 8) | 36n2 |
| (8, 8) | 18n2 − 12n |
2.1.1. Randić Entropy
where Rα for α = 1, −1, 1/2, −1/2 is written in (14), (15), (16), and (17), respectively.
2.1.2. ABC Entropy
where ABC index is written in (24).
2.1.3. GA Entropy
where GA index is written in (26).
2.2. Results on Triangular Prism Network
In this section, we will compute Randić, ABC, and GA entropies for triangular prism network. The edge partition of TP(n) is written in Table 2.
| Number of edges | |
|---|---|
| (3, 3) | 18n2 + 6n |
| (3, 6) | 18n2 + 6n |
| (6, 6) | 18n2 − 12n |
2.2.1. Randić Entropy
2.2.2. ABC Entropy
where ABC index is written in (37).
2.2.3. GA Entropy
where GA index is written in (39).
2.3. Results on Hex Planar Octahedron Network
In this section, we will compute Randić, ABC, and GA entropies for hex planar octahedron network. The edge partition of hex POH(n) is written in Table 3.
| Number of edges | |
|---|---|
| (4, 4) | 18n2 + 18n − 30 |
| (4, 8) | 36n2 − 48n + 12 |
| (8, 8) | 18n2 − 36n + 18 |
2.3.1. Randić Entropy
where Rα for α = 1, −1, 1/2, −1/2 is written in (42), (43), (44), and (45), respectively.
2.3.2. ABC Entropy
where ABC index is written in (51).
2.3.3. GA Entropy
where GA index is written in (53).
3. Discussion and Conclusion
In this article, we computed some degree-based topological indices of planar octahedron networks. After that, we used the definition of Shannon’s graph entropy to find some exact results of entropies for planar octahedron networks. For the variational change in the values of entropies for the degree-based indices, we construct some tables to enlist the numerical values for these networks. It is clear from Tables 4, 5, and 6 that the increase in the value of n causes a proportional increase or decrease in the values of entropies for Randić, ABC and GA indices. These formulae and their numerical values will help the researchers to predict physio- and biochemical activities of these networks. These numerical values of entropies can also predict the amount of energy that is unavailable for the work done in a chemical system. Furthermore, our future work will based on entropies of some other complex networks.
| n | ENTABC | ENTGA | ||||
|---|---|---|---|---|---|---|
| 6 | −2.525 × 10113 | −22.4506 | −6.546 × 105 | −1.2835 | 1.8516 | 2.3994 |
| 7 | −2.556 × 10113 | −22.4546 | −6.645 × 105 | −1.1628 | 1.9840 | 2.5333 |
| 8 | −2.578 × 10113 | −22.4431 | −6.717 × 105 | −1.0567 | 2.0988 | 2.6493 |
| 9 | −2.596 × 10113 | −22.4225 | −6.774 × 105 | −0.9622 | 2.2003 | 2.7516 |
| 10 | −2.611 × 10113 | −22.3968 | −6.819 × 105 | −0.8768 | 2.2911 | 2.8431 |
| 11 | −2.622 × 10113 | −22.3680 | −6.856 × 105 | −0.7992 | 2.3733 | 2.9258 |
| 12 | −2.632 × 10113 | −22.3376 | −6.886 × 105 | −0.7278 | 2.4484 | 3.0015 |
| 13 | −2.640 × 10113 | −22.3065 | −6.913 × 105 | −0.6619 | 2.5175 | 3.0709 |
| 14 | −2.647 × 10113 | −22.2751 | −6.935 × 105 | −0.6006 | 2.5815 | 3.1354 |
| 15 | −2.653 × 10113 | −22.2437 | −6.954 × 105 | −0.5434 | 2.6411 | 3.1953 |
| n | ENTABC | ENTGA | ||||
|---|---|---|---|---|---|---|
| 6 | −1.563 × 1054 | −10.4998 | −3228.67 | −0.1748 | 1.8579 | 2.2787 |
| 7 | −1.581 × 1054 | −10.4305 | −3275.76 | −0.0500 | 1.9905 | 2.4127 |
| 8 | −1.594 × 1054 | −10.3633 | −3310.88 | 0.0591 | 2.1055 | 2.5287 |
| 9 | −1.604 × 1054 | −10.2992 | −3338.08 | 0.1561 | 2.2070 | 2.6311 |
| 10 | −1.612 × 1054 | −10.2384 | −3359.77 | 0.2433 | 2.2979 | 2.7226 |
| 11 | −1.619 × 1054 | −10.1807 | −3377.46 | 0.3226 | 2.3802 | 2.8055 |
| 12 | −1.625 × 1054 | −10.1263 | −3392.17 | 0.3952 | 2.4554 | 2.8811 |
| 13 | −1.629 × 1054 | −10.0746 | −3404.58 | 0.4623 | 2.5245 | 2.9506 |
| 14 | −1.633 × 1054 | −10.0256 | −3415.21 | 0.5245 | 2.5886 | 3.0149 |
| 15 | −1.637 × 1054 | −9.9789 | −3424.39 | 0.5826 | 2.6482 | 3.0749 |
| n | ENTABC | ENTGA | ||||
|---|---|---|---|---|---|---|
| 6 | −2.425 × 10113 | −21.1812 | −617561.93 | −1.2328 | 1.7906 | 2.3284 |
| 7 | −2.472 × 10113 | −21.3481 | −632989.74 | −1.1176 | 1.9324 | 2.4732 |
| 8 | −2.507 × 10113 | −21.4624 | −644447.57 | −1.0161 | 2.0541 | 2.5972 |
| 9 | −2.533 × 10113 | −21.5419 | −653292.98 | −0.9252 | 2.1608 | 2.7056 |
| 10 | −2.555 × 10113 | −21.5978 | −660327.95 | −0.8430 | 2.2557 | 2.8019 |
| 11 | −2.572 × 10113 | −21.6368 | −666056.66 | −0.7679 | 2.3413 | 2.8886 |
| 12 | −2.586 × 10113 | −21.6636 | −670812.03 | −0.6988 | 2.4191 | 2.9675 |
| 13 | −2.597 × 10113 | −21.6814 | −674822.68 | −0.6348 | 2.4906 | 3.0397 |
| 14 | −2.608 × 10113 | −21.6922 | −678250.84 | −0.5752 | 2.5565 | 3.1064 |
| 15 | −2.617 × 10113 | −21.6978 | −681214.82 | −0.5194 | 2.6178 | 3.1683 |
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported in part by the Natural Science Fund of Education Department of Anhui Province under Grant KJ2020A0478.
Open Research
Data Availability
No data were used to support this study.




