Efficacy and Safety of Fire Needle Therapy for Flat Warts: Evidence from 29 Randomized Controlled Trials
Abstract
Flat warts are a common and recurrent skin disease that has no specific antiviral treatment. As an alternative or complementary therapy, fire needle therapy has been widely used in the treatment of flat warts. The objective of this study was to systematically evaluate the efficacy and safety of fire needle therapy for flat warts. Using the search terms “flat warts” and “fire needle,” we searched the PubMed, Embase, Cochrane, China National Knowledge Infrastructure, Wanfang Data Knowledge Service Platform, Chinese biomedical (SinoMed) database, and the China Science and Technology Journal databases for studies until March 12, 2020. Randomized controlled trials comparing fire needle therapies with conventional therapies were also included. We calculated the risk ratios (RR) and mean differences with a 95% confidence interval (CI). We analyzed 29 trials involving 2,666 patients. Results showed that the use of fire needle therapy alone may have a higher efficacy rate compared with that of an immunomodulator (RR = 1.11, 95% CI: 1.03 to 1.20, I2 = 0%, P = 0.006; RR = 1.19, 95% CI: 1.03 to 1.37, I2 = 70%, P = 0.02, respectively) or tretinoin (RR = 1.39, 95% CI: 1.25 to 1.55, I2 = 0%, P < 0.00001), with a lower risk of blisters (P = 0.03) or erythema (P = 0.04), but with a higher risk of pigmentation (P = 0.02). We also determined the efficacy rate of fire needle therapy in combination with traditional Chinese medicine (RR = 1.16, 95% CI: 1.10 to 1.23, I2 = 21%, P < 0.00001), immunomodulators (RR = 1.17, 95% CI: 1.07 to 1.28, I2 = 33%, P = 0.0005), imiquimod (RR = 1.21, 95% CI: 1.04 to 1.42, P = 0.02), or as multidrug therapies (RR = 1.15, 95% CI: 1.07 to 1.24, I2 = 0%, P = 0.0001) and found that the combination treatments could reduce recurrence rates (P < 0.00001) and provided a lower risk of desquamation (P = 0.006). In conclusion, fire needle therapy seems to be effective for flat warts, with a reduced incidence of adverse events, such as blisters, erythema, and desquamation, but may increase incidence of pigmentation.
1. Introduction
Flat warts, which appear as flat, light brown papules, are caused by human papillomavirus (HPV) infection, especially types 3/10 and 28 [1]. Epidemiological surveys have shown that the incidence of flat warts has reached 1.77% in recent years, accounting for 11.4% of facial skin diseases, mainly occurring in children and adolescents [2]. Although 65% to 78% of skin warts may resolve within 2 years, skin warts in adults rarely repair themselves and usually last 5 to 10 years [3, 4], affecting general appearance and mental health, and cause major psychosocial problems.
The stratum corneum of the flat wart lesions in the basal layer of the skin was observed to have epidermal thickening and hyperkeratinization [5]. Histopathological analysis showed that many vacuole-like clear cells were found in the upper and granular layers of the spinous layer [6]. The diagnosis of flat warts is usually based on clinical symptoms: apical papules, minimal scale, and a slight elevation of 2 to 4 mm in diameter of the papules [7].
The mechanisms linking HPV to flat warts have not yet been identified, but it is generally believed that flat warts are closely related to changes in the human immune system [8]. There is no antiviral treatment specific to HPV; however, cell-mediated immunity against viruses has been reported to have a significant effect on flat warts [9]. Current treatments for flat warts, including salicylic acid, cryotherapy, bleomycin, 5-fluorouracil, and lasers, destroy the wart body, correct abnormal proliferation, and differentiation, and stimulate the local or systemic immune response [10–15]. Although these therapies have been proven effective, treating the adverse effects of warts, including infection, blisters, or scars, requires the development of a formulation with similar therapeutic effects but fewer adverse events [8, 16].
In China, fire needle therapy, a type of acupuncture therapy, has been used to treat skin diseases. It is an external treatment method that uses a specific needle that is heated until it burns red and is quickly stabbed into diseased local lesions or acupuncture points. It could stimulate and dredge the meridians and accelerate the flow of Qi and blood thus dissipating nodules. On this basis, the fire needles therapy has been proven to treat nodular prurigo [17], moderate severe acne [18], vitiligo [19]and psoriasis [20, 21]. Recently, fire needle therapy has also been used to treat flat warts, and its possible therapeutic mechanism may be destroying the wart body, improving local circulation, and promoting the local immune response. Additionally, the 2014 British Dermatology Association guidelines recommended acupuncture as a treatment for flat warts on the hands or face [8], and the Chinese fire needle guidelines also recommend acupuncture for flat warts [22].
Nevertheless, there is still a lack of systematic reviews comparing the use of fire needle therapy combined with different medications for flat warts. Here, we conducted a systematic review of randomized controlled trials (RCTs) to evaluate the efficacy and safety of fire needle therapy for flat warts.
2. Materials and Methods
2.1. Materials and Methods for Fire Needle Therapy
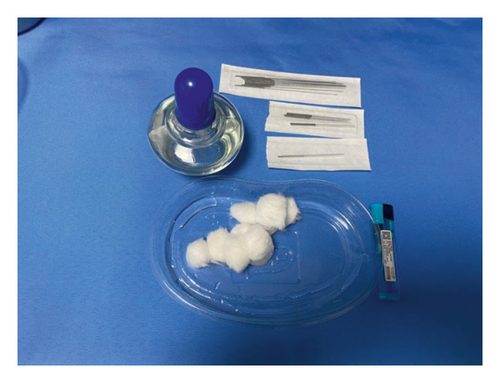
- (1)
An alcohol lamp and a disposable sterile fire needle were prepared (Figures 1(a) and 1(b)).
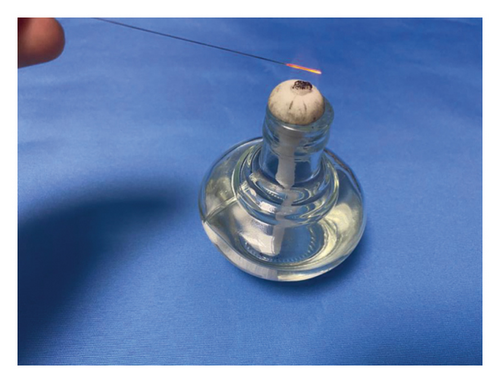
- (2)
The alcohol lamp was lit and was moved continuously from the needle root, along the needle body, to the needle tip (Figure 1(c)).
- (3)
The needle tip and the front of the needle body were heated over the outer flame, and the needle body was moved over the flame until it turned red (Figure 1(d)).
- (4)
The center of the wart body was quickly punctured vertically, and the needle was withdrawn. Small warts only needed to be pricked using one needle; large warts were punctured around the lesion with multiple needles, and the puncture depth did not exceed the base of the lesion.


2.2. Registration
This systematic review was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplementary file 1: Table S1) [23]. The PROSPERO registration number of this study is CRD42020185678.
2.3. Search Trials
Two reviewers (Le Kuai and Yue Luo) searched for relevant RCTs published in the following databases: PubMed, Embase, Cochrane, China National Knowledge Infrastructure (CNKI), Wanfang Medical Database, Chinese biomedical (SinoMed) database, and the China Science and Technology Journal Database (VIP), from their inception to March 12, 2020. Furthermore, the clinicaltrials.gov and the China Clinical Trial registry website were thoroughly searched to confirm the availability of relevant unpublished studies. Studies were restricted to the English and Chinese languages. The search strategy is listed in Supplementary File 2: Table S2. A total of 341 articles were retrieved.
2.4. Study Selection
We screened the titles, abstracts, and full texts of these 341 trials using the following inclusion criteria: participants, inclusion of patients diagnosed with flat warts, regardless of the age, sex, and disease duration; intervention, fire needle, or combined therapies as the intervention; comparison, control groups of conventional therapies; outcome, standardized therapeutic evaluation (efficacy rate) as the outcome; study design, RCTs. A total of 312 trials were excluded by the following exclusion criteria: case reports, reviews, animal studies, and studies containing fire needle therapy or combined therapies for a control group. The inclusion and exclusion of studies were formulated according to participants, intervention, comparison, outcome, and study design (PICOS) principle (Supplementary File 3: Table S3).
2.5. Data Extraction
Two investigators independently scrutinized the full texts of the selected studies. Two authors (Jia-le Chen and Yan-jiao Wang) completed the self-designed data extraction form (Table 1), including the general information (i.e., the first author, study design, and year of publication), participant characteristics (i.e., average age, sample size, and disease duration), diagnostic criteria, interventions, duration of treatments, primary or secondary outcomes, adverse events, and recurrence rates.
| Study | Average course duration of disease | Average age (years) | Treatment course | Sample size | Adverse events | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| E | C | E | C | E | C | E | C | |||
| Sheng 2019 | NR | NR | 23.5 (4.5) | 23.2 (4.4) | 4 w | 60 | 60 | NR | NR | |
| Zhu 2019 | NR | NR | 25.79 (2.52) | 25.81 (2.47) | 4 w | 48 | 48 | Itching (1), mild burning (1) | Infection (2), itching (2), mild burning (3) | |
| Yuan 2019 | 8.09 (3.11) m | 8.25 (3.04) m | 25.97 (6.78) | 25.36 (7.36) | 8 w | 39 | 39 | Itching (3), mild burning (5), erythema (2), pigmentation (2) | Itching (4), mild burning (4), erythema (3) | |
| Li 2019 | 9.0 (3.8) m | 8.8 (3.2) m | 30.0 (3.9) | 31.3 (3.6) | 30 d | 40 | 40 | Itching (2) | Itching (3) | |
| Li2 2019 | NR | NR | 26.8 (3.2); 26.4 (3.3) | 26.4 (3.1) | 4 w | 40; 40 | 40 | Scar (1); 0 | Scar (2), pigmentation (3) | |
| Huang 2019 | NR | NR | NR | NR | 10 w | 30; 30 | 30 | NR | NR | |
| Cui 2019 | 21.92 (6.65) m ; 22.75 (6.62) m | 22.47 (6.74) m | 26 (5.87); 24 (6.94) | 25 (6.05) | 6 w | 30; 30 | 30 | NR | NR | |
| Liang 2018 | 19.11 (6.23) m ; 19.43 (5.98) m | 18.76 (6.01) m | 26.59 (5.38); 25.55 (5.13) | 26.30 (4.88) | 4 w | 30; 30 | 30 | NR | NR | |
| Liao 2017 | 3.59 (1.91) y;3.35 (1.87) y | 3.41 (1.89) y | 25 (7); 26 (5) | 25 (8) | 4 w | 30; 30 | 30 | NR | NR | |
| Jia 2017 | NR | NR | NR | NR | 4 w | 50 | 50 | NR | NR | |
| Ruan 2017 | NR | NR | 20.5 | 20.3 | 4 w | 60 | 60 | Pigmentation (6) | 0 | |
| Jiang 2017 | 30.4 (6.45) m | 24.8 (6.24) | 8w | 60 | 55 | Pain (6) | 0 | |||
| He 2017 | 1.60 y | 1.53 y | 28.5 | 27.5 | 4 w | 60 | 58 | Pain (5) | 0 | |
| Fan 2016 | 1.60 y | 1.53 y | 28.5 | 27.5 | 4 w | 60 | 58 | NR | NR | |
| Wang 2016 | NR | NR | 20.8; 20.5 | 20.3 | 8 w | 38; 52 | 36 | NR | NR | |
| Huang 2016 | 1.8 (1.82) y | 26.8 (6.4) | 8 w | 49 | 46 | 0 | Mild burning (5), desquamation (5) | |||
| Guo 2016 | 1.8 (0.60) y | 1.70 (0.70) y | 23.86 (8.92) | 22.28 (9.35) | 30 d | 38 | 38 | NR | NR | |
| Liu 2016 | 6.9 m | 7.2 m | 18.3 | 19.7 | 4 w | 30 | 30 | NR | NR | |
| Shi 2015 | 30.03 (16.51) m | 29.48 (14.18) m | 26.46 (6.48) | 25.39 (6.27) | 30 d | 33 | 32 | Infection (2), mild burning (1), pigmentation (4), desquamation (2), isomorphic response (3) | Infection (2), mild burning (1), pigmentation (2), isomorphic response (3) | |
| He 2014 | 19.48 (12.95) m | 20.24 (13.14) m | 30.11 (6.47) | 29.87 (6.30) | 8 w | 46 | 46 | NR | NR | |
| Ma 2014 | NR | NR | NR | NR | 10 d | 38 | 30 | NR | NR | |
| Xu 2014 | 4.8 y | 4.5 y | 33 | 31 | 30 d | 30 | 30 | Pain (5) | Pain (3), blister (11) | |
| Jin 2013 | 32.89 (12.29) m | 31.89 (12.91) m | 27.06 (9.15) | 26.67 (7.89) | 4 w | 18 | 18 | NR | NR | |
| Pu 2011 | 18.2 m ; 19.6 m | 21.5 m | 23; 27 | 26 | 4 w | 32; 29 | 27 | Itching (4), desquamation (9), pigmentation (25); itching (4), desquamation (3), pigmentation (11); | Itching (6), pigmentation (8), desquamation (12), | |
| Zheng 2010 | NR | NR | NR | NR | 2 w | 60; 60 | 60 | 0; 0 | Itching (2) | |
| Chen 2009 | 4.8 y | 4.5 y | 31 | 31 | 30 d | 72 | 48 | Pigmentation (4), isomorphic response (2) | Mild burning (6), erythema (6) | |
| Yang 2008 | 2.28 (1.92) y | 1.80 (2.57) y | 22.75 (7.59) | 24.03 (5.69) | 4 w | 30 | 30 | NR | NR | |
| Zhang 2007 | 3.5 m | 3.55 m | 20.3 | 21.1 | 30 d | 52 | 57 | NR | NR | |
| Chen 2007 | 30.13 (17.61) m | 29.59 (14.28) m | 26.56 (6.58) | 25.29 (6.37) | 30 d | 48 | 48 | Infection (2), mild burning (2), pigmentation (4), isomorphic response (2) | Pigmentation (2), desquamation (8), isomorphic response (1), mild burning (8) | |
| Study | FUP (m) | Recurrence | Patients (M/F) | Interventions | Main outcomes | |||||
| E | C | E | C | E | C | |||||
| Sheng 2019 | NR | NR | NR | 19/41 | 25/35 | Fire needle | BCG nucleic acid injection | Efficacy rate | ||
| Zhu 2019 | 3 | 4 | 13 | 25/23 | 26/22 | 5% Imiquimod + fire needle | 5% Imiquimod | Efficacy rate, RER, AEs | ||
| Yuan 2019 | 3 | 2 | 3 | 22/17 | 25/14 | Fire needle + Isotretinoin Erythromycin Gel | Isotretinoin Erythromycin Gel | Efficacy rate + skin lesion scores + IL-2/IL-10/INF-γ, RER, AEs | ||
| Li 2019 | 3 | NR | NR | 13/27 | 11/29 | Fire needle | Tretinoin | Efficacy rate, AEs | ||
| Li2 2019 | 3 | 4; 0 | 11 | 22/18; 23/17 | 25/15 | Fire needle; fire needle + Xiaoyou Decoction | Recombinant human interferon alpha-2b gel | Efficacy rate + skin lesion scores, RER, AEs | ||
| Huang 2019 | NR | 3; 0 | 8 | 40/50 | Fire needle; Fire needle + Polymyosin injection | Polymyosin injection | Efficacy rate, RER | |||
| Cui 2019 | NR | NR | NR | 10/20; 13/17 | 11 /19 | Fire needle; Fire needle + Taohongsiwu Decoction | Taohongsiwu Decoction | Efficacy rate | ||
| Liang2018 | NR | NR | NR | 13/17; 14/16 | 15/15 | Fire needle; Fire needle + Xiaoyou Decoction | Xiaoyou Decoction | Efficacy rate + IL-2/IL-10/IFN-γ, DLQI | ||
| Liao 2017 | NR | NR | NR | 11/19; 13/17 | 12/18 | Fire needle; fire needle + mild moxibustion | Mild moxibustion | Efficacy rate | ||
| Jia 2017 | 3 | 7 | 10 | 22/28 | 24/26 | Fire needle + recombinant human interferon alpha 2 | Recombinant human interferon alpha 2 | Efficacy rate, RER | ||
| Ruan 2017 | NR | NR | NR | 20/40 | 18/42 | Fire needle + Chinese medicine inverted film | Chinese medicine inverted film | Efficacy rate | ||
| Jiang 2017 | NR | NR | NR | 52/63 | Fire needle | Mannan peptide | Efficacy rate | |||
| He 2017 | NR | NR | NR | 21/39 | 22/36 | Fire needle + Xiangfu lotion | Xiangfu lotion | Efficacy rate | ||
| Fan 2016 | NR | NR | NR | 21/39 | 22/36 | Fire needle + Xiangfu lotion | Xiangfu lotion | Efficacy rate | ||
| Wang 2016 | 3 | NR | NR | 15/23; 20/32 | 14/22 | Fire needle; fire needle + Chinese medicine inverted film | Chinese medicine inverted film | Efficacy rate | ||
| Huang 2016 | 3 | 3 | 13 | NR | NR | Fire needle + Chinese medicine inverted film + Imiquimod | Chinese medicine inverted film + Imiquimod | Efficacy rate, RER, AEs | ||
| Guo 2016 | NR | NR | NR | 22/16 | 20/18 | Fire needle | Recombinant human interferon alpha 2 | Efficacy rate | ||
| Liu 2016 | NR | NR | NR | 11/19 | 15/15 | Fire needle + recombinant human interferon alpha 2 | Recombinant human interferon alpha 2 | Efficacy rate | ||
| Shi 2015 | 3 | 1 | 2 | 18/15 | 17/15 | Fire needle | Tretinoin | Efficacy rate + skin lesion scores, RER, AEs | ||
| He 2014 | NR | NR | NR | 25/21 | 22/24 | Fire needle + Tazarotene gel | Tazarotene gel | Efficacy rate | ||
| Ma 2014 | NR | NR | NR | 23/45 | Fire needle + Quyou Decoction | Quyou Decoction | Efficacy rate | |||
| Xu 2014 | NR | NR | NR | 10/20 | 7/23 | Fire needle | Liquid nitrogen freezing | Efficacy rate, AEs | ||
| Jin 2013 | 1.5 | NR | NR | 9/9 | 5/13 | Fire needle | Photodynamic | Efficacy rate + skin lesion scores + IL-2/IL-10/INF-γ | ||
| Pu 2011 | NR | NR | NR | 16/16; 15/14 | 15/12 | Fire needle; fire needle + tretinoin + BCG polysaccharide nucleic acid | Tretinoin + BCG polysaccharide nucleic acid | Efficacy rate, AEs | ||
| Zheng 2010 | 6 | 10; 0 | 0 | 54/126 | Fire needle; Fire needle + Utlins injection | Utlins injection | Efficacy rate, RER, AEs | |||
| Chen 2009 | 3 | 2 | 3 | 33/39 | 20/28 | Fire needle | Tretinoin | Efficacy rate, RER, AEs | ||
| Yang 2008 | 3 | 1 | 3 | 9/21 | 12/18 | Fire needle + decoction | Fire needle + decoction | Efficacy rate, RER | ||
| Zhang 2007 | 12 | 2 | 4 | 19/33 | 20/37 | Fire needle + tretinoin + BCG polysaccharide nucleic acid | Tretinoin + BCG polysaccharide nucleic acid | Efficacy rate, RER | ||
| Chen 2007 | 3 | 1 | 2 | 23/25 | 20/28 | Fire needle | Tretinoin | Efficacy rate + skin lesion scores, RER, AEs | ||
- E: experimental group; C: control group; NR: no report; FUP: follow-up period; M: male; F: female; y: years; m: months; w: weeks; d: days; BCG: Bacillus Calmette–Guerin; RER: recurrence rate; AEs: adverse events; DLQI: Dermatology Life Quality Index; IL-2/10: interleukin-2/10; IFN–γ: interferon-γ. E, experimental group; C, control group; NR, no report; y, years; m, months; w, weeks; d, days.
2.6. Risk of Bias Assessment
Two authors (Meng Xing and Rong Xu) independently conducted risk assessments, using the Cochrane bias risk tool [24]. The evaluation items included random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (implementation bias), blinding of result evaluation (monitoring bias), incomplete result data (wear bias), selective reporting (reporting bias), and other biases. We assessed the risk of bias by using the terms “low risk,” “unclear risk,” and “high risk.” When disagreements occurred, the two authors had discussions to address these issues. If differences still existed, a third author, Bin Li, was invited to make a final decision.
2.7. Level of Evidence
The level of evidence combines considerations of risk of bias, directness, heterogeneity, precision, and publication bias classified into grades of recommendation, assessment, development, and evaluation working (GRADE) criteria: very low-quality evidence (+), low-quality evidence (++), moderate-quality evidence (+++), and high-quality evidence (++++). The GRADEpro guideline development tool (GDT) platform (https://gradepro.org) was adopted to create a summary of findings tables for Cochrane systematic reviews and assess the level of evidence of the outcomes.
2.8. Statistical Analysis
In this meta-analysis, we used RevMan 5.3 software (version 5.3, Cochrane Collaboration) to calculate the risk ratios (RR) and the mean differences (MD) with a 95% confidence interval (CI). Standard mean differences (SMD) were used when the measurement criteria were not the same. Heterogeneity was tested using the I2 statistic. A fixed effect model was used when P > 0.1 and I2 < 50%; otherwise, when I2 > 50%, subgroup analysis was adopted to resolve methodological and clinical heterogeneity. When there was heterogeneity that could not be readily explained, a random effect model was considered. We performed a sensitivity analysis of all indices to test the stability of the results when necessary. Since studies with negative results could remain unpublished, a funnel plot was used to analyze publication bias across the studies.
2.9. Outcomes
According to the PICOS principle, we use efficiency rate as an indicator that emphasizes the primary outcome of patients and include secondary outcomes. The primary outcome was divided into the following four categories: cured, defined by complete resolution of the skin lesions; significantly effective, defined by partial resolution of the skin lesions or if the skin lesion scores were ≥70% but <100%; effective, defined by partial resolution of the skin lesions or if the skin lesion scores were ≥30% but <70%; and ineffective, defined by an insignificant resolution of the skin lesions or if the skin lesion scores were <30% [25]. We used the following formula to calculate the total effective rate: total effective rate = (number of “cured” patients + number of “significantly effective” patients + number of “effective” patients)/total number of patients × 100%. The secondary outcomes were skin lesion scores, cytokine levels, Dermatology Life Quality Index (DLQI), recurrence rate, and adverse events.
3. Results
3.1. Included Studies and Their Characteristics
We obtained 341 relevant studies from seven databases; after the removal of 221 duplicate reports, 120 reports remained. After title and abstract filtering, 52 records were excluded, and 68 were left. Further, 39 articles were excluded from the full-text screening, and 29 articles that met the criteria were included in this review [2, 26–53]. The study flow is depicted in Figure 2.
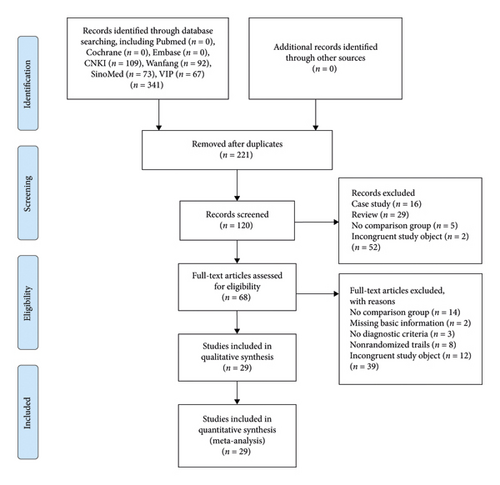
Participants: a total of 29 studies were included, with a total of 2,666 patients. All trials met the diagnostic criteria; 20 trials mentioned the diagnostic criteria used [2, 27, 32–40, 42–48, 50, 51, 53], and three were confirmed cases from hospitals [26, 28, 29, 31, 41, 49, 52]. None reported polycentric differentiation or syndrome differentiation, as described by traditional Chinese medicine (TCM).
Intervention: this systematic review included 17 interventions (Table 1). Seven of the articles included two experimental groups, including fire needle therapy alone and combined therapy, with one control group each [2, 27, 30, 32, 33, 39, 49].
Comparison: to present the results of the studies more concisely, we developed subgroups based on the different interventions. Seventeen RCTs used fire needle therapy only [2, 26, 27, 29–33, 36, 37, 39, 41, 43, 46–48, 51, 53], whereas ten studies were in combination with TCM [27, 31–33, 35, 37–39, 45, 51]. Four trials used fire needle therapy in combination with immunosuppressive agents [2, 34, 42, 49], one trial used fire needle therapy in combination with imiquimod [28], one used fire needle therapy and tretinoin [44], and the other four used a multidrug combination [29, 40, 48, 52]. The treatment course ranged from 4 to 10 weeks.
Outcome: the primary outcome indicator is efficacy rate. Four trials used the efficacy rate from the skin lesion scores as the outcome indicator [29, 31–33, 43, 53], whereas the other 25 studies used symptom assessment.
Study design: all included studies are RCTs.
3.2. Risk of Bias Assessment
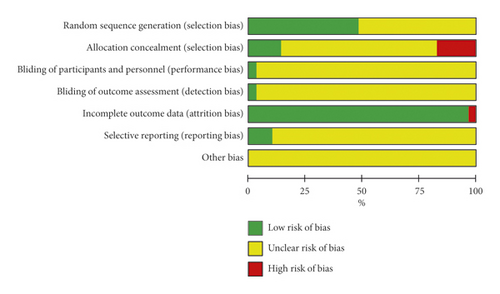
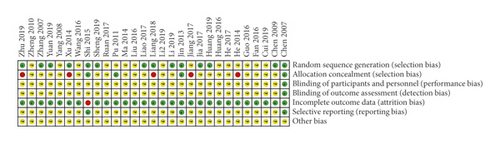
Fifteen trials reported the generation of random sequences, nine of which used a random number table [2, 26–29, 34, 44, 50, 52], four used a computer random number generator (SAS) [33, 43, 47, 53], and one used a lottery method [31]. One trial used sequential sampling inspection and introduced artificial evaluation to the process of case inclusion, which was then identified as high risk [40]. The other 15 trials mentioned random only, without further explanation; thus, we identified them as unclear. Eight trials used the random concealment method, three of which used sequentially coded opaque envelopes and were thus identified as low-risk [43, 47, 53], whereas five experiments used an open consultation order, which were identified as high risk [28, 33, 36, 44, 46]. Only one study reported the implementation and monitoring of blinding [53]. Since one trial had no explanation regarding the amount of data loss or the reason for data loss, it was considered high risk [43]. Three trials provided detailed protocols and result reports followed by the research plan, and these were identified as low risk [43, 47, 53]. No study described other biases (Figure 3). The risk of publication bias across studies is presented in a funnel plot (Supplementary file 4: Figure S1), implying low-quality methodology and that publication bias related to insufficient sample size may exist.
3.3. Level of Evidence
Based on the GRADE system, the evidence on the safety and efficacy of fire needle therapy for flat warts was evaluated using the GRADEpro GDT platform. The evidence of efficacy rate of fire needle alone compared with control groups was level C (Table 2), whereas the evidence of fire needle combined therapies was level B. In addition, the results of secondary indices indicated that the evidence of skin lesion scores and adverse events was level C, and the evidence of cytokine expression levels and recurrence rate was level B. All of them were moderate or weak recommendations.
| Certainty assessment | Number of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Fire needle | Control group | Relative (95% CI) | Absolute (95% CI) | ||
| Efficacy rate of fire needle alone vs. control groups | ||||||||||||
| 18 | Randomized trials | Serious | Serious | Not serious | Not serious | None | 686/749 (91.6%) | 424/568 (74.6%) | RR 1.18 (1.09 to 1.28) | 134 more per 1,000 (from 67 more to 209 more) | ⊕⊕⃝⃝Low | Important |
| Efficacy rate of fire needle combined therapies vs. control groups | ||||||||||||
| 20 | Randomized trials | Serious | Not serious | Not serious | Not serious | None | 831/863 (96.3%) | 579/694 (83.4%) | RR 1.16 (1.12 to 1.20) | 133 more per 1,000 (from 100 more to 167 more) | ⊕⊕⊕⃝Moderate | Important |
| Skin lesions scores | ||||||||||||
| 9 | Randomized trials | Serious | Serious | Not serious | Not serious | None | 725 | 619 | — | SMD 1 lower (1.43 lower to 0.57 lower) | ⊕⊕⃝⃝Low | Important |
| Cytokine levels | ||||||||||||
| 2 | Randomized trials | Serious | Not serious | Not serious | Not serious | None | 207 | 162 | — | MD 0.4 higher (0.19 lower to 1 higher) | ⊕⊕⊕⃝Moderate | Important |
| Recurrence rate | ||||||||||||
| 14 | Randomized trials | Serious | Not serious | Not serious | Not serious | None | 40/621 (6.4%) | 72/508 (14.2%) | RR 0.44 (0.30 to 0.63) | 79 fewer per 1,000 (from 99 fewer to 52 fewer) | ⊕⊕⊕⃝Moderate | Important |
| Adverse events | ||||||||||||
| 15 | Randomized trials | Serious | Serious | Not serious | Not serious | None | 123/1794 (6.9%) | 109/1478 (7.4%) | RR 0.83 (0.56 to 1.23) | 13 fewer per 1,000 (from 32 fewer to 17 more) | ⊕⊕⃝⃝Low | Important |
- CI, confidence interval; RR, risk ratio; SMD, standardized mean difference; MD, mean difference.
3.4. Primary Outcomes
3.4.1. Efficacy Rate
Twenty-six trials used the clinical evaluation criteria of clinical dermatology that defined the reduction of symptoms or scores of more than 30% as effective, and less than 30% as invalid [25]. One study was based on the evaluation criteria for treatment of flat warts given in the Standards for Diagnosis and Treatment of Traditional Chinese Medical Diseases that defines the degree of skin loss of more than 20% as effective, and otherwise invalid [51]. The evaluation criteria for other two studies were unclear [47, 53]. The results of the meta-analysis showed that the efficacy rate of fire needle therapy alone was higher when compared with that of an immunomodulator (RR = 1.19, 95% CI: 1.03 to 1.37, I2 = 70%, P = 0.02; Table 3; Supplementary file 5: Figure S2) or tretinoin (RR = 1.39, 95% CI: 1.25 to 1.55, I2 = 0%, P < 0.00001). Regarding the different subgroups, the efficacy rate of combination of fire needle therapy with other TCMs was significantly higher than that of TCM alone (RR = 1.16, 95% CI: 1.10 to 1.23, I2 = 21%, P < 0.00001; Supplementary file 6: Figure S3). The groups that used fire needle therapy combined with an immunomodulator (RR = 1.17, 95% CI: 1.07 to 1.28, I2 = 33%, P = 0.0005), imiquimod (RR = 1.21, 95% CI: 1.04 to 1.42, P = 0.02), and multidrug therapy (RR = 1.15, 95% CI: 1.07–1.24, I2 = 0%, P = 0.0001) also exhibited statistically significant differences.
| Trials | Comparisons | Effect estimates (95% CI) | P value | |
|---|---|---|---|---|
| 1. Fire needle versus control group | ||||
| 1.1. Fire needle versus traditional Chinese medicine | ||||
| Wang 2016 | Fire needle versus traditional Chinese medicine | RR | 1.01 [0.83, 1.23] | |
| He 2017 | Fire needle versus traditional Chinese medicine | RR | 1.12 [1.01, 1.24] | |
| Liao 2017 | Fire needle versus traditional Chinese medicine | RR | 1.79 [1.02, 3.14] | |
| Liang 2018 | Fire needle versus traditional Chinese medicine | RR | 1.00 [0.69, 1.45] | |
| Cui 2019 | Fire needle versus traditional Chinese medicine | RR | 1.09 [0.77, 1.55] | |
| Meta-analysis | RR | 1.10 [0.99, 1.21] | 0.07 | |
| 1.2. Fire needle versus immunomodulator | ||||
| Zheng 2010 | Fire needle versus immunomodulator | RR | 1.06 [0.92, 1.21] | |
| Guo 2016 | Fire needle versus immunomodulator | RR | 1.20 [1.00, 1.44] | |
| Jiang 2017 | Fire needle versus immunomodulator | RR | 1.14 [1.00, 1.29] | |
| Huang 2019 | Fire needle versus immunomodulator | RR | 1.09 [0.77, 1.55] | |
| Li 2019 | Fire needle versus immunomodulator | RR | 1.09 [0.89, 1.33] | |
| Sheng 2019 | Fire needle versus immunomodulator | RR | 1.89 [1.40, 2.55] | |
| Meta-analysis | RR | 1.19 [1.03,1.37] | 0.02 | |
| 1.3 Fire needle versus tretinoin | ||||
| Chen 2007 | Fire needle versus tretinoin | RR | 1.48 [1.19, 1.84] | |
| Chen 2009 | Fire needle versus tretinoin | RR | 1.48 [1.20, 1.84] | |
| Shi 2015 | Fire needle versus tretinoin | RR | 1.25 [1.01, 1.56] | |
| Li 2019 | Fire needle versus tretinoin | RR | 1.36 [1.09, 1.68] | |
| Meta-analysis | RR | 1.39 [1.25, 1.55] | < 0.00001 | |
| 1.4. Fire needle versus liquid nitrogen freezing | ||||
| Xu 2014 | Fire needle versus liquid nitrogen freezing | RR | 0.96 [0.83, 1.12] | 0.64 |
| 1.5 Fire needle versus photodynamic | ||||
| Jin 2013 | Fire needle versus photodynamic | RR | 1.00 [0.85, 1.17] | 1 |
| 1.6. Fire needle versus multi drug therapy | ||||
| Pu 2011 | Fire needle versus multidrug therapy | RR | 1.20 [0.94, 1.28] | 0.15 |
| 2. Fire needle combined with conventional therapies versus control group | ||||
| 2.1 Fire needle combined with traditional Chinese medicine versus traditional Chinese medicine | ||||
| Yang 2006 | Fire needle combined with traditional Chinese medicine versus traditional Chinese medicine | RR | 1.29 [0.99, 1.67] | |
| Ma 2014 | Fire needle combined with traditional Chinese medicine versus traditional Chinese medicine | RR | 1.33 [1.06, 1.66] | |
| Fan 2016 | Fire needle combined with traditional Chinese medicine versus traditional Chinese medicine | RR | 1.12 [1.01, 1.24] | |
| Wang 2016 | Fire needle combined with traditional Chinese medicine versus traditional Chinese medicine | RR | 1.18 [0.95, 1.45] | |
| Ruan 2017 | Fire needle combined with traditional Chinese medicine versus traditional Chinese medicine | RR | 1.05 [0.98, 1.14] | |
| He 2017 | Fire needle combined with traditional Chinese medicine versus traditional Chinese medicine | RR | 1.12 [1.01, 1.24] | |
| Liao 2017 | Fire needle combined with traditional Chinese medicine versus traditional Chinese medicine | RR | 1.19 [0.69, 2.05] | |
| Liang 2018 | Fire needle combined with traditional Chinese medicine versus traditional Chinese medicine | RR | 1.21 [0.93, 1.57] | |
| Cui 2019 | Fire needle combined with traditional Chinese medicine versus traditional Chinese medicine | RR | 1.32 [0.96, 1.80] | |
| Meta-analysis | RR | 1.16 [1.10, 1.23] | < 0.00001 | |
| 2.2. Fire needle combined with traditional Chinese medicine versus immunomodulator | ||||
| Li 2019 | Fire needle combined with traditional Chinese medicine versus immunomodulator | RR | 1.19 [0.98, 1.44] | 0.09 |
| 2.3 Fire needle combined with immunomodulator versus immunomodulator | ||||
| Zheng 2010 | Fire needle combined with immunomodulator versus immunomodulator | RR | 1.08 [0.97, 1.20] | |
| Liu 2016 | Fire needle combined with immunomodulator versus immunomodulator | RR | 1.21 [1.00, 1.46] | |
| Jia 2017 | Fire needle combined with immunomodulator versus immunomodulator | RR | 1.12 [0.96, 1.31] | |
| Huang 2019 | Fire needle combined with immunomodulator versus immunomodulator | RR | 1.50 [1.05, 2.14] | |
| Meta-analysis | RR | 1.17 [1.07, 1.28] | 0.0005 | |
| 2.4. Fire needle combined with tretinoin versus tretinoin | ||||
| He 2014 | Fire needle combined with trunnion versus tretinoin | RR | 1.09 [0.99, 1.21] | 0.07 |
| 2.5. Fire needle combined with imiquimod versus imiquimod | ||||
| Zhu 2019 | Fire needle combined with imiquimod versus imiquimod | RR | 1.21 [1.04, 1.42] | 0.02 |
| 2.6. Fire needle combined with multidrug therapy versus multidrug therapy | ||||
| Zhang 2007 | Fire needle combined with multidrug therapy versus multidrug therapy | RR | 1.10 [0.98, 1.23] | |
| Pu 2011 | Fire needle combined with multidrug therapy versus multidrug therapy | RR | 1.18 [0.94, 1.49] | |
| Huang 2016 | Fire needle combined with multidrug therapy versus multidrug therapy | RR | 1.13 [0.99, 1.30] | |
| Yuan 2019 | Fire needle combined with multidrug therapy versus multidrug therapy | RR | 1.25 [1.06, 1.48] | |
| Meta-analysis | RR | 1.15 [1.07, 1.24] | 0.0001 | |
When heterogeneity of the subgroups was observed in the efficacy rate of fire needle therapy compared with an immunomodulator, sensitivity analysis was performed (Supplementary file 7: Figure S4). We excluded studies [26] on the sensitivity analysis that led to a reduction in the heterogeneity of the subgroups (I2 = 0), yet the result was still statistically significant (P = 0.006).
3.5. Secondary Outcomes
3.5.1. Skin Lesion Scores
Five trials used skin lesion scores as the criteria to assess disease severity [29, 30, 32, 33, 54], as recommended by the Chinese Dermatology Monograph [25]. The overall skin lesion scores of the fire needle group were similar to those of the control group (P = 0.15; Table 4; Supplementary file 8: Figure S5), whereas the combined groups had lower scores (SMD = −2.66, 95% CI: −4.55 to −0.78, I2 = 97%, P = 0.006; Supplementary file 9: Figure S6). Compared with TCM and tretinoin, the group with fire needle therapy alone had reduced scores with respect to the size (SMD = −1.20, 95% CI: −1.54 to −0.87, I2 = 0%, P < 0.00001), thickness (SMD = −0.94, 95% CI: −1.26 to −0.61, I2 = 0%, P < 0.00001), and itching (SMD = −0.44, 95% CI: −0.75 to −0.13, I2 = 0%, P = 0.006). However, there was no statistically significant difference in the number of warts (P = 0.30), color of the skin lesions (P = 0.57), and isomorphic response (P = 0.23). We conducted a sensitivity analysis of the two subgroups with high heterogeneity, but the result could not be considered significant because there were an insufficient number of trials (Supplementary file 10: Figure S7; Supplementary file 11: Figure S8).
| Trials | Comparisons | Effect Estimates (95% CI) | P value | |
|---|---|---|---|---|
| 1. Skin lesion scores | ||||
| 1.1 Fire needle versus control group | ||||
| 1.1.1 Overall | ||||
| Liang 2018 | Fire needle versus traditional Chinese medicine | RR | −0.05 [−0.67, 0.57] | |
| Cui 2019 | Fire needle versus traditional Chinese medicine | RR | −4.86 [−5.91, −3.82] | |
| Li2 2019 | Fire needle versus immunomodulator | RR | −0.47 [−1.10, 0.16] | |
| Meta-analysis | RR | −1.75 [−4.15,0.64] | 0.15 | |
| 1.1.2 Number of warts | ||||
| Chen 2007 | Fire needle versus tretinoin | RR | −1.31 [−1.75, −0.86] | |
| Shi 2015 | Fire needle versus tretinoin | RR | −0.02 [−0.51, 0.46] | |
| Meta-analysis | RR | −0.67 [−1.93, 0.59] | 0.3 | |
| 1.1.3 Size | ||||
| Chen 2007 | Fire needle versus tretinoin | RR | −1.17 [−1.61, −0.74] | |
| Shi 2015 | Fire needle versus tretinoin | RR | −1.25 [−1.79, −0.72] | |
| Meta-analysis | RR | −1.20 [−1.54, −0.87] | <0.00001 | |
| 1.1.4 Thickness | ||||
| Chen 2007 | Fire needle versus tretinoin | RR | −0.97 [−1.39, −0.55] | |
| Shi 2015 | Fire needle versus tretinoin | RR | −0.89 [−1.40, −0.38] | |
| Meta-analysis | RR | −0.94 [−1.26, −0.61] | < 0.00001 | |
| 1.1.5 Skin lesion color | ||||
| Chen 2007 | Fire needle versus tretinoin | RR | −0.89 [−1.31, −0.47] | |
| Shi 2015 | Fire needle versus tretinoin | RR | 0.25 [−0.24, 0.74] | |
| Meta-analysis | RR | −0.32 [−1.44, 0.79] | 0.57 | |
| 1.1.6 Itching | ||||
| Chen 2007 | Fire needle versus tretinoin | RR | −0.42 [−0.83, −0.02] | |
| Shi 2015 | Fire needle versus tretinoin | RR | −0.47 [−0.96, 0.03] | |
| Meta-analysis | RR | −0.44 [−0.75, −0.13] | 0.006 | |
| 1.1.7 Isomorphic response | ||||
| Chen 2007 | Fire needle versus tretinoin | RR | 0.17 [−0.23, 0.57] | |
| Shi 2015 | Fire needle versus tretinoin | RR | 0.22 [−0.27, 0.71] | |
| Meta-analysis | RR | 0.19 [−0.12, 0.50] | 0.23 | |
| 1.2 Fire needle combined with control group versus control group | ||||
| 1.2.1 Skin lesions overall scores | ||||
| Liang 2018 | Fire needle combined with traditional Chinese medicine versus traditional Chinese medicine | RR | −0.54 [−1.18, 0.09] | |
| Cui 2019 | Fire needle combined with traditional Chinese medicine versus traditional Chinese medicine | RR | −0.58 [−1.21, 0.05] | |
| Li2 2019 | Fire needle combined with traditional Chinese medicine versus immunomodulator | RR | −9.84 [−11.74, −7.94] | |
| Yuan 2019 | Fire needle combined with multidrug therapy versus multidrug therapy | RR | −1.09 [−1.57, −0.61] | |
| Meta-analysis | RR | −2.66 [−4.55, −0.78] | 0.006 | |
| 2. Cytokine levels | ||||
| 2.1 Fire needle combined with control group versus control group | ||||
| 2.1.1 Interleukin-2 | ||||
| Liang 2018 | Fire needle combined with traditional Chinese medicine versus traditional Chinese medicine | RR | 5.56 [3.05, 8.07] | |
| Yuan 2019 | Fire needle combined with multidrug therapy versus multidrug therapy | RR | 4.94 [3.17, 6.71] | |
| Meta-analysis | RR | 5.15 [3.70, 6.59] | <0.00001 | |
| 2.1.2 Interleukin-10 | ||||
| Liang 2018 | Fire needle combined with traditional Chinese medicine versus traditional Chinese medicine | RR | −2.48 [−3.71, −1.25] | |
| Yuan 2019 | Fire needle combined with multidrug therapy versus multidrug therapy | RR | −1.41 [−2.26, −0.56] | |
| Meta-analysis | RR | −1.75 [−2.45, −1.05] | <0.00001 | |
| 2.1.3 Interferon-γ | ||||
| Liang 2018 | Fire needle combined with traditional Chinese medicine versus traditional Chinese medicine | RR | 7.75 [4.24, 11.26] | |
| Yuan 2019 | Fire needle combined with multidrug therapy versus multidrug therapy | RR | 7.64 [5.48, 9.80] | |
| Meta-analysis | RR | 7.67 [5.83, 9.51] | <0.00001 | |
| 3 Dermatology Life Quality Index | ||||
| 3.1 Fire needle versus control group | ||||
| Liang 2018 | Fire needle versus traditional Chinese medicine | RR | −0.03 [−2.65, 2.59] | 0.98 |
| 3.1 Fire needle combined with control group versus control group | ||||
| Liang 2018 | Fire needle combined with traditional Chinese medicine versus traditional Chinese medicine | RR | −3.82 [−6.32, −1.32] | 0.003 |
| 4. Recurrence rate | ||||
| 4.1 Fire needle versus control group | ||||
| Chen 2007 | Fire needle versus tretinoin | RR | 0.50 [0.05, 5.33] | |
| Chen 2009 | Fire needle versus tretinoin | RR | 0.44 [0.08, 2.56] | |
| Shi 2015 | Fire needle versus tretinoin | RR | 0.48 [0.05, 5.09] | |
| Zheng 2010 | Fire needle versus immunomodulator | RR | 10.67 [0.65, 176.19] | |
| Li2 2019 | Fire needle versus immunomodulator | RR | 0.33 [0.11, 1.05] | |
| Huang 2019 | Fire needle versus immunomodulator | RR | 0.38 [0.10, 1.46] | |
| Meta-analysis | RR | 0.71 [0.38, 1.31] | 0.27 | |
| 4.2 Fire needle combined with control group versus control group | ||||
| Yang 2008 | Fire needle combined with traditional Chinese medicine versus traditional Chinese medicine | RR | 0.33 [0.04, 3.03] | |
| Li2 2019 | Fire needle combined with traditional Chinese medicine versus immunomodulator | RR | 0.05 [0.00, 0.80] | |
| Huang 2016 | Fire needle combined with immunomodulator versus immunomodulator | RR | 0.22 [0.07, 0.71] | |
| Huang 2019 | Fire needle combined with immunomodulator versus immunomodulator | RR | 0.06 [0.00, 1.00] | |
| Zhu 2019 | Fire needle combined with imiquimod versus imiquimod | RR | 0.31 [0.11, 0.88] | |
| Zhang 2007 | Fire needle combined with multidrug therapy versus multidrug therapy | RR | 0.55 [0.10, 2.87] | |
| Jia 2017 | Fire needle combined with multidrug therapy versus multidrug therapy | RR | 0.70 [0.29, 1.69] | |
| Yuan 2019 | Fire needle combined with multidrug therapy versus multidrug therapy | RR | 0.84 [0.15, 4.77] | |
| Meta-analysis | RR | 0.34 [0.21, 0.54] | <0.00001 | |
| 5. Adverse events | ||||
| 5.1 Fire needle versus control group | ||||
| 5.1.1 Infection | ||||
| Chen 2007 | Fire needle versus tretinoin | RR | 5.00 [0.25, 101.48] | |
| Shi 2015 | Fire needle versus tretinoin | RR | 0.97 [0.15, 6.47] | |
| meta-analysis | RR | 1.55 [0.31, 7.71] | 0.59 | |
| 5.1.2 Itching | ||||
| Zheng 2010 | Fire needle versus immunomodulator | RR | 0.10 [0.00, 2.07] | |
| Pu 2011 | Fire needle versus multidrug therapy | RR | 0.54 [0.14, 2.09] | |
| Li 2019 | Fire needle versus tretinoin | RR | 0.67 [0.12, 3.78] | |
| Meta-analysis | RR | 0.48 [0.18, 1.32] | 0.15 | |
| 5.1.3 Pain | ||||
| Xu 2014 | Fire needle versus liquid nitrogen freezing | RR | 1.67 [0.44, 6.36] | |
| He 2017 | Fire needle versus traditional Chinese medicine | RR | 1−.64 [0.60, 188.18] | |
| Jiang 2017 | Fire needle versus immunomodulator | RR | 11.93 [0.69, 207.04] | |
| Meta-analysis | RR | 3.73 [0.87, 16.06] | 0.08 | |
| 5.1.4 Mild burning | ||||
| Chen 2007 | Fire needle versus tretinoin | RR | 5.00 [0.25, 101.48] | |
| Chen 2009 | Fire needle versus tretinoin | RR | 0.05 [0.00, 0.90] | |
| Shi 2015 | Fire needle versus tretinoin | RR | 0.97 [0.06, 14.85] | |
| Meta-analysis | RR | 0.61 [0.04, 8.47] | 0.72 | |
| 5.1.5 Erythema | ||||
| Chen 2009 | Fire needle versus tretinoin | RR | 0.05 [0.00, 0.90] | 0.04 |
| 5.1.6 Pigmentation | ||||
| Chen 2007 | Fire needle versus tretinoin | RR | 2.00 [0.38, 10.41] | |
| Chen 2009 | Fire needle versus tretinoin | RR | 6.04 [0.33, 109.71] | |
| Shi 2015 | Fire needle versus tretinoin | RR | 1.94 [0.38, 9.86] | |
| Pu 2011 | Fire needle versus multidrug therapy | RR | 2.54 [1.10, 5.86] | |
| Li 2019 | Fire needle versus immunomodulator | RR | 0.17 [0.01, 4.01] | |
| Meta-analysis | RR | 2.19 [1.15, 4.17] | 0.02 | |
| 5.2 Fire needle combined with control group versus control group | ||||
| 5.2.1 Infection | ||||
| Zhu 2019 | Fire needle combined with imiquimod versus imiquimod | RR | 0.20 [0.01, 4.06] | 0.29 |
| 5.2.2 Itching | ||||
| Zhu 2019 | Fire needle combined with imiquimod versus imiquimod | RR | 0.50 [0.05, 5.33] | |
| Pu 2011 | Fire needle combined with multidrug therapy versus multidrug therapy | RR | 0.64 [0.17, 2.49] | |
| Yuan 2019 | Fire needle combined with multidrug therapy versus multidrug therapy | RR | 0.75 [0.18, 3.13] | |
| Meta-analysis | RR | 0.66 [0.27, 1.64] | 0.37 | |
| 5.2.3 Mild burning | ||||
| Zhu 2019 | Fire needle combined with imiquimod versus imiquimod | RR | 0.33 [0.04, 3.09] | |
| Huang 2016 | Fire needle combined with multidrug therapy versus multidrug therapy | RR | 0.09 [0.00, 1.50] | |
| Yuan 2019 | Fire needle combined with multidrug therapy versus multidrug therapy | RR | 1.25 [0.36, 4.31] | |
| Meta-analysis | RR | 0.48 [0.10, 2.31] | 0.36 | |
| 5.2.4 Erythema | ||||
| Yuan 2019 | Fire needle combined with multidrug therapy versus multidrug therapy | RR | 0.67 [0.12, 3.77] | 0.65 |
| 5.2.5 Pigmentation | ||||
| Ruan 2017 | Fire needle combined with traditional Chinese medicine versus traditional Chinese medicine | RR | 13.00 [0.75, 225.75] | |
| Li2 2019 | Fire needle combined with traditional Chinese medicine versus immunomodulator | RR | 0.10 [0.01, 2.04] | |
| Pu 2011 | Fire needle combined with multidrug therapy versus multidrug therapy | RR | 1.33 [0.51, 3.43] | |
| Yuan 2019 | Fire needle combined with multi drug therapy versus multidrug therapy | RR | 5.00 [0.25, 100.89] | |
| Meta-analysis | RR | 1.65 [0.32, 8.48] | 0.55 | |
| 5.2.6 Desquamation | ||||
| Pu 2011 | Fire needle combined with multidrug therapy versus multidrug therapy | RR | 0.24 [0.07, 0.83] | |
| Huang 2016 | Fire needle combined with multidrug therapy versus multidrug therapy | RR | 0.09 [0.00, 1.50] | |
| Meta-analysis | RR | 0.21 [0.07, 0.64] | 0.006 | |
3.5.2. Cytokine Levels
Two studies reported that increased levels of cytokines, including interleukin-2 (IL-2), interleukin-10 (IL-10), and interferon-γ (IFN-γ), were associated with flat warts [29, 33] (Table 4; Supplementary file 10: Figure S9). After combined treatments, the levels of IL-2 (MD = 5.15, 95% CI: 3.70 to 6.59, I2 = 0%, P < 0.00001) or IFN-γ (MD = 7.67, 95% CI: 5.83 to 9.51, I2 = 0%, P < 0.00001) were significantly increased, whereas the level of IL-10 was decreased, compared with that of other drugs (MD = −1.75, 95% CI: −2.45 to −1.05, I2 = 49%, P < 0.00001).
3.5.3. DLQI
One study used DLQI to evaluate the effects of the treatment [33]; it indicated no significant difference when comparing fire needle therapy and TCM (P = 0.98; Table 4). However, when compared to the effects of TCM alone, the combination of fire needle therapy and TCM showed a statistical difference (RR = −3.82, 95% CI: −6.32 to −1.32, P = 0.003).
3.5.4. Recurrence Rates
Twelve trials reported recurrence rates [2, 28, 29, 31, 34, 40, 43, 49–53]. The follow-up period for 10 studies was 3 months, one was 6 months [49], and one was 12 months [52]. The results of the meta-analysis (Table 4; Supplementary file 13: Figure S10) showed that the recurrence rate in the groups that used fire needle therapy alone was similar to that in the control groups (RR = 0.71, 95% CI: 0.38 to 1.31, I2 = 24%, P = 0.27); however, recurrence rates were significantly lower in the combined therapies (RR = 0.34, 95% CI: 0.21 to 0.54, I2 = 12%, P < 0.00001).
3.5.5. Adverse Events
Fourteen trials evaluated the safety of treatment by assessing various types of adverse events [28–31, 35–37, 40, 43, 46, 48–50, 53]. Subgroup analysis (Table 4; Supplementary file 14: Figure S11) showed that patients treated with fire needle therapy were at a reduced risk of erythema (RR = 0.05, 95% CI: 0.00 to 0.90, P = 0.04) and blisters (RR = 0.04, 95% CI: 0.00 to 0.71, P = 0.03), but were more likely to develop pigmentation (RR = 2.19, 95% CI: 1.15 to 4.17, I2 = 0%, P = 0.02). Furthermore, comparisons of desquamation in the fire needle combined with multidrug therapy group and multidrug therapy alone demonstrated a significant difference (RR = 0.21, 95% CI: 0.07 to 0.64, I2 = 0%, P = 0.006; Supplementary file 15: Figure S12). In the sensitivity analysis, the subgroup of mild burning and pigmentation showed high heterogeneity, which may be related to two studies [30, 50], but the conclusion is still valid with statistical significance (Supplementary file 16: Figure S13; Supplementary file 17: Figure S14).
4. Discussion
This systematic review included 29 trials comparing the effectiveness of fire needle therapy alone and combined treatments for flat warts. We found evidence to support that fire needle therapy showed significantly better therapeutic potential than immunomodulators or tretinoin, especially in terms of wart size, thickness, and itching. The reason for this may be related to the mechanism of high temperature directly destroying warts and degenerative proteins and killing viruses in epidermal spinous processes, which are possibly the most common methods of inducing cell death and antigen exposure [8, 40, 55]. Based on the high heterogeneity in the efficacy rate of the subgroup that received fire needle therapy compared with that of the immunomodulator, we found that the intervention frequency might be the determining factor responsible for the differences. The results of the sensitivity analysis resolved the heterogeneity and proved that the results are still significant. However, the efficacy rate between fire needle therapy and liquid nitrogen or photodynamic therapy did not significantly differ, but the evidence was insufficient because each of the comparisons was made using only one study. Furthermore, our results showed that the total efficacy rate of fire needle therapy alone was as effective as TCM, whereas combination therapies significantly improved effectiveness.
In terms of combined therapies, the total efficacy rates of TCM, immunomodulators, imiquimod, or multi drug therapy were significantly improved after using fire needle therapy, with lower skin lesion scores. The mechanism of fire needle combination therapy is still unclear and may be related to thermal effects that improve microcirculation and enhance drug absorption [35, 40]. Additionally, local or systemic cellular immune responses generated by natural killer (NK) cells have been reported as an important mechanism of fire needle therapy for flat warts [2]. In this meta-analysis, we found that groups that received fire needle therapy combined with tretinoin or TCM treatment were more likely to have decreased IL-10 and increased IL-2 or IFN-γ levels after peripheral blood testing. Consistent with this result, an experiment confirmed that HPV infection inhibits NK cell activation by fluorescence quantitative polymerase chain reaction (PCR) and western blot analysis of lesion tissues and peripheral blood samples. The peripheral blood of infected patients analyzed using enzyme-linked immunosorbent assays revealed that IL-2 and IFN-γ protein levels were significantly lower than those in normal subjects, whereas the IL-10 levels of the patients were higher [54]. The expression levels of IFN-γ and IL-2 mRNA were correlated with wart remission, as evidenced by real-time PCR of select punch biopsy specimens, and IL-2 or IFN-γ mRNA levels were significantly increased in tissues of effectively treated viral warts [56]. Treatments of flat warts that regulate the levels of these cytokines have been reported, including retinoids [57]. Fire needle therapy may assist retinoids in enhancing this effect, increasing IL-2 and IFN-γ expression and enhancing cell-mediated immune responses. The levels of IL-2 or IFN-γ were positively correlated with promoting the activation of NK cells and removing the target cells infected with viruses [58]. As a cell synthesis inhibitor, IL-10 can directly inhibit IFN-γ synthesis, thereby reducing their ability to eliminate toxins [59]. The result of the reduction of IL-10 was consistent with the hypothesis of immunosuppression in infected patients [54].
Combined medication significantly reduced the clinical recurrence rate of flat warts. In addition to their destructive effects, the reduction of recurrence rates through fire needle therapy may be related to the regulation of immune function. According to previous reports, the recurrence rate of flat warts after combined therapy was lower than that of fire needle therapy alone, and it is recommended that, after fire needle treatment, antiviral therapy be commenced to further reduce the recurrence rates [29].
Regarding adverse events, we found that patients were less likely to have blisters and erythema when they were treated with fire needle therapy alone, and desquamation was less likely to occur in groups that received combined therapy. The main adverse events caused by the treatment of flat warts were irritating cautery, hypertrophic scar formation, local or systemic infection, itching, allergies, and increased sensitivity to pain [60–62]. Skin lesions, including pigmentation, scar formation, erythema, desquamation, and blistering, were also common [8]. This study included only 10 adverse events. Blisters have been reported as adverse events in cryotherapy, and erythema and desquamation have been reported as adverse events of squaric acid dibutyl ester or diphencyprone therapy [8]. We demonstrated that, to a limited extent, fire needle therapy can be used as an alternative or complementary therapy to avoid these adverse events. Pigmentation was the main adverse reaction to fire needle therapy alone, whereas our results showed that using combined therapies may lower this risk. Furthermore, some preventive measures for pigmentation have been proposed and implemented clinically. For example, the use of asiaticoside ointment could significantly relieve the pigmentation caused by fire needle therapy [63].
The present study is the first systematic review of the efficacy of fire needle treatment for flat warts that follows the PRISMA statement and is formulated according to the PICOS framework. The heterogeneity of each index factor was evaluated using sensitivity analysis to test the stability of the approach. The GRADE system was adopted to evaluate the level of evidence and make the results more credible. Evidence from 29 clinical trials illustrates that using fire needle therapy could significantly improve the efficacy rate with fewer side effects. This article provides evidence and guidance for the clinical practice of fire needles in flat warts.
However, this study had some limitations. First, the quality of trials was not very good; only fourteen trials reported specific randomization, and only nine mentioned allocation concealment. Only one trial reported blinding, and no studies reported other biases. Second, we did not distinguish between flat warts on the hands and faces. Third, flat warts from children and adults were discussed together; the difference among them requires further research.
5. Conclusion
In conclusion, fire needle therapy seems to be more effective against flat warts when compared with an immunomodulator or tretinoin and can reduce adverse events, such as blisters and erythema, but may cause pigmentation. Using fire needle therapy in combination with conventional therapies could significantly improve the treatment efficacy rate and lower the risk of desquamation. However, the quality of the included literature was not good, and large multicenter studies with a large number of samples and high-quality RCTs should be conducted to confirm the efficacy and safety of fire needle therapy in the treatment of flat warts.
Abbreviations
-
- CD4:
-
- Cluster of differentiation 4
-
- CI:
-
- Confidence intervals
-
- CNKI:
-
- China National Knowledge Infrastructure
-
- SinoMed:
-
- Chinese Biomedical Database
-
- VIP:
-
- China Science and Technology Journal Database
-
- DLQI:
-
- Dermatology Life Quality Index
-
- HPV:
-
- Human papillomavirus
-
- IL-2:
-
- Interleukin-2
-
- IL-10:
-
- Interleukin-10
-
- IFN-γ:
-
- interferon-γ
-
- MD:
-
- Mean difference
-
- NK:
-
- Natural killer
-
- PCR:
-
- Polymerase chain reaction
-
- PRISMA:
-
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
-
- RCT:
-
- Randomized controlled trial
-
- GRADE:
-
- Grades according to grades of recommendation, assessment, development, and evaluation working group
-
- GDT:
-
- Guideline development tool
-
- RR:
-
- Risk ratios
-
- SMD:
-
- Standard mean difference
-
- TCM:
-
- Traditional Chinese medicine
-
- PICOS:
-
- Participants, intervention, comparison, outcome, and study design.
Disclosure
The funders had no role in study design, data collection, analysis, decision to publish, or manuscript preparation.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors’ Contributions
YZ and JSJ conceived this study. XL and BL designed this study. LK and YL performed the searches. JLC and YJW extracted the data. MX and RX assessed the quality of the trials. MX and LL analyzed the data. YZ and JSJ wrote the original draft. YZ, LK, YL, XL, and BL contributed to the revision of the manuscript. All authors have read and approved the final manuscript. Ying Zhang and Jing-si Jiang contributed equally to this work.
Acknowledgments
This project was supported by a grant from the National Key Research and Development Program of China (no. 2018YFC1705301). This was also supported by the NSFC of China (nos. 81973860, 81904214, 81874470, 82074427, and 82004235). This study was also supported by the Shanghai Key Clinical Specialty Construction Project (No. shslczdzk05001), the Shanghai Three-Year Action Plan for the Development of Traditional Chinese Medicine (No. ZY(2018-2020)-FWTX-4010, ZY(2018-2020)-FWTX-100), the Shanghai Pujiang Talent Plan (No. 2020PJD067), and the Shanghai Science and Technology Commission (Nos. 20YF1450400, and 18401932300).
Open Research
Data Availability
All of the data used to support the findings of this study are available from the corresponding author upon request.




