Visible-Light-Driven SO42-/TiO2 Photocatalyst Synthesized from Binh Dinh (Vietnam) Ilmenite Ore for Rhodamine B Degradation
Abstract
A low-cost and simplistic approach for the synthesis of nanosized SO42-/TiO2 photocatalyst was successfully performed using Binh Dinh ilmenite ore and H2SO4 as titanium and sulfur sources, respectively. The experimental results indicate that the obtained material exists in the form of particles with a size of about 22 nm and has a specific surface area of about 49 m2 g-1. Compared with the TiO2 sample, the SO42-/TiO2 sample shows much higher photocatalytic degradation of rhodamine B (RhB) under the sunlight irradiation. In more details, the nanosized SO42-/TiO2 sample obtained is capable of completely decomposing RhB after 9 hours of irradiation by a 60 W LED lamp with a corresponding intensity of 9,500 Lux. However, when the SO42-/TiO2 is irradiated by the sunlight with the intensity of 65,000 Lux, it only takes 2 hours to completely decompose rhodamine B (RhB), facilitating the use of SO42-/TiO2 as a potential photocatalyst for the RhB photodegradation.
1. Introduction
Coupled with the exploitation of resources to produce a series of different products to serve life, people have been releasing many pollutants into the environment. Among these pollutants, the persistent organic compounds in the water environment are substances that have negative impacts on humans as well as all other living creatures on earth. In order to meet the urgent requirement for waterwaste treatment, a variety of water treatment technologies have been proposed such as adsorption [1–3], microwave catalysis [4, 5], and advanced oxidation processes including photocatalysis [6].
In recent years, the interest in the heterogeneous photocatalysis, which allows the use of the sunlight for the photocatalytic degradation of organic pollutants, has noticeably increased due to their potential applications in the water remediation. Compared to other water treatment technologies, this technology has more advantages in terms of environmental benefits and operation costs. Thus, photocatalysis is considered one of the most promising technologies to replace the current wastewater treatment technologies in the near future [7–9].
From a practical point of view, a desirable semiconductor for photocatalysis should have the following properties: (i) high photostability, (ii) low toxicity, (iii) suitable bandgap, (iv) resistance to photocorrosion, and (v) affordable fabrication costs [10]. In this respect, among the reported candidates as photocatalysts like ZnO- [11], WO3- [12], CdS- [13], MoS2- [14], BiVO4- [15], and TiO2-based materials turn out to be one of the highly promising due to its excellent physical and chemical properties including high photocatalytic activity, nontoxicity, high thermal and chemical stability, and high reproducibility [16, 17].
However, the main reason that limits the use of TiO2 for the photodegradation in wastewater treatment under solar irradiation is its large bandgap (3.20 eV for anatase, 3.0 eV for rultile, and about 3.2 eV for brookite). In fact, TiO2 can only be activated upon irradiation with a photon of light < 390 nm, which only accounting for 3–5% of the solar spectrum. Hence, it is highly desirable to extend the light response of TiO2 towards the visible region, enhancing its photocatalytic activity under the visible light irradiation. This can be achieved by (i) metal doping [18–20], (ii) nonmetal doping [19–22], and designing composites based on TiO2 [23–33].
In this present work, TiO2 was first successfully synthesized from Binh Dinh (Vietnam) ilmenite ore to reduce the fabrication costs of TiO2. After that, the obtained TiO2 was hydrothermalized with H2SO4 to fabricate the SO42-/TiO2 with the aim to extend the light response of TiO2 in the visible light region, enhancing its photocatalytic activities under the visible light irradiation. This work is devoted to investigating the effect of the SO42- doping on the structural, optical, and photocatalytic activities for the RhB degradation of TiO2.
2. Experiments
2.1. Preparation of Nanosized TiO2 from Binh Dinh Ilmenite Ore
- (Step 1)
10 g of ilmenite ore (titanium accounting for 52% of TiO2) and 70 mL HF 20% (diluted from HF 48%, Sigma-Aldrich) were put into a plastic beaker and magnetically stirred with a speed of 300 rpm for 5 hours before settling and filtering to remove the solid residue (r1) and collect the filtrate (l1)
- (Step 2)
KCl saturated solution, which was diluted from 99% KCl(s), Sigma-Aldrich, was slowly added into the filtrate (l1) under continously stirring, leading to the formation of a white K2TiF6 precipitate. With the aim at eliminating the impurities and purify the K2TiF6 precipitate, the precipitate was filtered and dissolved in hot water at 80°C before rapidly cooled down to room temperature to obtain again the K2TiF6 precipitate (r2). The obtained K2TiF6 precipitate was dried at 105°C for 2 hours
- (Step 3)
5 g of the K2TiF6 precipitate was dissolved in 500 mL of distilled water at 80°C before slowly adding 4 M NH3 solution (prepared from ammonium hydroxide solution 28%, Sigma-Aldrich) until pH = 9. At the end of the hydrolysis process, the suspension was filtrated and washed on a vacuum filter to collect Ti(OH)4 solid (r3), which was then dried at 105°C for 2 hours before annealing at 550°C for 3 hours to obtain the nanosized TiO2 (denoted as TiO2)
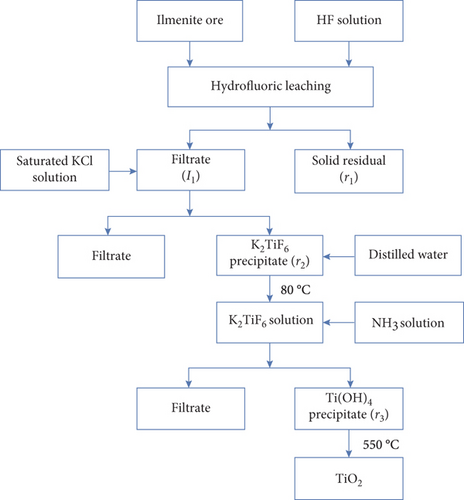
2.2. Synthesis of Nanosized SO42-/TiO2
1 g aboveobtained TiO2 was put into a Teflon flask before adding 150 mL of H2SO4 0.7 M (diluted from H2SO4 99%, Sigma-Aldrich). After that, the mixture was hydrothermalized at 170°C for 24 hours using an autoclave. At the end of the hydrothermal process, the suspension was washed and dried at 105°C for 2 hours, obtaining the nanosized SO42-/TiO2 material.
2.3. Catalyst Characterization
XRD patterns of obtained samples were recorded at room temperature in the 2θ range of 20-70° using a D8 Advance Brucker diffractometer (Cu Kα radiation, λ = 1.540 Å); operated at 40 kV and 0.04 A. The average crystallite size was determined from XRD measurements by applying the Debye–Scheerer equation: [34], to the highest intensity peak (101), which was fitted with Origin software to identify the full-width-at-half-maximum and the peak position.
FT-IR spectra were collected by means of an IRAffinity-1S spectrometer in the spectra range of 4000-400 cm-1. The UV-vis diffuse reflectance spectra were recorded using U4100 UV-Vis-NIR Hitachi.
The specific surface area and porous properties of obtained sample were determined by N2 adsorption-desorption isotherms methods at 77 K (BET) using Micromeritics ASAP 2000.
XPS measurements were carried out on the ESCALab 250 spectrometer (Thermo VG, UK) using Al Kα radiation with the photon energy of 1486.6 eV to identify the element, composition, and oxidation state on the surface of the photocatalyst. A sufficient amount of powder was immobilized on carbon tape before loading into the XPS chamber. Survey scans were acquired using 0.48 eV resolution energy and 0.1 eV/step with charge neutralization. The peaks positions were calibrated according to the C1s peak at 284.6 eV.
Particle size and surface morphology of obtained samples were investigated by SEM and TEM using Nano SEM–450 and JOEL-JEM-1010, respectively. The measurement of photoluminescence (PL) was performed by a spectrometer Horiba FL3-22 over the wavelength of 400–600 nm with a 450 W Xenon lamp (the excitation wavelength of 380 nm).
2.4. Photocatalytic Measurements for SO42-/TiO2
Photodegradation of RhB was performed in order to evaluate the photocatalytic activities of the TiO2 and SO42-/TiO2 photocatalysts. 60 mg SO42-/TiO2 photocatalyst was added into a 250 mL containing 100 mL RhB solution (10 mg/L). The suspension was stirred for 60 minutes in the dark to reach the adsorption-desorption equilibrium before irradiating the mixture by the 60 W LED lamp with a corresponding intensity of 9,500 Lux or the sunlight with the intensity of 65,000 Lux. After that, 2 mL of the solution was taken out every 60 minutes and centrifuged (6000 rpm, 20 minutes) for subsequent measurements. The solution obtained after centrifugation was protected in the dark before determining the concentration of RhB with a UV-Vis spectrometer (UV-1800 Shimazu) by monitoring the absorbance of RhB at 553 nm.
In which, C0 is the initial concentration of RhB before illumination and Ct is the remaining concentration of RhB after each corresponding irradiation time.
3. Results and Discussions
3.1. Determination of Crystal Phase Structure by X-Ray Diffraction
Figure 2 shows the XRD patterns of the TiO2 and SO42-/TiO2 samples. As can be seen in Figure 2, the two XRD patterns exhibit the characteristic diffraction peaks of both the anatase and rutile phases. In more details, the diffraction peaks at 2θ = 25.26, 37.78, 38.56, 48.5, and 53.90° are characteristic peaks for the anatase-type TiO2(JCPDS 21-1272) while the diffraction peaks at 2θ = 27.50 and 53.9° are typical peaks for the rutile-type TiO2(JCPDS 21-1276) and are indexed as (110) and (101).
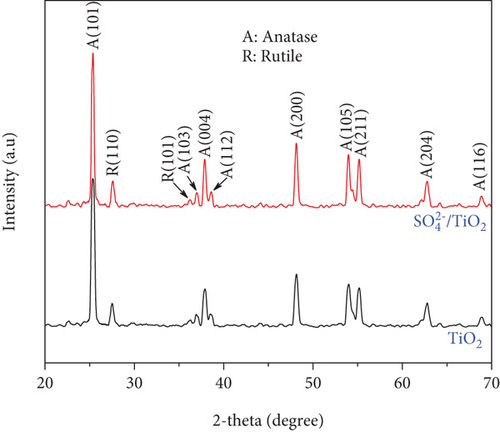
Table 1 shows the average crystallite size, the ratio of the anatase to rutile phase, the crystallinity, the specific surface area, and the RhB photodegradation efficiency for the TiO2 and SO42-/TiO2 samples. As can be seen in Table 1, the ratio of the anatase to rutile phase hardly changes while there is a slight decrease in the crystallinity of the obtained sample after the SO42- doping. This suggests that the hydrothermal process of the TiO2 in H2SO4 at 170°C for 24 hours enhances the crystallinity of the SO42-/TiO2 obtained.
| Sample | Average crystallite size (nm) | Ratio of the anatase to rutile phase | Specific surface area (m2g-1) | RhB photodegradation efficiency (%) | |
|---|---|---|---|---|---|
| After 9 hours of the visible light irradiation | After 2 hours of the sunlight irradiation | ||||
| TiO2 | 19.09 | 83.16/16.84 | 89.4135 | 11.77 | 26.09 |
| SO42-/TiO2 | 22.19 | 82.95/17.05 | 49.9120 | 99.47 | 99.63 |
3.2. Surface Morphology and Porous Properties
The particle size and surface morphology of the TiO2 and SO42-/TiO2 samples were characterized by SEM and TEM. Obtained SEM and TEM images are presented in Figures 3 and 4, respectively.
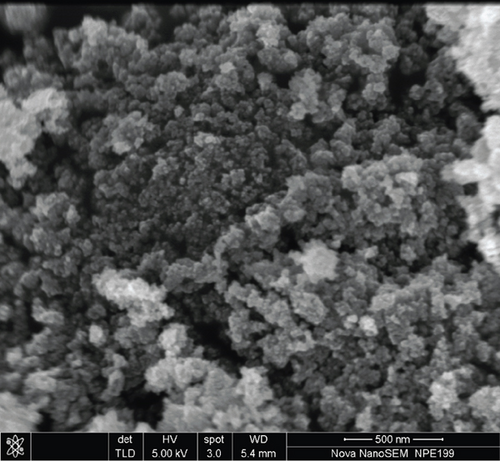
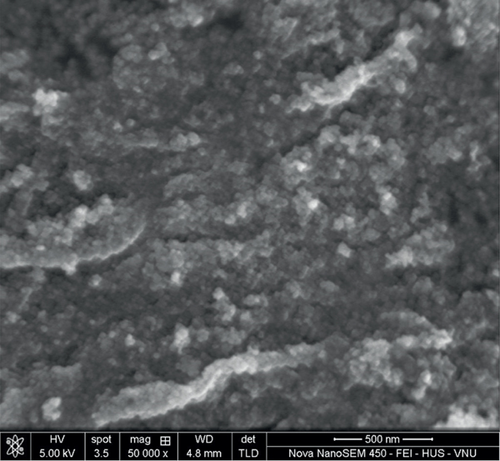


SEM images shown in Figure 3 indicate that all samples exist in the form of granules and are quite uniform with relatively small particle size.
TEM images shown in Figure 4 indicate that the presence of SO42- on the surface of TiO2 not only increases the particle size but also changes the surface morphology of the SO42-/TiO2 sample in comparison with the unmodified TiO2.
It is well known that the photocatalytic degradation process of organic pollutants is affected by their adsorption on the photocatalyst; thus, the impact of the surface area is critical in the entire process. In this present work, the porous properties and specific surface area of the obtained samples were characterized by N2 adsorption-desorption isotherms methods using Brunauer–Emmett–Teller (BET) surface area analysis and the results are shown in Figure 5.
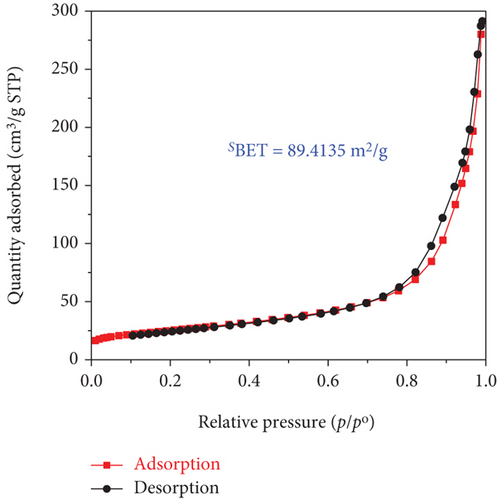
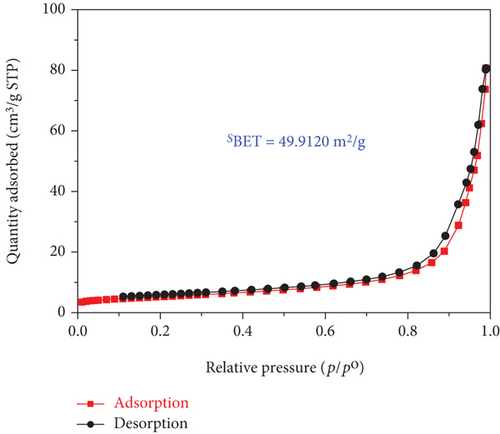
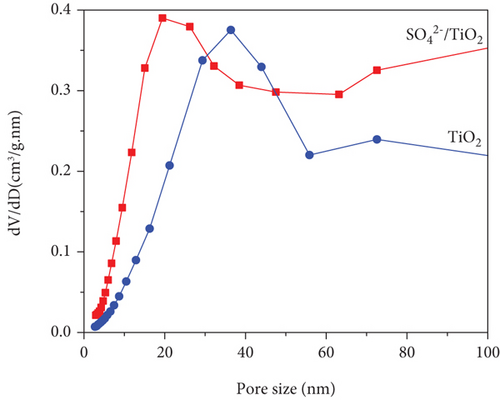
The adsorption-desorption isotherms of both the TiO2 and SO42-/TiO2 samples shown in Figure 5 had characteristic features of the type IV isotherm with the type H3 loop according to IUPAC showing structure of the material samples consist by an assemblage of particles joined together. Indeed, the mall hysteresis loops occur in the area of relative pressure (p/p0 = 0.7-0.98) higher compared with materials of mesoporous construction (their hysteresis loops usually present at relative pressure zones p/p0 = 0.4-0.8). This suggests that the capillary structure inside the particles of the TiO2 and SO42- materials is not dominant (the material particles are in the solid form). In other words, the materials have the dominant particle rather than porosity. The adsorption-desorption isothermal hysteresis loops of the two samples shown in Figure 5 are caused by adsorption-desorption in the interparticle space with dimensions in the range of 20-100 nm as determined by the capillary distribution curves in Figure 6. In addition, the peak in the capillary distribution curve of the SO42-/TiO2 material shifts significantly towards the small capillary size compared with the TiO2 material. This can be explained by the fact that the modification of the TiO2 materials leads to an increase in surface defects and a decrease in surface bond saturation, and thereby decreasing the strength of free particles and increasing the interaction between particles. As the results, the specific surface area of the particles significantly decreases (89.4135 m2/g and 49.9120 m2/g for the TiO2 and SO42-/TiO2 materials, respectively) due to the decrease in the spacing between the particles.
3.3. Optical Absorption Capacity
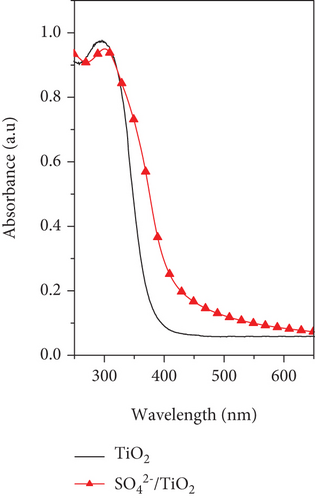
Optical properties of the TiO2 and SO42-/TiO2 samples were investigated by UV-Vis DRS and their UV-Vis DRS spectra are shown in Figure 7(a). It can be seen that the unmodified TiO2 sample only absorbs photons with the wavelength below 400 nm while the modified SO42-/TiO2 sample can absorb photons in the visible light region with the optical absorption edge spreading to about 550 nm. In other words, compared with the TiO2 sample, the SO42-/TiO2 sample has an absorption band shifting to the lower energy region (higher wavelength), thereby probably utilizing more efficiently the visible light.
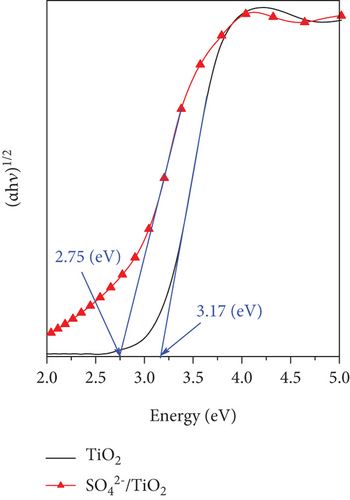
Kubelka–Munk function was employed to estimate the bandgap energy of the TiO2 and SO42-/TiO2 samples by plotting (αhν)1/2 as a function of the phonon energy (Figure 7(b)). The bandgap energies of the TiO2 and SO42-/TiO2 samples are 3.17 and 2.75 eV, respectively. Thus, when modification of TiO2 by sulfur in the form of sulfate ions, the bandgap energy of the obtained sample has been significantly reduced. This is in a good agreement with previous reports [36, 37]. The absorption in the visible light region of the SO42-/TiO2 sample is enhanced compared with the undoped TiO2 sample may be attributed to the formation of an intermediate energy level below the conduction band of TiO2 after the SO42- doping [38].
3.4. Surface Chemical State of Samples
Bonding characteristics in the obtained samples were characterized by FT-IR and FT-IR spectra are shown in Figure 8.
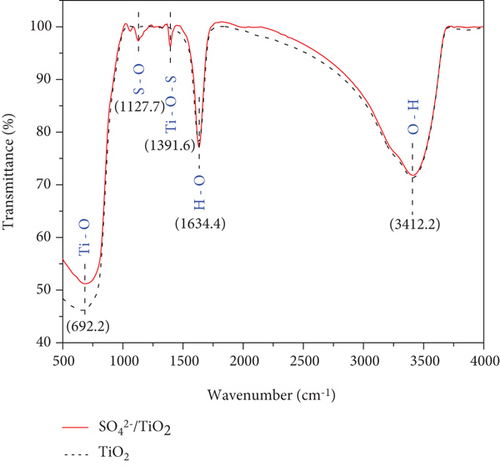
The FT-IR spectra for both the TiO2 and SO42-/TiO2 samples appear modes of oscillation originating from the OH-stretching (around 1634.4 cm-1), the OH-bending of adsorbed water (around 3412.2 cm-1) [39, 40], and tetrahedral/octahedral TiO-stretching (around 692.2 cm-1). However, coupled with these peaks, the SO42-/TiO2 sample exhibits two additional peaks at 1391.6 and 1127.7 cm-1 which are attributed to the characteristic modes of ocisllation of the SO42- with bidentate bond [41, 42]. This suggests that there is the chemical adsorption or penetration of SO42- ions into the crystal lattice of TiO2.
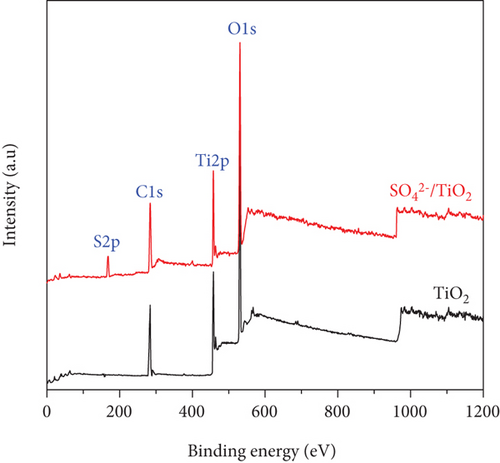
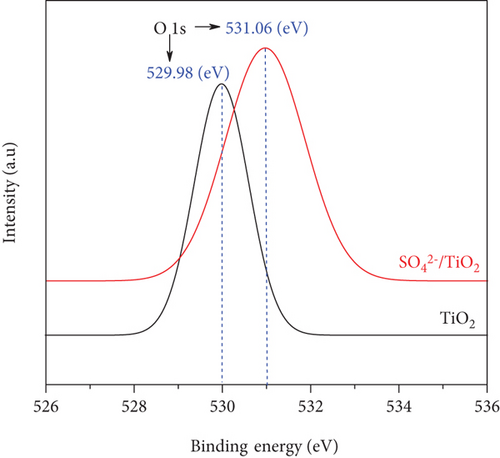
The chemical states of the TiO2 and SO42-/TiO2 surfaces were investigated by XPS. Figure 9(a) presents XPS survey spectra of the TiO2 and SO42-/TiO2 samples. Compared with the XPS survey spectrum of the TiO2 sample, that of the SO42-/TiO2 sample shows an additional peak of S2p, indicating the presence of sulfur in the modified sample. The XPS survey spectra of both samples show a strong peak of C1s at 284.6 eV corresponding to the elemental carbon (graphite) from the substrate. Figure 9(b) shows the XPS O1s core level for the TiO2 and SO42-/TiO2 samples. As can be seen, the characteristic peaks are at 529.98 and 531.06 eV of O1s in TiO2 and SO42-/TiO2, respectively. The XPS Ti2p core level shown in Figure 9(c) has two characteristic peaks at around 458.2 eV for Ti2p3/2 and 464 eV for Ti2p1/2, confirming that the valence state of titanium exists in Ti4+ form. Figure 9(d) shows the S2p doublet core peak locating at 167.06 eV for S2p1/2 and 168.28 eV for S2p3/2, indicating that the valence states of sulfur are S4+ (in Ti-O-S bonding) and S6+ (in SO42-), respectively, [42]. Hence, the presence of SO42- ions on the surface of TiO2 did not significantly affect the peak position at Ti2p but greatly affected the binding energy of O1s and S2p. It is noted that the binding energies of Ti2p3/2 and O1s in pure TiO2 are 458.5 and 530.28 eV, respectively, and the binding energy of S2p3/2 in pure SO42- is 169 eV [43]. The above results show that there is an upward shift in the binding energy of O1s and a downward shift in the binding energy of S2p3/2 (about 0.7 eV) after the SO42- doping. This can be explained by the fact that a part of the electron is transferred from oxygen to sulfur [44], resulting in a change in the valence state of a part S6+ to S4+ and the formation of a new band in energy structure [37].
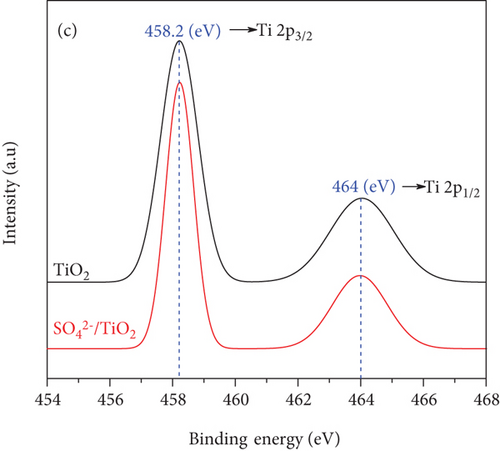
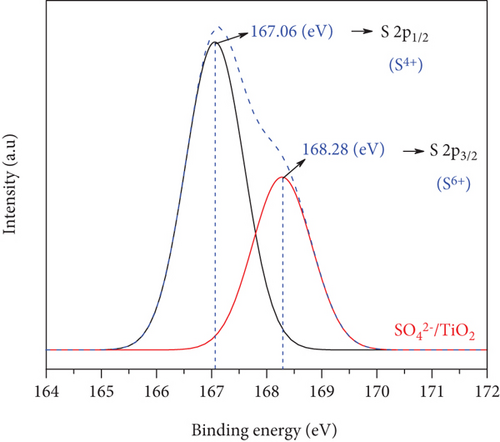
The XPS spectra demonstrate the formation of bonds among titanium, oxygen, and sulfur atoms. This result is also consistent with the perception of the connection between the elements shown on the FT-IR spectra in Figure 8.
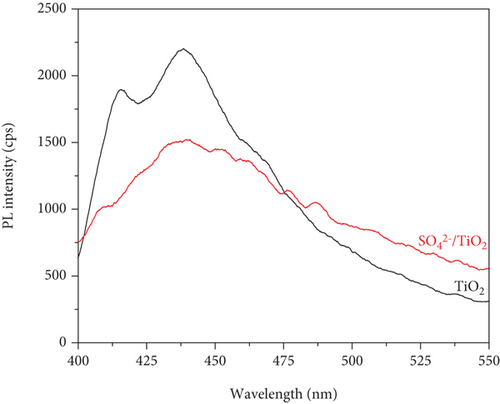
PL measurements were conducted to evaluate the recombination rate of the photoinduced electrons and holes of the obtained samples. As can be seen shown in Figure 10, the luminescent intensity of the SO42-/TiO2 sample is lower than that of the TiO2 sample, indicating a different electron-hole recombination path. This may due to the fact that doping SO42- on the surface of TiO2 is suitable for trapping photoinduced electrons and thereby suppressing the recombination of photoinduced elontrons and holes [38]. In a nutshell, the SO42- doping on TiO2 improves the charge separation efficiency and consequently promotes the formation of oxidizing agents such as hydroxyl radical, which may increase the photocatalytic activity of the SO42-/TiO2 sample.
3.5. Photocatalytic Degradation
Evaluation of RhB photodegradation was conducted as mentioned in Section 2.4 and the RhB photocatalytic degradation of the blank sample, TiO2, and SO42-/TiO2 under the irradiation of the 60 W LED lamp and the sunlight is shown in Figure 11. It is noted that the C/C0 as a function of irradiation time for the blank sample hardly changes under both the visible light and sunlight irradiation, indicating that the presence of TiO2 and SO42-/TiO2 plays a major role in the RhB photodegradation.
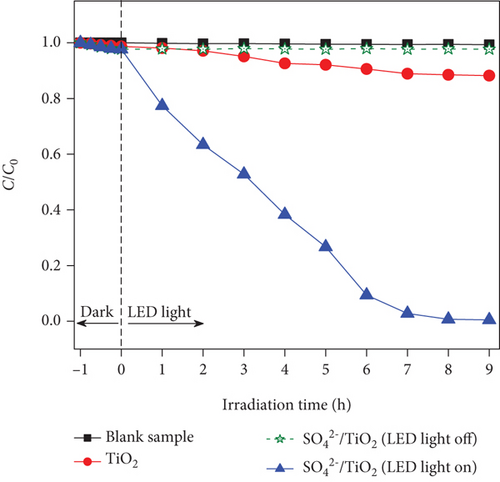
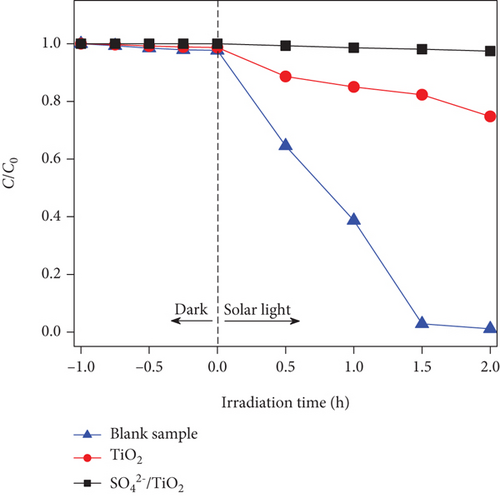
UV-Vis molecular absorption spectra of RhB solution as a function of irradiation time shown in Figure 12 indicate that RhB was completely decomposed in the visible light region when the SO42-/TiO2 sample was used as a photocatalyst. Indeed, when continuously irradiating the reaction mixture between 01 and 09 hours by a 60 W LED lamp, the RhB photodegradation efficiency increases from 15.65 to 99.47% (Figure 11(a)). It is worth noting that the improvement of the photocatalytic activies over the SO42-/TiO2 sample due to electrostatic interaction between negatively charge sulphate titania and rhodamine B can be excluded since the C/C0 in the case of the SO42-/TiO2 presence hardly change if the LED light was turned off (Figure 11(a)).
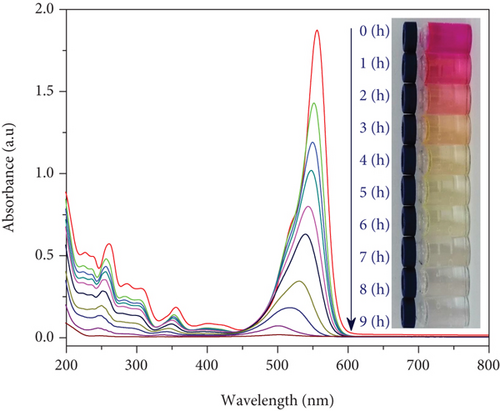
The results from Figure 12 show that the absorption peak position of RhB at 553 nm is gradually shifted towards short wavelengths. The shift of absorption peaks during the photodegradation process of RhB solution can be explained by the fact that the RhB decomposition process will form a series of intermediates with a shorter conjugate π. The process of destroying the chromophore cleavage structure of the dye molecule is quite easy by dividing the conjugate π system into π shorter aromatic, homologous phenol intermediate products, etc. so that RhB will eventually be completely decomposed into CO2 and H2O [45].
When using the sunlight as a light source (intensity of 65,000 Lux), the RhB photodegradation efficiency for the SO42-/TiO2 significantly increases. UV-Vis absorption spectra for the RhB photodegradation process of the SO42-/TiO2 sample presented in Figure 13(a) reveal that after 2 hours of irradiation by the sunlight, the absorption peaks of RhB in both the visible and ultraviolet regions are almost no longer detected. It is worth noting that the RhB photodegradation efficiency for the SO42-/TiO2 sample is about 99.63% while that for the TiO2-550 sample is only 26.09% (Table 1). Hence, the SO42- doping enhances the photocatalytic activities of the obtained sample.
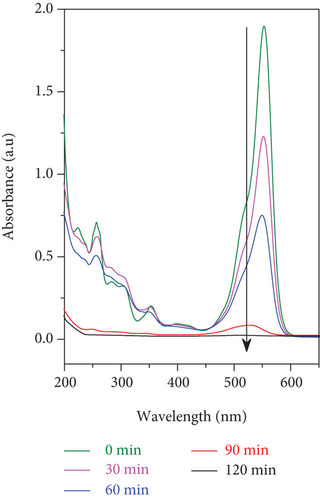
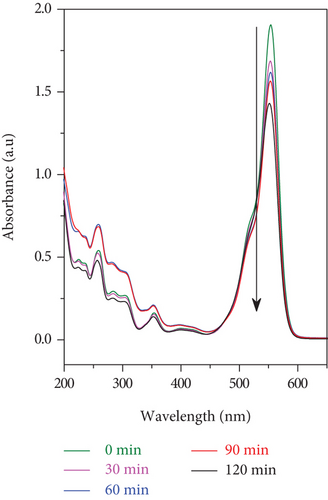
The photocatalytic ability of the SO42-/TiO2 sample when stimulated by the sunlight is much better than that of the TiO2 sample may be ascribed to two factors. Firstly, the TiO2 nondenatured sample only adsorbs photons in the ultraviolet light region while the SO42-/TiO2 sample is capable of absorbing photons of light in both ultraviolet and visible regions. Since the radiation from the sunlight contains only about 5% of UV rays, so the SO42-/TiO2 sample will have better optical absorption performance and thereby exhibiting higher photocatalytic activity compared with the TiO2 sample. Secondly, SO42- ions doped on the surface of the TiO2 have a strong electron affinity and thereby hindering the electron-hole recombination by capturing the photoinduced electrons [38]. This is in good agreement with the results of the PL spectra.
It is found that RhB was almost completely decomposed in the presence of the SO42-/TiO2 sample as a photocatalyst and either the sunlight (Figure 13(a)) or the 60 W LED lamp (Figure 13) was used as an irradiation light source. However, the processing time for the RhB photodegradation of the SO42-/TiO2 sample shortens when illuminated with the sunlight rather than the 60 W LED lamp (2 and 9 hours, respectively). This is due to the fact that in the experimental conditions, the sunlight has a higher illumination than incandescent light sources. Therefore, under the irradiation of the sunlight, the SO42-/TiO2 sample absorbs both visible and UV energies to catalyze the reaction.
All things considered, the SO42- doping in the TiO2 narrows the bandgap and reduces the recombination of the photoinduced electrons and holes, thereby making the SO42-/TiO2 sample such a promising candidate for the photodegradation of RhB solution under the illumination of both the visible light and sunlight.
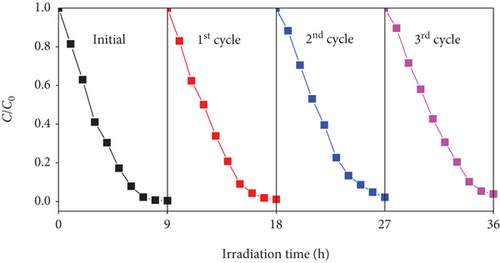
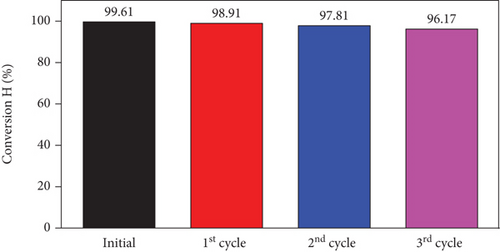
Reusability plays a pivotal role in choosing a photocatalyst for practical applications. The used SO42-/TiO2 material was washed with distilled water at least three times before dried at 80° C for 12 hours for regeneration. The photodegradation efficiency of RhB over reused photocatalyst is presented in Figure 14. This result shows a slight decrease in the RhB decomposition efficiency as a function of recycling cycle. However, the RhB photodegradation efficiency still reached over 96.0% after three cycles of recycling. The XRD pattern of the SO42-/TiO2 material (Figure 15) hardly changes, suggesting that the SO42-/TiO2 material possesses excellent structural stability after three cycles of recycling.

4. Conclusions
Nanosized SO42-/TiO2 nanoparticles have been successfully synthesized from TiO2 (prepared from Binh Dinh ilmenite ore) and characterized by modern chemical-physical methods. The experimental results indicate that the formation of Ti-O-S and the chemical adsorption of SO42- ions on the TiO2 surface have shifted the optical absorption band of the obtained sample into the visible light region with the bandgap energy (Eg) of 2.75 eV. With the purpose of manufacturing samples with high photocatalytic activities in the visible light region, the nanosized SO42-/TiO2 sample is considered as a highly promising candidate for the RhB photodegradation owing to its high photodegradation efficiency under the illumination of both the visible light and sunlight. It is noted that the time treatment is greatly reduced when using the sunlight as an illumination source rather than the 60 W LED lamp.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This research is sponsored by Vietnamese Ministry of Education and Training under the project with code number B2019-DQN-13.
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.




