Diarylalkanoids as Potent Tyrosinase Inhibitors from the Stems of Semecarpus caudata
Abstract
From a CHCl3-soluble extract of the stems of Semecarpus caudata (Anacardiaceae), two new diarylalkanoids, semedienone (1) and semetrienone (2), were isolated. Their structures were elucidated based on NMR spectroscopic data interpretation. These compounds possess strong tyrosinase inhibitory activity with the IC50 values of 0.033 and 0.11 μM, respectively. Docking studies of 1 and 2 with oxy-tyrosinase were carried out to analyze their interactions. Accordingly, semedienone (1) showed good interactions with the peroxide group and amino acid residues. The biosynthesis of the isolated diarylalkanoids was proposed.
1. Introduction
Melanin is a pigment that is essential for protecting human skin against UV radiation. However, the abnormal accumulation of melanin induced skin pigmentation disorders. Melanogenesis is a complex process to produce melanin under control of tyrosinase. Tyrosinase (EC 1.14.18.1) is a binuclear copper-containing monooxygenase, which catalyzes the oxidation of phenol to the corresponding o-quinone [1,2]. Tyrosinase is the main factor causing some dermatological diseases including freckles, age spots, and melasma. Hydroquinone, arbutin, kojic acid, azelaic acid, L-ascorbic acid, ellagic acid, and tranexamic acid are commercial tyrosinase inhibitors, which have been used as skin-whitening agents, but these compounds have certain drawbacks [3]. Thus, the finding of new efficient and safe antityrosinase agents is necessary for anti-hyperpigmentation drug development.
A previous study on the chemical constituents of Semecarpus caudata (Anacardiaceae), collected at Dong Nai Province in Vietnam, led to the isolation of six flavonoid derivatives and the evaluation of their tyrosinase inhibitory activity [4]. Our continued phytochemical study on the stems of S. caudata was carried out, leading to the isolation of seven compounds (1–7) including two new diarylalkanoids named semedienone (1) and semetrienone (2). These compounds were found to possess tyrosinase inhibitory activity. Semedienone (1) showed a strong effect with an IC50 value of 0.033 μM, which makes it 1300 times more potent than that of kojic acid (IC50, 44.6 μM). In addition, molecular docking studies of 1 and 2 with the oxy-form of the copper-bound Streptomyces castaneoglobisporus tyrosinase were performed.
2. Materials and Methods
2.1. General Experimental Procedures
Optical values were measured on a Shimadzu UV-1800 spectrophotometer (Shimadzu Pte., Ltd., Singapore). IR spectra were measured with a Shimadzu IR-408 infrared spectrometer (Shimadzu Pte., Ltd., Singapore). NMR spectra were acquired on a Bruker Avance III 500 spectrometer (Bruker BioSpin AG, Bangkok, Thailand). Chemical shifts are expressed as δ values. HRESIMS data were acquired on Bruker micrOTOF-QII mass spectrometer (Bruker Singapore Pte., Ltd., Singapore). Column chromatography was carried out using silica gel 60, 0.06–0.2 mm (Scharlau, Barcelona, Spain) and LiChroprep RP-18, 40−63 μm (Merck KGaA, Darmstadt, Germany). Kieselgel 60 F254 or RP-18 F254 plates for TLC were purchased from Merck (Merck KGaA, Darmstadt, Germany). Tyrosinase (EC 1.14.18.1) from mushroom (3933 U·mL−1) and L-dihydroxyphenylalanine (l-DOPA) were obtained from Sigma-Aldrich (Sigma-Aldrich Pte., Ltd., Singapore). Other chemicals were of the highest grade available.
2.2. Plant Material
The stems of Semecarpus caudata were collected in the Ma Da Forest, Dong Nai Culture and Nature Reserve, Dong Nai Province, Vietnam, in April 2014. The plant was identified by Assoc. Prof. Dr. Hop Tran, Institute of Tropical Biology, Ho Chi Minh City, Vietnam. A voucher sample (MCE0002) has been deposited at the Division of Medicinal Chemistry, Faculty of Chemistry, University of Science, Ho Chi Minh City, Vietnam.
2.3. Extraction and Isolation
The dried powdered stems of S. caudata (7.0 kg) were exhaustively extracted in a Soxhlet extractor with MeOH (20 L, 3 h × 3) to yield MeOH-soluble extract (700 g). This extract was suspended in H2O (5 L) and successively partitioned with n-hexane (2 L) and CHCl3 (3 L) to give n-hexane (37 g)- and CHCl3 (500 g)-soluble fractions. The CHCl3-soluble fraction was chromatographed by silica gel column chromatography (15 × 150 cm) and eluted with EtOAc-n-hexane (0 : 100 ⟶ 100 : 0) and MeOH-CHCl3 (0 : 100 ⟶ 20 : 80) to afford 12 fractions (Fr.1−Fr.12). Fraction Fr.3 (4.5 g) was subjected to further silica gel column chromatography and was eluted with EtOAc-n-hexane (0–100%) to yield 4 subfractions (Fr.3.1−Fr.3.4). Subfractions Fr.3.2 (1.1 g) and Fr.3.3 (540 mg) were chromatographed over a silica gel column with EtOAc-n-hexane (0–100%) and purified by preparative TLC with EtOAc-n-hexane (20 : 80) and EtOAc-CHCl3 (10 : 90) to afford 5 (5.0 mg) and 7 (6.0 mg), respectively. Fraction Fr.5 (5.2 g) was separated by silica gel column chromatography with EtOAc-n-hexane (0–100%) and MeOH-CHCl3 (0–20%) to yield 5 subfractions (Fr.5.1−Fr.5.5). Subfraction Fr.5.3 (850 mg) was subjected to further silica gel column chromatography, eluted with Me2CO-CHCl3 (0–80%) to give 4 (5.0 mg). Fraction Fr.6 (0.9 g) was separated by normal-phase chromatography with EtOAc-n-hexane (0 : 100 ⟶ 80 : 20) and MeOH-CHCl3 (0 : 100 ⟶ 5 : 95) and reversed phase chromatography with H2O-MeOH (0–100%) and then purified by preparative TLC with AcOH-EtOAc-PhMe (4 : 16 : 80) to obtain 3 (4.0 mg) and 6 (4.0 mg). Fraction Fr.8 (4.5 g) was loaded onto a silica gel column and eluted with CHCl3-Me2CO (0–80%) and CHCl3-MeOH (0–20%) to yield 5 subfractions (Fr.8.1–Fr.8.5). Subfraction Fr.8.2 (630 mg) was further purified using a silica gel column with EtOAc-CHCl3 (0–80%) and preparative TLC with MeOH-CHCl3 (5 : 95) to give 1 (2.0 mg) and 2 (2.0 mg).
2.3.1. Semedienone (1)
Yellow, amorphous solid; IR vmax (CHCl3): 3455, 1620, 1485, 1250, 1091 cm−1; 1H and 13C NMR (500 MHz, acetone-d6, see Table 1); HRESIMS m/z 321.0752 [M + Na]+ (calcd. for C17H14O5Na, 321.0739).
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC, type C | δH (J, Hz) | δC, type C | δH (J, Hz) | |
| 1′ | 114.5, C | 114.9, C | ||
| 2′ | 167.6, C | 167.4, C | ||
| 3′ | 103.8, CH | 6.35, d (2.4) | 103.8, CH | 6.35, d (2.4) |
| 4′ | 165.5, C | 165.9, C | ||
| 5′ | 108.7, CH | 6.45, dd (8.9, 2.4) | 108.8, CH | 6.45, dd (8.9, 2.4) |
| 6′ | 132.9, CH | 7.94, d (8.9) | 132.6, CH | 7.92, d (8.9) |
| C=O | 192.8, C | 192.6, C | ||
| α | 122.1, CH | 7.27, d (14.5) | 122.8, CH | 7.26, d (14.5) |
| β | 147.3, CH | 7.68, dd (14.5, 11.3) | 145.8, CH | 7.61, dd (14.5, 11.5) |
| γ | 125.0, CH | 7.17, dd (15.6, 11.3) | 129.4, CH | 6.62, dd (13.7, 11.5) |
| δ | 139.5, CH | 7.35, d (15.6) | 145.8, CH | 7.00, m |
| ε | 126.1, CH | 7.00, m | ||
| ζ | 134.3, CH | 7.13, d (14.6) | ||
| 1 | 116.5, C | 116.6, C | ||
| 2 | 158.5, C | 157.8, C | ||
| 3 | 103.7, CH | 6.47, d (2.4) | 103.7, CH | 6.45, d (2.4) |
| 4 | 161.0, C | 161.0, C | ||
| 5 | 108.9, CH | 6.42, dd (8.5, 2.4) | 108.8, CH | 6.39, dd (8.5, 2.4) |
| 6 | 130.2, CH | 7.43, d (8.5) | 129.3, CH | 7.39, d (8.5) |
| OH-2′ | 13.68, s | 13.64, s | ||
2.3.2. Semetrienone (2)
Yellow, amorphous solid; IR vmax (CHCl3): 3305, 1650, 1430, 1245, 1085 cm−1; 1H and 13C NMR (500 MHz, acetone-d6, see Table 1); HRESIMS m/z 347.0902 [M + Na]+ (calcd. for C19H16O5Na, 347.0895).
2.4. Synthesis of 2,4,2′,4′-Tetrahydroxychalcone (8)
2,4-Dihydroxybenzaldehyde (276.3 mg, 2.0 mmol) and 2ʹ,4ʹ-dihydroxyacetophenone (152.1 mg, 1.0 mmol) were dissolved in 1 mL·H2O, and then 1 mL KOH 14 M was added. The resulting mixture was kept in the ultrasonic water bath at 80°C for 8 h. This reaction was monitored by TLC using MeOH-CHCl3 (1 : 9) mixture. After completion, the reaction mixture was quenched by acidification with HCl 3 M to pH ∼5 and cooled to 0°C to precipitate crude product, which was recrystallized with MeOH-H2O (1 : 3) mixture to afford pure chalcone. It was identified as 2,4,2′,4′-tetrahydroxychalcone (8), by comparison with authentic sample.
2.5. Tyrosinase Inhibitory Assay
All pure compounds were dissolved in DMSO and tested at concentrations ranging from 0.01 to 100 μM. Assay mixtures in 0.1 M phosphate buffer pH 6.8 were prepared immediately before use, consisting of 100 μL of tyrosinase solution (15 U/mL) and 1900 μL of test solution. These mixtures were preincubated at room temperature for 30 min, followed by addition of 1000 μL of l-DOPA 1.5 mM in pH 6.8 phosphate buffer and incubated at room temperature for 7 min. The absorbances (A) at 475 nm were acquired on Shimadzu UV-1800 spectrophotometer. The inhibitory percentage (I%) was calculated according to the formula: I% = ((Acontrol − Asample)/Acontrol) × 100%. Data were represented as means ± standard error (n = 3). The IC50 values were determined by using GraphPad Prism software with multivariate nonlinear regression and R2 > 0.9. Kojic acid was used as positive control.
2.6. Molecular Docking
Docking studies of 1, 2, 8, and the positive reference (kojic acid) were performed with Molecular Operating Environment 2016 (MOE 2016.0802) suite. The structures of these compounds were constructed by using the Builder module. Subsequently, all compounds were minimized up to 0.0001 gradients using the Amber12 : EHT force field. The crystal structure of the oxy-tyrosinase was taken from the Protein Data Bank (PDB code 1WX2). The caddie protein (ORF378) and water molecules were removed. The enzyme structure was prepared using the QuickPrep module. The binding site was determined based on the PLB (Propensity for Ligand Binding) score in the Site Finder module. The molecular docking was performed by Dock module, using Triangle Matcher placement, Induced Fit refinement, London dG, and GBVI/WSA dG scoring methods. Five top poses showed up based on the negative binding free energy value (S value). The best pose was selected to analyze the receptor-ligand interactions by using BIOVIA Discovery Studio Visualizer 2016.
3. Results and Discussion
3.1. Extraction and Isolation
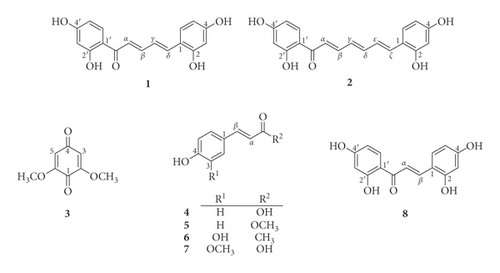
The dried powdered stems of S. caudata were exhaustively extracted in a Soxhlet extractor with MeOH to yield MeOH-soluble extract (700 g). This extract was successively partitioned to give the n-hexane (37 g)- and CHCl3 (500 g)-soluble fractions. The CHCl3-soluble extract of stems of S. caudata was repeatedly chromatographed using silica gel CC and preparative TLC to obtain seven compounds including two new diarylalkanoids named semedienone (1) and semetrienone (2). The known compounds were identified as 2,6-dimethoxybenzoquinone (3) [5], p-coumaric acid (4) [6], methyl p-coumarate (5) [7], trans-4-(3,4-dihydroxyphenyl)but-3-en-2-one (6) [8], and ferulic acid (7) [9] (Figure 1).
3.2. Structural Elucidation of Two New Isolated Compounds from S. caudata
Compound 1 showed a molecular formula to be C17H14O5 based on the HRESIMS ion at m/z 321.0752 [M + Na]+ (calcd. for C17H14O5Na, 321.0739). The IR spectrum exhibited the presence of hydroxy (3455 cm−1) and carbonyl (1620 cm−1) functionalities. The 1H NMR spectrum showed signals for two 1,2,4-trisubstituted aromatic rings (δH 7.94 (d, J = 8.9 Hz, H-6′), 6.45 (dd, J = 8.9, 2.4 Hz, H-5′), 6.35 (d, J = 2.4 Hz, H-3′), 7.43 (d, J = 8.5 Hz, H-6), 6.47 (d, J = 2.4 Hz, H-3), and 6.42 (dd, J = 8.5, 2.4 Hz, H-5)), four olefinic protons (δH 7.27 (d, J = 14.5 Hz, H-α), 7.68 (dd, J = 14.5, 11.3 Hz, H-β), 7.17 (dd, J = 15.6, 11.3 Hz, H-γ), and 7.35 (d, J = 15.6 Hz, H-δ)), and a distinctive signal of a hydrogen-bonded hydroxy group (δH 13.68). The 13C NMR data (Table 1) exhibited resonances for a keto-carbonyl (δC 192.8), twelve aromatic carbons (δC 103.7–167.6), and four olefinic carbons (δC 122.1 (C-α), 147.3 (C-β), 125.0 (C-γ), and 139.5 (C-δ)). These were characteristic of those reported for (2E,4E)-1,5-diarylpenta-2,4-dien-1-one [10,11]. The HMBC correlations (Figure 2) from OH-2′ to C-1′ and C-3′, from H-3′ to C-1′ and C-4′, from H-5′ to C-1′, from H-6′ to C-2′ and C-4′, from H-3 to C-1, C-2, and C-4, from H-5 to C-1, and from H-6 to C-2 and C-4 indicated that four hydroxy groups were located at C-2′, C-4′, C-2, and C-4. Moreover, the HMBC correlations from H-6′, H-α, and H-β to C=O, from H-α to C-γ, from H-β to C-δ, from H-γ to C-α, from H-γ to C-β and C-1, and from H-δ to C-β and C-6 suggested the presence of the α,β,γ,δ-unsaturated carbonyl moiety in 1. The NOESY correlations between H-5′ and H-6′, H-6′ and H-α, H-β and H-δ, H-γ and H-6, and H-6 and H-5 indicated the relative configuration of 1 as shown in Figure 2. Thus, the structure of semedienone (1) was concluded as 2E,4E-1,5-bis(2,4-dihydroxyphenyl)penta-2,4-dien-1-one.

Compound 2 showed the HRESIMS ion at m/z 347.0902 [M + Na]+ (calcd. for C19H16O5Na, 347.0895). Its IR spectrum showed absorption bands for hydroxy (3305 cm−1) and carbonyl (1650 cm−1) groups. The 1H and 13C NMR spectra of 2 (Table 1) showed signals for two 1,2,4-trisubstituted aromatic rings, which resembled those of 1. Compound 2 showed the presence of six olefinic protons (δH 7.26 (d, J = 14.5 Hz, H-α), 7.61 (dd, J = 14.5, 11.5 Hz, H-β), 6.62 (dd, J = 13.7, 11.5 Hz, H-γ), 7.00 (2H, m, H-δ and H-ε), and 7.13 (d, J = 14.6 Hz, H-ζ)) in the 1H NMR spectrum, and six olefinic carbons (δC 122.8 (C-α), 145.8 (C-β), 129.4 (C-γ), 145.8 (C-δ), 126.1 (C-ε), 134.3 (C-ζ)) in the 13C NMR spectrum. These were characteristic of those reported for (2E,4E,6E)-1,7-diarylhepta-2,4,6-trien-1-one [12]. The locations of four hydroxy groups were assigned at C-2′, C-4′, C-2, and C-4 by the observed HMBC correlations (Figure 1). Moreover, the HMBC correlations from H-6′, H-α, and H-β to C=O, from H-α to C-γ, from H-γ to C-β, from H-δ to C-ζ, from H-ε to C-ζ and C-1, and from H-ζ to C-δ and C-ε suggested the presence of the α,β,γ,δ,ε,ζ-unsaturated carbonyl moiety in 2. The relative configuration of 2 was deduced based on the NOESY correlations between H-5′ and H-6′, H-6′ and H-α, H-α and H-γ, H-β and H-δ, H-γ and H-ε, H-ε and H-6, and H-6 and H-5 (Figure 2). Thus, the structure of semetrienone (2) was established as 2E,4E,6E-1,7-bis(2,4-dihydroxyphenyl)hepta-2,4,6-trien-1-one.
3.3. Tyrosinase Inhibitory Activity of Isolated Compounds from S. caudata
Compounds (1–7) were tested for their tyrosinase inhibitory activity [13]. Kojic acid, a purported skin-lightening agent, was used as a positive control. 2,4,2′,4′-Tetrahydroxychalcone (8), which was synthesized following our previous procedure [14], showed potent activity with an IC50 value of 0.016 μM (Table 2). Semedienone (1) and semetrienone (2) exhibited remarkable inhibitory effect with the IC50 values of 0.033 and 0.11 μM, respectively, more potent than that of kojic acid (IC50, 44.6 μM). Additionally, compounds 4 and 6 were found to possess tyrosinase inhibitory activity with the IC50 values of 2.35 and 27.0 μM, respectively.
| Compound | IC50 (μM) |
|---|---|
| 1 | 0.033 |
| 2 | 0.11 |
| 3 | >100 |
| 4 | 2.35 |
| 5 | >100 |
| 6 | 27.0 |
| 7 | >100 |
| 8 | 0.016 |
| Kojic acid a | 44.6 |
- aPositive control.
The presence of α,β-unsaturated hydroxycarbonyl groups in cinnamic acid derivatives were found to enhance activity (2 ≫ 3). Additionally, the occurrence of a C-3 methoxy group decreased the inhibitory activity (2 ≫ 5) [15,16]. Diarylalkanoids with 2,4-disubstituted resorcinol subunit on ring B contributed the most to inhibitory activity [17]. Moreover, the length of the conjugated carbon chain in diarylalkanoids led to a change of activity (8 > 1 > 2). This result reaffirmed the (Z)-β-phenyl-α,β-unsaturated carbonyl scaffold plays an important role for tyrosinase inhibition [18,19]. In previous reports, diarylpentanoids such as diarylpentadiene-3-one were not significantly inhibiting tyrosinase activity [20], but some analogues showed moderate antimelanogenesis activity [21]. Some cyclic diarylheptanoids were found to have melanogenesis-inhibitory activity [22]. In this regard, semedienone (1) and semetrienone (2) could be the potent structural templates for developing new skin-lightening agents.
3.4. Docking Study of the Active Compounds 1, 2, and 8
Tyrosinase has four possible oxidation states (deoxy-, oxy-, met-, and deact-form) [23]. Met-tyrosinase, having a hydroxy and the two Cu2+ ions in the binding site, is responsible for the oxidation of catechols. In this oxidizing process, met-tyrosinase is reduced to deoxy-tyrosinase which rapidly binds dioxygen to give oxy-tyrosinase form. Oxy-tyrosinase, which is the primary form of the enzyme, oxidizes both phenols and catechols to o-quinones by the monooxygenase and oxidase mechanisms, respectively. In the active site of oxy-tyrosinase, two bound Cu2+ ions and the peroxide group play a catalytic oxidation role. Mushroom tyrosinase (EC 1.14.18.1), which was used in the inhibitory assay, plays the same role with respect to oxy-tyrosinase form. Thus, in this study, the molecular docking studies of 1, 2, and 8, respectively, with oxy-tyrosinase (PDB ID : 1WX2) [24] were carried out to explore their interactions and inhibition mechanisms.
In molecular docking study, the imperfect scoring results (false-positive hits), which may be considered as decoys, can be occurred by predicting incorrect ligand geometries or by applying nonbinding molecules. The active and decoy ligands are similar according to some physicochemical properties (molecular weight, number of rotational bonds, total hydrogen bond donors, total hydrogen bond acceptors, topological polar surface area, and the octanol-water partition coefficient), but decoy was presumed to be inactive against a target. According to Choi et al. [25], kojic acid and hypoxanthine showed the tyrosinase inhibitory constant (Ki) values of 13 μM and >1000 μM, respectively [25]. Thus, in this docking study, kojic acid and hypoxanthine was selected as the active inhibitor and the decoy molecule, respectively, to validate our docking protocol.
The docking studies were performed with Molecular Operating Environment 2016 (MOE 2016.0802) suite [26]. The top-ranked pose with the highest negative binding free energy value (S value) was selected for further interaction analysis with BIOVIA Discovery Studio Visualizer 2016 [27].
Compounds 1, 2, and 8 showed an H-donor interaction between a hydroxy group and a peroxide bridge PER404, presenting the distances of 1.85, 1.88, and 1.78 Å, respectively, whereas kojic acid showed the interactions with a Cu2+ ion, HIS194, and THR203 residues (Figure 3). In the binding pocket, compounds 1 and 8 showed more interactions with targeting residues than those of 2 (Table 3). These analysis results were consistent with their experimental inhibitory activities (8 > 1 > 2). The C-2 hydroxy group of 1 exhibited H-bonding interactions with ASN191 and GLY183 residues. Moreover, the aromatic ring A of 1 formed π-π T-shaped and π-σ interactions with TRP184 and ILE42 residues, respectively. Compound 2 showed an H-acceptor interaction between C=O group and ASN188 residue. In addition, the aromatic ring B of 2 interacted with HIS194 residue via a π-π stacking interaction. Thus, the S values and these interactions suggested that 1 and 2 showed high binding affinity for oxy-tyrosinase than those of kojic acid. Hypoxanthine showed the lower negative S value and the longer-distance interactions than that of kojic acid. Apparently, these results could be used to validate the abovementioned docking procedure in this study.
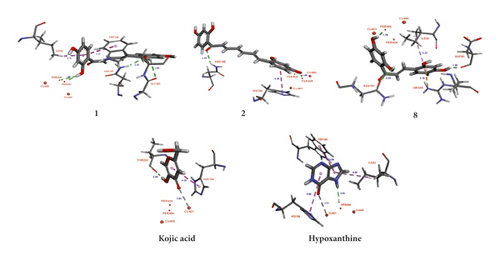
| Compound | oxy-tyrosinase (1WX2) | |||
|---|---|---|---|---|
| S values | Interactions | Targeting residues | Distance (Å) | |
| 1 | –5.75 | H-donor | PER404 | 1.85 |
| ASN191 | 2.25 | |||
| GLY183 | 2.85 | |||
| π-σ | ILE42 | 2.75 | ||
| π-π | TRP184 | 5.17 | ||
| 2 | –6.37 | H-donor | PER404 | 1.88 |
| H-acceptor | ASN188 | 2.30 | ||
| π-π | HIS194 | 4.30 | ||
| 8 | –5.54 | H-donor | PER404 | 1.78 |
| ASP45 | 1.96 | |||
| ASN191 | 2.83 | |||
| π-alkyl | ILE42 | 5.22 | ||
| π-cation | ARG55 | 3.10 | ||
| Kojic acid a | –4.50 | H-donor | THR203 | 2.04 |
| Metal-acceptor | CU401 | 2.92 | ||
| π-π | HIS194 | 4.30 | ||
| Hypoxanthine b | –4.34 | H-donor | PER404 | 2.05 |
| Metal-acceptor | CU401 | 3.11 | ||
| π-π | HIS194 | 5.40 | ||
| TRP184 | 5.04 | |||
| π-alkyl | ILE42 | 5.04 | ||
- aPositive control. bDecoy molecule.
3.5. Proposed Biosynthetic Pathways of 1 and 2
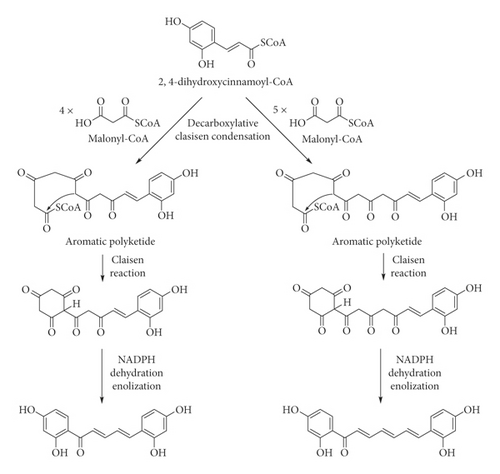
We have proposed plausible biogenetic pathways for two new diarylalkanoids (1 and 2) (Figure 4) via the shikimate and acetate pathways [28]. α-Ketoglutarate-dependent hydroxylase is responsible for the C-2 hydroxylation of p-coumaroyl-CoA to give 2,4-dihydroxycinnamoyl-CoA [29]. It is condensed with four or five malonyl-CoA moieties to afford the corresponding polyketides, which undergo the intramolecular ring closure via Claisen reaction. After that, reduction, dehydration, and enolization must occur to give rise to 1 and 2.
4. Conclusions
From the CHCl3-soluble extract of the stems of S. caudata, two new diarylalkanoids were isolated together with five known compounds. Compounds 1 and 2 were found to possess potent tyrosinase inhibitory activity with the IC50 values of 0.033 and 0.11 μM, respectively. Binding interaction analyses between the oxy-tyrosinase active site and the active compounds (1 and 2) have been performed. Plausible biogenetic pathways for formation of two new diarylalkanoids (1 and 2) were also proposed.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Acknowledgments
This research was funded by Vietnam National University Ho Chi Minh City (VNU-HCM) under grant no. NCM2020-18-01.
Open Research
Data Availability
The data used to support the findings of this study are included within the article.




