Efficacy of Xuebijing Injection for Acute Pancreatitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
Background and Aim. Xuebijing injection is a traditional Chinese medicine compound for the improvement of systemic inflammation response. This meta-analysis of randomized controlled trials (RCTs) aimed to explore the clinical efficacy and safety of Xuebijing injection for the treatment of acute pancreatitis (AP). Methods. PubMed Medline, Embase, Cochrane Library, China National Knowledge Infrastructure, China Biology Medicine disc, VIP, and Wanfang databases were searched. The primary outcome was treatment response. The secondary outcomes included changes in clinical and laboratory indicators and incidence of AP-related complications. Meta-analyses were performed by using a random-effect model. Risk ratios (RRs) with 95% confidence intervals (CIs) or weighted mean differences (WMDs) with 95% CIs were calculated. Results. Overall, 23 RCTs were included. The rates of overall (RR = 1.16; 95% CI = 1.12 to 1.20; P < 0.00001) and complete (RR = 1.40; 95% CI = 1.30 to 1.50; P < 0.00001) responses were significantly higher in the Xuebijing injection group. After treatment, the levels of interleukin-6 (WMD = −18.22; 95% CI = −23.36 to −13.08; P < 0.00001), tumor necrosis factor-α (WMD = −16.44; 95% CI = −20.49 to −12.40; P < 0.00001), serum amylase (WMD = −105.61; 95% CI = −173.77 to −37.46; P = 0.002), white blood cell (WMD = −1.51; 95% CI = −1.66 to −1.36; P < 0.00001), and C-reactive protein (WMD = −11.05; 95% CI = −14.32 to −7.78; P < 0.00001) were significantly lower in the Xuebijing injection group. Abdominal pain (WMD = −1.74; 95% CI = −1.96 to −1.52; P < 0.00001), abdominal distension (WMD = −1.56; 95% CI = −2.07 to −1.04; P < 0.00001), gastrointestinal function (WMD = −2.60; 95% CI = −3.07 to −2.13; P < 0.00001), body temperature (WMD = −2.16; 95% CI = −2.83 to −1.49; P < 0.00001), serum amylase level (WMD = −1.81; 95% CI = −2.66 to −0.96; P < 0.0001), and white blood cell (WMD = −2.16; 95% CI = −2.99 to −1.32; P < 0.00001) recovered more rapidly in the Xuebijing injection group. The incidence of multiple organ dysfunction syndrome (RR = 0.18; 95% CI = 0.05 to 0.62; P = 0.006), pancreatic pseudocyst (RR = 0.17; 95% CI = 0.04 to 0.77; P = 0.02), and renal failure (RR = 0.16; 95% CI = 0.05 to 0.60; P = 0.006) was significantly lower in the Xuebijing injection group. Conclusions. Xuebijing injection added on the basis of conventional treatment has a potential benefit for improving the outcomes of AP.
1. Introduction
Acute pancreatitis (AP) is one of the most common gastrointestinal diseases that require urgent hospitalization [1, 2]. Its global incidence is 34 cases per 100,000 general population per year [3]. About 20% of AP cases are moderately severe or severe [4]. At present, the overall mortality of AP is about 5% [5], and the mortality of severe AP is up to 30% [6], despite the fact that fluid resuscitation, nutritional support, enzyme suppression, antibiotics, analgesia, and treatment of local and systemic complications have been widely employed [7–10].
Xuebijing injection is a traditional Chinese medicine compound developed by Professor Jinda Wang on the basis of the ancient blood regulating formula and the theory of “simultaneous treatment of bacteria, toxin, and inflammation” [11]. It consists of five Chinese herbs, as follows: Carthami Flos (hong hua), Paeoniae Radix Rubra (chi shao), Chuanxiong Rhizoma (chuan xiong), Salviae Miltiorrhizae Radix et Rhizoma (dan shen), and Angelicae Sinensis Radix (dang gui), which can promote blood circulation, strengthen and consolidate body resistance, clear heat, and remove toxicity [12]. Xuebijing injection has been widely employed for the clinical treatment of AP in China, but its effectiveness still needs to be further confirmed. Additionally, it should be noted that published studies are of poor quality, thereby influencing the reliability of previous findings. Herein, we selected relatively high-quality randomized controlled trials (RCTs) and performed a meta-analysis to clarify the role of Xuebijing injection for the treatment of AP.
2. Methods
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The PRIMSA checklist is shown in Supplementary Table 1.
2.1. Registration
The meta-analysis was registered in the PROSPERO with a registration number of CRD42020219118.
2.2. Search Strategy
PubMed Medline, Embase, Cochrane Library, China National Knowledge Infrastructure (CNKI), China Biology Medicine disc (CBMdisc), VIP, and Wanfang databases were searched. The search items are as follows: “Xuebijing” AND “pancreatitis” (Supplementary Material 1 (available here)). The last search was conducted on November 07, 2020.
2.3. Study Selection
There was neither publication language nor publication status restriction. All RCTs regarding Xuebijing injection for the treatment of AP were included. The Xuebijing injection group should include patients, who received Xuebijing injection combined with conventional treatment. The control group should be patients, who received conventional treatment alone.
Exclusion criteria were as follows: (1) duplicates; (2) catalogue, indexes, guidelines, and conferences; (3) reviews or meta-analyses; (4) animal experiments; (5) non-RCTs; (6) irrelevant papers; (7) low-quality RCTs; and (8) absence of efficacy data.
2.4. Outcomes of Interest
The primary outcome should be treatment response. The secondary outcomes include laboratory indicators after treatment; recovery time of clinical symptoms and signs and laboratory indicators after treatment; and incidence of AP-related complications.
2.5. Data Extraction
The following data were extracted from the included studies: first author; journal; publication year; region; study design; usage and dosage of Xuebijing injection; study population; follow-up duration; classification of AP; laboratory indicators after treatment; recovery time of clinical symptoms and signs and laboratory indicators after treatment; incidence of AP-related complications; and outcomes.
2.6. Definitions
The conventional treatment of AP was mainly in accordance with the treatment strategy employed in each included study. Briefly, major treatment strategy included fasting for solids and liquids, fluid resuscitation, gastrointestinal decompression, nutritional support, inhibition of acid and pancreatic secretion, improvement of pancreatic microcirculation, and prophylaxis of infection.
Assessment of response was mainly in accordance with the definitions made by every individual study. In detail, overall response included complete response and partial response; complete response was defined as significant remission or disappearance of clinical symptoms and signs and significant improvement or normalization of laboratory indicators after treatment; partial response was defined as improvement of clinical symptoms and signs and laboratory indicators after treatment; no response was defined as no improvement or even deterioration of clinical symptoms and signs and laboratory indicators after treatment.
2.7. Risk of Bias Assessment
The quality of RCTs was assessed using the Cochrane Collaboration’s Risk of Bias tool [13], which includes random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias.
2.8. Statistical Analysis
We performed the meta-analysis by the Review Manager 5.2 (Cochrane collaboration, the Nordic Cochrane Centre, Copenhagen, Denmark) and STATA 12.0 (Stata Corp, College Station, Texas, USA). A random-effect model was employed. P value < 0.05 was considered statistically significant. Dichotomous outcomes were expressed as risk ratios (RRs) with 95% confidence intervals (CIs), and continuous outcomes were expressed as weighted mean differences (WMDs) with 95% CI. The Cochran’s Q test and I2 statistics were employed to assess the heterogeneity. P < 0.1 and/or I2 > 50% was considered as a statistically significant heterogeneity. Subgroup analyses were conducted in patients diagnosed with severe AP. Publication bias was performed with Egger test. P < 0.1 was considered as a statistically significant publication bias. The meta-regression analyses and sensitivity analyses were used to explore the sources of heterogeneity. Covariates used for meta-regression analyses included the year of publication, region, and dosage of Xuebijing injection every time. Sensitivity analyses were performed by omitting a single study in turn.
3. Results
3.1. Study Selection
Overall, 3051 publications were identified via the 7 databases. Finally, 23 RCTs [14–36] were included in this meta-analysis (Figure 1).
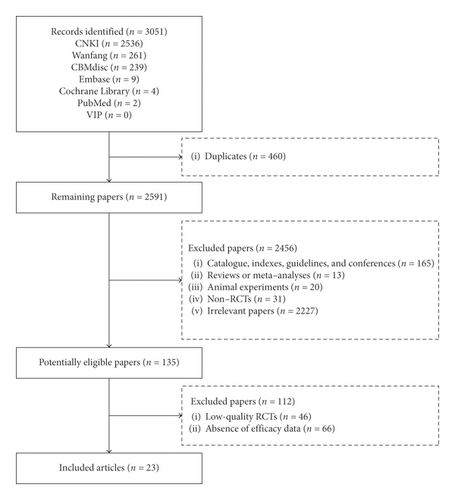
3.2. Study Characteristics
The characteristics of these studies are shown in Tables 1 and 2. All of them were conducted in China and published from 2012 to 2020 as full texts. A total of 1882 AP patients were included with a sample size ranging from 39 to 146 among studies.
| First author (year) | Region | Journal | Study design | Classification of AP | Groups | Interventions | Duration of treatment (days) |
|---|---|---|---|---|---|---|---|
| Li (2020) | Shanxi | Guangming Journal of Chinese Medicine | RCT | SAP | XBJ group | Conventional treatment; Xuebijing injection 100 ml, 2 time/day | 14 |
| Control group | Conventional treatment | ||||||
| Zhan (2019) | Beijing | Chinese Archives of Traditional Chinese Medicine | RCT | SAP | XBJ group | Conventional treatment; Xuebijing injection 100 ml, 2 times/day | 10 |
| Control group | Conventional treatment | ||||||
| Yuan (2019) | Hubei | Chinese and Foreign Medical Research | RCT | SAP | XBJ group | Conventional treatment; Xuebijing injection 100 ml, 2 times/day | 7 |
| Control group | Conventional treatment | ||||||
| Hu (2019) | Zhejiang | Chinese Journal of Surgery of Integrated Traditional and Western Medicine | RCT | SAP | XBJ group | Conventional treatment; Xuebijing injection 100 ml, 1 time/day | 10 |
| Control group | Conventional treatment | ||||||
| Zhang (2018) | Henan | Chinese Journal of New Clinical Medicine | RCT | SAP | XBJ group | Conventional treatment; Xuebijing injection 100 ml, 2 times/day | 7 |
| Control group | Conventional treatment | ||||||
| Zhang (a) (2018) | Shaanxi | Modern Journal of Integrated Traditional Chinese and Western Medicine | RCT | SAP | XBJ group | Conventional treatment; Xuebijing injection 100 ml, 2 times/day | 7 |
| Control group | Conventional treatment | ||||||
| Zhang (b) (2018) | Shaanxi | Medical Journal of West China | RCT | SAP | XBJ group | Conventional treatment; Xuebijing injection 50 ml, 2 times/day | 7 |
| Control group | Conventional treatment | ||||||
| Fan (2018) | Chongqing | Journal of Clinical Medical | RCT | SAP | XBJ group | Conventional treatment; Xuebijing injection 100 ml, 2 times/day | 7 |
| Control group | Conventional treatment | ||||||
| Zha (2018) | Henan | Chongqing Medicine | RCT | SAP | XBJ group | Conventional treatment; Xuebijing injection 100 ml, 2 times/day | 7 |
| Control group | Conventional treatment | ||||||
| Ji (2017) | Qinghai | Shaanxi Journal of Traditional Chinese Medicine | RCT | SAP | XBJ group | Conventional treatment; Xuebijing injection 50 ml, 2–3 times/day | 7 |
| Control group | Conventional treatment | ||||||
| Chen (2017) | Henan | Modern Diagnosis and Treatment | RCT | SAP | XBJ group | Conventional treatment; Xuebijing injection 100 ml, 2 times/day | 7 |
| Control group | Conventional treatment | ||||||
| Liu (2017) | Hubei | World Chinese Journal of Digestology | RCT | SAP | XBJ group | Conventional treatment; Xuebijing injection 100 ml, 2 times/day | 7 |
| Control group | Conventional treatment | ||||||
| Gao (2016) | Liaoning | Chinese Traditional Patent Medicine | RCT | SAP | XBJ group | Conventional treatment; Xuebijing injection 100 ml, 2 times/day | 7 |
| Control group | Conventional treatment | ||||||
| Chen (2015) | Hubei | World Chinese Journal of Digestology | RCT | SAP | XBJ group | Conventional treatment; Xuebijing injection 100 ml, 2 times/day | 7 |
| Control group | Conventional treatment | ||||||
| Chen (2015) | Hebei | International Journal of Traditional Chinese Medicine | RCT | SAP | XBJ group | Conventional treatment; Xuebijing injection 50 ml, 2 times/day | 7 |
| Control group | Conventional treatment | ||||||
| Zhang (2015) | Liaoning | Medical Information | RCT | AP | XBJ group | Conventional treatment; Xuebijing injection 50 ml, 2 times/day | NA |
| Control group | Conventional treatment | ||||||
| Zhu (2015) | Jiangsu | The World Clinical Medicine | RCT | AP | XBJ group | Conventional treatment; Xuebijing injection 50 ml, 2 times/day | NA |
| Control group | Conventional treatment | ||||||
| Liu (2015) | Zhejiang | Journal of Emergency in Traditional Chinese Medicine | RCT | AP | XBJ group | Conventional treatment; Xuebijing injection 100 ml, 2 times/day | 7 |
| Control group | Conventional treatment | ||||||
| Bai (2015) | Nei Mongol | China Foreign Medical Treatment | RCT | AP | XBJ group | Conventional treatment; Xuebijing injection 50 ml, 2 times/day | 7 |
| Control group | Conventional treatment | ||||||
| Yang (2014) | Fujian | Fujian Journal of Traditional Chinese Medicine | RCT | AP | XBJ group | Conventional treatment; Xuebijing injection 50 ml, 2 times/day | 5–7 |
| Control group | Conventional treatment | ||||||
| Liu (2014) | Henan | Modern Journal of Integrated Traditional Chinese and Western Medicine | RCT | SAP | XBJ group | Conventional treatment; Xuebijing injection 50 ml, 2 times/day | 7 |
| Control group | Conventional treatment | ||||||
| Lin (2012) | Xinjiang | Nei Mongol Journal of Traditional Chinese Medicine | RCT | SAP | XBJ group | Conventional treatment; Xuebijing injection 100 ml, 2 times/day | 7 |
| Control group | Conventional treatment | ||||||
| Hong (2012) | Zhejiang | Journal of Emergency in Traditional Chinese Medicine | RCT | AP | XBJ group | Conventional treatment; Xuebijing injection 100 ml, 2 times/day | 7 |
| Control group | Conventional treatment |
- Abbreviations: RCT: randomized controlled trial; AP: acute pancreatitis; SAP: severe acute pancreatitis; XBJ: Xuebijing injection.
| First author (year) | No. pts | Etiology of acute pancreatitis | Sex (male/female) (n) | Age (mean) | Groups | Follow-up (days) | Overall response (n) | Complete response (n) | No response (n) |
|---|---|---|---|---|---|---|---|---|---|
| Li (2020) | 32 | NA | 16/16 | 43.1 | XBJ group | 14 | 28 | 15 | 4 |
| 32 | NA | 17/15 | 43.6 | Control group | 14 | 20 | 8 | 12 | |
| Zhan (2019) | 39 | Biliary diseases 14; alcohol 11; surgery 4; overeating 10 | 28/11 | 46.6 | XBJ group | 10 | 39 | 32 | 0 |
| 39 | Biliary diseases 15; alcohol 11; surgery 5; overeating 7; other 1 | 24/15 | 45.9 | Control group | 10 | 37 | 23 | 2 | |
| Yuan (2019) | 35 | Biliary diseases 13; alcohol 15; hyperlipidemia 7 | 19/16 | 50.7 | XBJ group | 7 | 34 | 28 | 1 |
| 35 | Biliary diseases 13; alcohol 13; hyperlipidemia 9 | 21/14 | 49.8 | Control group | 7 | 28 | 15 | 7 | |
| Hu (2019) | 67 | NA | 40/27 | 41.5 | XBJ group | 10 | 64 | 53 | 3 |
| 67 | NA | 39/28 | 41.4 | Control group | 10 | 56 | 41 | 11 | |
| Zhang (2018) | 35 | NA | 19/16 | 42.8 | XBJ group | 7 | 33 | 29 | 2 |
| 35 | NA | 20/15 | 43.1 | Control group | 7 | 27 | 22 | 8 | |
| Zhang (a) (2018) | 37 | NA | 18/19 | 39.3 | XBJ group | 7 | 31 | 20 | 6 |
| 37 | NA | 17/20 | 38.0 | Control group | 7 | 23 | 13 | 14 | |
| Zhang (b) (2018) | 40 | Biliary diseases 14; alcohol 11; surgery 4; overeating 10 | 23/17 | 42.1 | XBJ group | 7 | 39 | 34 | 1 |
| 40 | Biliary diseases 20; alcohol 10; overeating 9; other 1 | 25/15 | 40.5 | Control group | 7 | 32 | 25 | 8 | |
| Fan (2018) | 20 | NA | 11/9 | 38.6 | XBJ group | 7 | 17 | 9 | 3 |
| 20 | NA | 12/8 | 38.9 | Control group | 7 | 12 | 6 | 8 | |
| Zha (2018) | 49 | NA | NA | NA | XBJ group | 7 | 46 | 28 | 3 |
| 49 | NA | NA | NA | Control group | 7 | 39 | 18 | 10 | |
| Ji (2017) | 61 | NA | 48/13 | 48.0 | XBJ group | 7 | 59 | 50 | 2 |
| 61 | NA | 46/15 | 48.2 | Control group | 7 | 51 | 39 | 10 | |
| Chen (2017) | 43 | NA | 24/19 | 43.1 | XBJ group | 7 | 40 | 29 | 3 |
| 43 | NA | 25/18 | 43.3 | Control group | 7 | 33 | 21 | 10 | |
| Liu (2017) | 52 | NA | 29/23 | 42.5 | XBJ group | 7 | 49 | 35 | 3 |
| 42 | NA | 24/18 | 40.5 | Control group | 7 | 31 | 19 | 11 | |
| Gao (2016) | 44 | NA | 23/21 | 41.9 | XBJ group | 7 | 41 | 31 | 3 |
| 44 | NA | 24/20 | 42.6 | Control group | 7 | 32 | 21 | 12 | |
| Chen (2015) | 73 | Biliary diseases 43; alcohol or overeating 30 | 45/28 | 42.7 | XBJ group | 7 | 72 | 51 | 1 |
| 73 | Biliary diseases 42; alcohol or overeating 31 | 46/27 | 42.8 | Control group | 7 | 65 | 34 | 8 | |
| Chen (2015) | 52 | Biliary diseases 25; alcohol 109; overeating 8; hyperlipidemia 6; other 3 | 30/22 | 53.6 | XBJ group | 7 | 48 | 43 | 4 |
| 52 | Biliary diseases 24; alcohol 9; overeating 9; hyperlipidemia 8; other 2 | 31/21 | 53.7 | Control group | 7 | 38 | 31 | 14 | |
| Zhang (2015) | 21 | NA | NA | NA | XBJ group | NA | 19 | 14 | 2 |
| 18 | NA | NA | NA | Control group | NA | 15 | 9 | 3 | |
| Zhu (2015) | 25 | NA | 17/8 | 36.5 | XBJ group | NA | 23 | 15 | 2 |
| 25 | NA | 18/7 | 36.7 | Control group | NA | 18 | 7 | 7 | |
| Liu (2015) | 40 | NA | 28/12 | 32.6 | XBJ group | 7 | 38 | 30 | 2 |
| 40 | NA | 26/14 | 30.4 | Control group | 7 | 34 | 22 | 6 | |
| Bai (2015) | 50 | NA | 33/17 | 46.7 | XBJ group | 7 | 48 | 29 | 2 |
| 48 | NA | 30/18 | 49.0 | Control group | 7 | 37 | 20 | 11 | |
| Yang (2014) | 31 | NA | 22/9 | 40.3 | XBJ group | 5–7 | 29 | 26 | 2 |
| 31 | NA | 20/11 | 41.2 | Control group | 5–7 | 23 | 18 | 8 | |
| Liu (2014) | 25 | Biliary diseases 2; alcohol 12; overeating 9; other 2 | 16/9 | 48.2 | XBJ group | 7 | 23 | 16 | 2 |
| 20 | Biliary diseases 4; alcohol 9; overeating 5; other 2 | 13/7 | 49.1 | Control group | 7 | 14 | 10 | 6 | |
| Lin (2012) | 43 | NA | 26/17 | 38.8 | XBJ group | 7 | 41 | 24 | 2 |
| 43 | NA | 28/15 | 39.2 | Control group | 7 | 34 | 18 | 9 | |
| Hong (2012) | 62 | NA | 40/22 | 44.5 | XBJ group | 7 | 60 | 50 | 2 |
| 62 | NA | 40/22 | 44.9 | Control group | 7 | 56 | 37 | 6 |
- Abbreviations: Pts: patients; XBJ: Xuebijing injection.
3.3. Risk of Bias
We used the Cochrane Collaboration’s Risk of Bias tool to evaluate a total of 69 potentially eligible papers. Among them, 23 RCTs, which had 3 items at a low risk, were included; the remaining 46 papers, which had 2 or even fewer items at a low risk, were excluded (Supplementary Figure 1).
In the 23 included RCTs, regarding random sequence generation, all studies had a low risk of bias, of which 20 employed a random number table, 1 employed a computer random number generator, and 2 employed a drawing of lots. Regarding incomplete outcome data and selective reporting, all studies had a low risk of bias. Regarding allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and other bias, all studies had an unclear risk of bias (Figure 2).
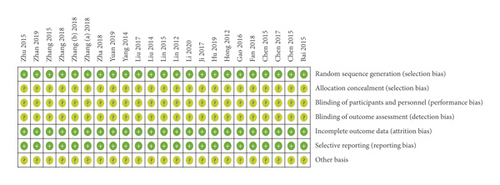
3.4. Outcomes
3.4.1. Overall Response
Twenty-three studies reported the data regarding overall response. The overall response rate was 94.4% (921/976) in the Xuebijing injection group, and 79.0% (755/956) in the control group. Meta-analysis showed that the Xuebijing injection group had a significantly higher overall response rate than the control group (RR = 1.16; 95% CI = 1.12 to 1.20; P < 0.00001) (Figure 3). There was no significant heterogeneity among studies (I2 = 7%; P = 0.37).
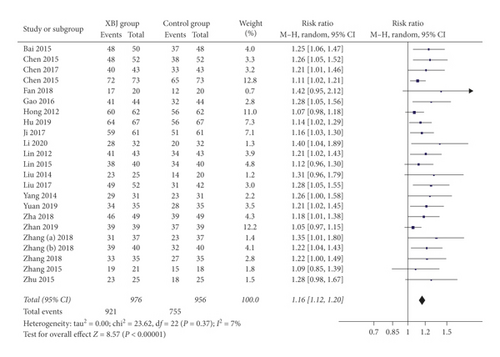
In the subgroup analysis of severe AP, the Xuebijing injection group also had a significantly higher overall response rate than the control group (RR = 1.17; 95% CI = 1.12 to 1.22; P < 0.00001) (Supplementary Figure 2). There was no significant heterogeneity among studies (I2 = 11%; P = 0.33).
3.4.2. Complete Response
Twenty-three studies reported the data regarding complete response. The complete response rate was 70.8% (691/976) in the Xuebijing injection group and 49.9% (477/956) in the control group. Meta-analysis showed that the Xuebijing injection group had a significantly higher complete response rate than the control group (RR = 1.40; 95% CI = 1.30 to 1.50; P < 0.00001) (Figure 4). There was no significant heterogeneity among studies (I2 = 0%; P = 1.00).
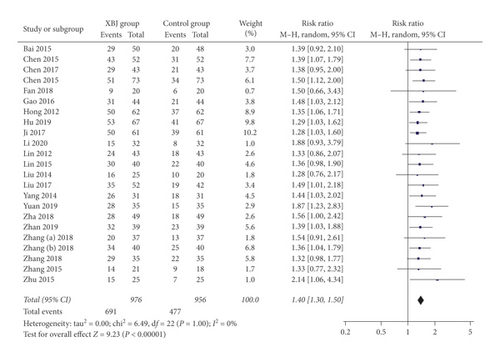
In the subgroup analysis of severe AP, the Xuebijing injection group also had a significantly higher complete response rate than the control group (RR = 1.40; 95% CI = 1.29 to 1.51; P < 0.00001) (Supplementary Figure 3). There was no significant heterogeneity among studies (I2 = 0%; P = 1.00).
3.4.3. No Response
Twenty-three studies reported the data regarding no response. The non-response rate was 5.6% (55/976) in the Xuebijing injection group and 21.0% (201/956) in the control group. Meta-analysis showed that the Xuebijing injection group had a significantly lower rate of non-response than the control group (RR = 0.28; 95% CI = 0.21 to 0.37; P < 0.00001) (Figure 5). There was no significant heterogeneity among studies (I2 = 0%; P = 1.00).
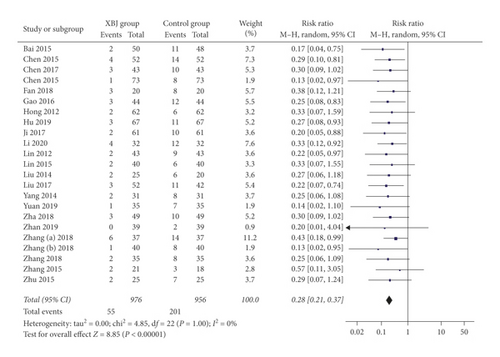
In the subgroup analysis of severe AP, the Xuebijing injection group also had a significantly lower rate of non-response than the control group (RR = 0.28; 95% CI = 0.20 to 0.38; P < 0.00001) (Supplementary Figure 4). There was no significant heterogeneity among studies (I2 = 0%; P = 1.00).
3.5. Laboratory Indicators after Treatment
3.5.1. Interleukin-6 Level
Ten studies including 984 patients reported the data regarding interleukin- (IL-) 6 level after treatment. Meta-analysis showed that the Xuebijing injection group had a significantly lower IL-6 level than the control group (WMD = −18.22; 95% CI = −23.36 to −13.08; P < 0.00001) (Table 3). There was a significant heterogeneity among studies (I2 = 97%; P < 0.00001). Meta-regression analysis and sensitivity analysis did not find any source of heterogeneity (Supplementary Tables 2 and 3).
| Endpoints | No. studies | Pooled proportion using random-effects model | Heterogeneity | ||
|---|---|---|---|---|---|
| WMD | P | I2 (%) | P | ||
| IL-6 level | 10 | −18.22 (95% CI = −23.36 to −13.08) | <0.00001 | 97 | <0.00001 |
| TNF-α level | 12 | −16.44 (95% CI = −20.49 to −12.40) | <0.00001 | 97 | <0.00001 |
| AMS level | 5 | −105.61 (95% CI = −173.77 to −37.46) | 0.002 | 95 | <0.00001 |
| WBC | 6 | −1.51 (95% CI = −1.66 to −1.36) | <0.00001 | 88 | <0.00001 |
| CRP level | 5 | −11.05 (95% CI = −14.32 to −7.78) | <0.00001 | 95 | <0.00001 |
| hs-CRP level | 4 | −12.39 (95% CI = −19.34 to −5.44) | 0.0005 | 96 | <0.00001 |
- Abbreviations: WMD: weighted mean difference; CI: confidence Interval; IL-6: interleukin-6; TNF-α: tumor necrosis factor-α; AMS: serum amylase; WBC: white blood cell; CRP: C-reactive protein; hs-CRP: hypersensitive C-reactive protein.
3.5.2. Tumor Necrosis Factor-α Level
Twelve studies including 1152 patients reported the data regarding tumor necrosis factor-α (TNF-α) level after treatment. Meta-analysis showed that the Xuebijing injection group had a significantly lower level of TNF-α than the control group (WMD = −16.44; 95% CI = −20.49 to −12.40; P < 0.00001) (Table 3). There was a significant heterogeneity among studies (I2 = 97%; P < 0.00001). Meta-regression analysis and sensitivity analysis did not find the source of heterogeneity (Supplementary Tables 2 and 3).
3.5.3. Serum Amylase Level
Five studies including 508 patients reported the data regarding serum amylase level after treatment. Meta-analysis showed that the Xuebijing injection group had a significantly lower level of serum amylase than the control group (WMD = −105.61; 95% CI = −173.77 to −37.46; P = 0.002) (Table 3). There was a significant heterogeneity among studies (I2 = 95%; P < 0.00001). Meta-regression analysis and sensitivity analysis did not find the source of heterogeneity (Supplementary Tables 2 and 3).
3.5.4. White Blood Cell
Six studies including 586 patients reported the data regarding white blood cell (WBC) after treatment. Meta-analysis showed that the Xuebijing injection group had a significantly lower WBC than the control group (WMD = −1.51; 95% CI = −1.66 to −1.36; P < 0.00001) (Table 3). There was a significant heterogeneity among studies (I2 = 88%; P < 0.00001). Meta-regression analysis and sensitivity analysis did not find the source of heterogeneity (Supplementary Tables 2 and 3).
3.5.5. C-Reactive Protein Level
Five studies including 560 patients reported the data regarding C-reactive protein level after treatment. Meta-analysis showed that the Xuebijing injection group had a significantly lower level of C-reactive protein value than the control group (WMD = −11.05; 95% CI = −14.32 to −7.78; P < 0.00001) (Table 3). There was a significant heterogeneity among studies (I2 = 95%; P < 0.00001). Meta-regression analysis and sensitivity analysis did not find the source of heterogeneity (Supplementary Tables 2 and 3).
3.5.6. Hypersensitive C-Reactive Protein Level
Four studies including 380 patients reported the data regarding hypersensitive C-reactive protein level after treatment. Meta-analysis showed that the Xuebijing injection group had a significantly lower level of hypersensitive C-reactive protein than the control group (WMD = −12.39; 95% CI = −19.34 to −5.44; P = 0.0005) (Table 3). There was a significant heterogeneity among studies (I2 = 96%; P < 0.00001). Meta-regression analysis and sensitivity analysis did not find the source of heterogeneity (Supplementary Tables 2 and 3).
3.6. Recovery Time of Clinical Symptoms and Signs and Laboratory Indicators after Treatment
3.6.1. Abdominal Pain
Twelve studies including 1093 patients reported the recovery time of abdominal pain. Meta-analysis showed that the Xuebijing injection group had a significantly shorter recovery time of abdominal pain than the control group (WMD = −1.74; 95% CI = −1.96 to −1.52; P < 0.00001) (Table 4). There was a significant heterogeneity among studies (I2 = 41%; P = 0.07). Meta-regression analysis did not find the source of heterogeneity (Supplementary Table 2). Sensitivity analysis found that the heterogeneity became not significant after excluding the study by Lin et al. (I2 = 37%; P = 0.10) and Zhang et al. (I2 = 19%; P = 0.26) (Supplementary Table 3).
| Endpoints | No. of studies | Pooled proportion using random-effects model | Heterogeneity | ||
|---|---|---|---|---|---|
| WMD | P | I2 (%) | P | ||
| Abdominal pain | 12 | −1.74 (95% CI = −1.96 to −1.52) | <0.00001 | 41 | 0.07 |
| Abdominal distension | 7 | −1.56 (95% CI = −2.07 to −1.04) | <0.00001 | 79 | <0.0001 |
| Gastrointestinal function | 6 | −2.60 (95% CI = −3.07 to −2.13) | <0.00001 | 89 | <0.00001 |
| Body temperature | 6 | −2.16 (95% CI = −2.83 to −1.49) | <0.00001 | 85 | <0.00001 |
| AMS level | 5 | −1.81 (95% CI = −2.66 to −0.96) | <0.0001 | 84 | <0.0001 |
| WBC | 8 | −2.16 (95% CI = −2.99 to −1.32) | <0.00001 | 86 | <0.00001 |
- Abbreviations: WMD: weighted mean difference; CI: confidence Interval; AMS: serum amylase; WBC: white blood cell.
3.6.2. Abdominal Distension
Seven studies including 637 patients reported the recovery time of abdominal distension. Meta-analysis showed that the Xuebijing injection group had a significantly shorter recovery time of abdominal distension than the control group (WMD = −1.56; 95% CI = −2.07 to −1.04; P < 0.00001) (Table 4). There was a significant heterogeneity among studies (I2 = 79%; P < 0.0001). Meta-regression analysis and sensitivity analysis did not find the source of heterogeneity (Supplementary Tables 2 and 3).
3.6.3. Gastrointestinal Function
Six studies including 536 patients reported the recovery time of gastrointestinal function. Meta-analysis showed that the Xuebijing injection group had a significantly shorter recovery time of gastrointestinal function than the control group (WMD = −2.60; 95% CI = −3.07 to −2.13; P < 0.00001) (Table 4). There was a significant heterogeneity among studies (I2 = 89%; P < 0.00001). Meta-regression analysis and sensitivity analysis did not find the source of heterogeneity (Supplementary Tables 2 and 3).
3.6.4. Body Temperature
Six studies including 551 patients reported the recovery time of body temperature. Meta-analysis showed that the Xuebijing injection group had a significantly shorter recovery time of body temperature than the control group (WMD = −2.16; 95% CI = −2.83 to −1.49; P < 0.00001) (Table 4). There was a significant heterogeneity among studies (I2 = 85%; P < 0.00001). Meta-regression analysis and sensitivity analysis did not find the source of heterogeneity (Supplementary Tables 2 and 3).
3.6.5. Serum Amylase Level
Five studies including 385 patients reported the recovery time of serum amylase level. Meta-analysis showed that the Xuebijing injection group had a significantly shorter recovery time of serum amylase level than the control group (WMD = −1.81; 95% CI = −2.66 to −0.96; P < 0.0001) (Table 4). There was a significant heterogeneity among studies (I2 = 84%; P < 0.0001). Meta-regression analysis and sensitivity analysis did not find the source of heterogeneity (Supplementary Tables 2 and 3).
3.6.6. WBC
Eight studies including 631 patients reported the recovery time of WBC. Meta-analysis showed that the Xuebijing injection group had a significantly shorter recovery time of WBC than the control group (WMD = −2.16; 95% CI = −2.99 to −1.32; P < 0.00001) (Table 4). There was a significant heterogeneity among studies (I2 = 86%; P < 0.00001). Meta-regression analysis and sensitivity analysis did not find the source of heterogeneity (Supplementary Tables 2 and 3).
3.7. AP-Related Complications
3.7.1. Multiple Organ Dysfunction Syndrome
Four studies including 368 patients reported the incidence of multiple organ dysfunction syndrome. Meta-analysis showed that the Xuebijing injection group had a significantly lower incidence of multiple organ dysfunction syndrome than the control group (RR = 0.18; 95% CI = 0.05 to 0.62; P = 0.006) (Table 5). There was no significant heterogeneity among studies (I2 = 0%; P = 0.94).
| Endpoints | No. of studies | Pooled proportion using random-effects model | Heterogeneity | ||
|---|---|---|---|---|---|
| RR | P | I2 (%) | P | ||
| MODS | 4 | 0.18 (95% CI = 0.05 to 0.62) | 0.006 | 0 | 0.94 |
| ARDS | 3 | 0.38 (95% CI = 0.14 to 1.04) | 0.06 | 0 | 0.55 |
| Septicemia | 3 | 0.67 (95% CI = 0.19 to 2.36) | 0.54 | 0 | 0.54 |
| Pancreatic pseudocyst | 3 | 0.17 (95% CI = 0.04 to 0.77) | 0.02 | 0 | 0.80 |
| Shock | 2 | 0.30 (95% CI = 0.08 to 1.12) | 0.07 | 23 | 0.25 |
| Renal failure | 2 | 0.16 (95% CI = 0.05 to 0.60) | 0.006 | 0 | 0.55 |
| Pleural effusion or ascites | 2 | 0.35 (95% CI = 0.12 to 1.01) | 0.05 | 6 | 0.30 |
- Abbreviations: AP: acute pancreatitis; RR: risk ratio; CI: confidence Interval; MODS: multiple organ dysfunction syndrome; ARDS: acute respiratory distress syndrome.
3.7.2. Acute Respiratory Distress Syndrome
Three studies including 193 patients reported the incidence of acute respiratory distress syndrome. Meta-analysis showed that the Xuebijing injection group had a lower incidence of acute respiratory distress syndrome than the control group, but there was no significant difference between the two groups (RR = 0.38; 95% CI = 0.14 to 1.04; P = 0.06) (Table 5). There was no significant heterogeneity among studies (I2 = 0%; P = 0.55).
3.7.3. Septicemia
Three studies including 294 patients reported the incidence of septicemia. Meta-analysis showed that the Xuebijing injection group had a lower incidence of septicemia than the control group, but there was no significant difference between the two groups (RR = 0.67; 95% CI = 0.19 to 2.36; P = 0.54) (Table 5). There was no significant heterogeneity among studies (I2 = 0%; P = 0.54).
3.7.4. Pancreatic Pseudocyst
Three studies including 265 patients reported the incidence of pancreatic pseudocyst. Meta-analysis showed that the Xuebijing injection group had a significantly lower incidence of pancreatic pseudocyst than the control group (RR = 0.17; 95% CI = 0.04 to 0.77; P = 0.02) (Table 5). There was no significant heterogeneity among studies (I2 = 0%; P = 0.80).
3.7.5. Shock
Two studies including 123 patients reported the incidence of shock. Meta-analysis showed that the Xuebijing injection group had a lower incidence of shock than the control group, but there was no significant difference between the two groups (RR = 0.30; 95% CI = 0.08 to1.12; P = 0.07) (Table 5). There was no significant heterogeneity among studies (I2 = 23%; P = 0.25).
3.7.6. Renal Failure
Two studies including 152 patients reported the incidence of renal failure. Meta-analysis showed that the Xuebijing injection group had a significantly lower incidence of renal failure than the control group (RR = 0.16; 95% CI = 0.05 to 0.60; P = 0.006) (Table 5). There was no significant heterogeneity among studies (I2 = 0%; P = 0.55).
3.7.7. Pleural Effusion or Ascites
Two studies including 119 patients reported the incidence of pleural effusion or ascites. Meta-analysis showed that the Xuebijing injection group had a lower incidence of pleural effusion or ascites than the control group, but there was no significant difference between the two groups (RR = 0.35; 95% CI = 0.12 to 1.01; P = 0.05) (Table 5). There was no significant heterogeneity among studies (I2 = 6%; P = 0.30).
3.8. Safety
In our included studies, none reported the data regarding adverse events related to Xuebijing injection.
3.9. Publication Bias
Publication bias is reported in Supplementary Table 4.
4. Discussion
Our systematic review suggests that Xuebijing injection combined with conventional treatment is more effective for AP than conventional treatment alone. Similar findings could be observed in the subgroup analysis of severe AP. Our study has several major features in study design and statistical analysis. First, we conducted more extensive literature search and included a larger number of publications. Second, we excluded low-quality RCTs. Third, we explored the efficacy outcomes in more details, including overall response, complete response, and no response. Fourth, we explored the laboratory indicators after treatment between Xuebijing injection and control groups. Fifth, we analyzed the incidence of various complications of AP between the two groups. Sixth, we conducted the meta-regression analyses to explore the source of heterogeneity.
In AP, the initial mediator that induces inflammatory cell response is TNF-α, which is the earliest promoter of inflammatory mediator chain reaction [37]. TNF-α induces the expression of many inflammatory factors, such as IL-6 [38], which react on macrophages, thereby producing more TNF-α [39]. This vicious cycle triggers the inflammatory cascade reaction, causing toxic damage to pancreas and other organs [40, 41].
It has been confirmed that Xuebijing injection can improve microcirculation, increase blood flow, reduce inflammation and capillary permeability, decrease inflammatory exudation, promote inflammation absorption, and inhibit the formation of inflammatory granulomas, which can alleviate the pathological damage during the infection [42]. Xuebijing injection is the only patented Chinese medicine officially authorized for the treatment of systemic inflammatory response syndrome and multiple organ dysfunction syndrome [43]. In the current clinical practice, Xuebijing injection has been widely used to treat critical diseases, such as sepsis [12], acute respiratory distress syndrome [44], severe pneumonia [45], and spontaneous bacterial peritonitis [46]. Xuebijing injection has a strong ability to antagonize endotoxin, which can effectively block the uncontrolled release of endogenous inflammatory mediators produced by endotoxin-induced monocytes/macrophages, thereby preventing from inflammation reaction [42, 46]. In addition, it can effectively reduce the levels of TNF-α, hypersensitive C-reactive protein, and IL-6, which are pro-inflammatory factors, upregulate the IL-10 level, which is an anti-inflammatory factor, block the cascade reaction mediated by inflammatory factors, and finally reduce the inflammatory reaction [11]. Our meta-analysis showed that Xuebijing injection can significantly reduce the levels of inflammatory mediators.
The incidence of adverse reactions of Xuebijing injection is about 0.3%, which often develops within 30 minutes of medication [47]. Most of them are mild. Adverse reactions are mainly located at the respiratory system, skin, and accessories. Main manifestations are skin pruritus, erythema, and chest tightness [48]. Based on the findings of the present systematic review, no adverse reactions related to Xuebijing injection have been reported.
Our meta-analysis has several major limitations. First, the treatment strategy of the control group was not completely equal. Second, the follow-up duration was too short. The average duration is always 7 days, and the longest duration is only 14 days. Third, blinding and allocation concealment were not reported in all included studies. Fourth, most of the included studies had a small sample size and were conducted at a single center. Fifth, all of included studies were conducted in China.
In conclusion, the application of Xuebijing injection on the basis of conventional treatment can improve the outcomes of AP. However, Xuebijing injection is currently used in China alone. In the future, more high-quality, well-designed, multi-center, and large-scale RCTs are still needed to validate the clinical efficacy and safety of Xuebijing injection for the treatment of AP.
Abbreviations
-
- AP:
-
- Acute pancreatitis
-
- CBMdisc:
-
- China Biology Medicine disc
-
- CNKI:
-
- China National Knowledge Infrastructure
-
- CIs:
-
- Confidence intervals
-
- IL:
-
- Interleukin
-
- RRs:
-
- Risk ratios
-
- RCTs:
-
- Randomized controlled trials
-
- TNF-α:
-
- Tumor necrosis factor-α
-
- WBC:
-
- White blood cell
-
- WMDs:
-
- Weighted mean differences.
Disclosure
Hongxin Chen, Zhaohui Bai, and Hongyu Li are the co-first authors.
Conflicts of Interest
The authors declare that they have no conflicts of interest.




