Chemically Active Elements of Reservoir Quartz Cement Trace Hydrocarbon Migration in the Mahu Sag, Junggar Basin, NW China
Abstract
Element exchange and enrichment during fluid-rock interactions are common, providing potentially novel proxies to trace hydrocarbon migration in addition to the traditional organic geochemistry tracers. However, the processes, mechanisms, and geological and geochemical fingerprints of these interactions are complex, hampering the applications of hydrocarbon migration tracers. To investigate such interactions, we conducted a petrological, mineralogical, and in situ and bulk geochemical study of authigenic quartz and whole-rock samples from the Mahu Sag, northwestern Junggar Basin, northwest China. We found that dissolution, clay and chlorite formation, and overgrowth occurred on quartz grains in hydrocarbon fluid migration pathways, suggestive of strong fluid-rock interactions. In situ quantitative elemental analysis of quartz grains revealed elemental enrichment (e.g., Mn, Fe, Al, Sr, and W) in quartz overgrowth rims compared with their cores, indicating that migration of hydrocarbon-bearing fluids in reservoirs may promote elemental exchange between fluids and minerals. Whole-rock geochemical analysis showed that decreasing contents of some elements may reflect the direction of hydrocarbon-bearing fluid migration and can be monitored with three geochemical proxies, which are the MnO contents and MnO/Zr and Y/Ho ratios. Our data provide new constraints on fluid-rock interactions in petroleum reservoirs and have implications for using inorganic geochemical methods to trace hydrocarbon migration.
1. Introduction
Hydrocarbon fluid migration is the key link between hydrocarbon generation and accumulation. Numerous studies have been undertaken on hydrocarbon migration, including physical experiments [1–5], numerical simulations [6–12], coupling analysis of thermal, stress, and pressure fields [13–18], and organic geochemical and isotope studies [19, 20]. Physical and numerical simulations build a model from the perspective of forward modeling and then observe the migration process of hydrocarbon fluids in reservoirs by using experimental facilities or computer models [8, 9]. Thermal, stress, and pressure field coupling analysis mainly involves assessing the direction of hydrocarbon migration using the fluid potential, crude oil density, formation water salinity, and other parameters based on the physical properties of hydrocarbon-bearing fluids [21–25]. Organic geochemistry indicators, including maturity indicators, nitrogen-containing compounds in crude oil, and fractionation effects on carbazole, pyrrole, and other compounds, are amongst the most efficient methods to trace hydrocarbon fluid migration [26–31]. However, due to mixing of multisource and multiphase hydrocarbons in superimposed basins and the effects of secondary modification, organic geochemical indicators are susceptible to complex geological processes [32, 33]. Therefore, it is difficult to determine the direction of hydrocarbon migration based on only one indicator.
Studies have shown that strata through which fluids have migrated experience strong physical and chemical interactions between fluids and rocks, which then lead to changes in the composition, morphology, and other aspects of the rocks and minerals [34–36]. As such, these interactions can leave a record of the processes, direction, and strength of fluid migration [37–40]. When hydrocarbon-bearing fluids migrate in strata, organic inclusions can form in authigenic minerals in reservoirs. An increasing number of studies have investigated the effects of fluid-rock interactions and, in particular, the elemental and isotope geochemistry of authigenic minerals [41, 42], sources of hydrothermal fluids [43, 44], and rates and mechanisms of fluid-rock interactions [45]. In theory, geochemical changes recorded by authigenic minerals can record the migration of hydrocarbon-bearing fluids and reveal the processes of hydrocarbon migration. Therefore, such information is of significance in determining the migration direction of hydrocarbon fluids. However, the whole-rock geochemistry records only the averaged history of a hydrothermally modified rock. In contrast, in situ geochemical studies of authigenic minerals can provide more resolved information regarding fluid migration.
Elemental geochemistry has been used in recent studies to trace hydrocarbon fluid migration, based on rationale of interactions between organic fluids and inorganic minerals [46–48]. Most of these studies have focused on calcite or calcium carbonate cements in fault zones. However, studies of fluid migration in reservoirs with low contents of calcite and other diagenetic minerals remain limited. In addition, the distribution of elements in diagenetic minerals is heterogeneous, and hydrothermal fluids are likely to modify parts of the minerals, which requires an in situ analytical approach.
In addition to calcite cement, quartz is common in terrigenous clastic sedimentary rocks, and diagenesis and hydrothermal fluids result in quartz overgrowths. Recent studies have proposed that the geochemistry of quartz overgrowths may reflect the characteristics of hydrothermal fluids [38]. However, the relationship between the geochemical composition of the quartz overgrowths and hydrocarbon migration remains unclear and requires further investigation.
Therefore, in this paper, we present a petrological, mineralogical, and in situ and whole-rock geochemical study of the Lower Triassic Baikouquan Formation, which is a new exploration target on the slope of the Mahu Sag, Junggar Basin, northwest China. We use these data to investigate the migration of hydrocarbon fluids in reservoirs based on the geochemistry of quartz cements.
2. Geological Setting
The Junggar Basin is located in northern Xinjiang Province and is an important part of the Central Asian Orogenic Belt, covering an area of ~130,000 km2. The basin is surrounded by three fold and thrust belts, including the Zhayier and Hala’alat Mountains along its northwestern margin, the Qinggelidi and Kelameili Mountains along its northeastern margin, and the Bogda and Yilinheibiergen Mountains of the Tianshan along its southern margin (Figure 1(a)). The Junggar Basin comprises Carboniferous folded basement [49, 50] and sedimentary cover rocks, including Paleozoic, Mesozoic, and Cenozoic strata. The basin has experienced three main stages: development of a foreland basin in the late Carboniferous-Permian, an intracontinental depressional basin in the Triassic-Paleogene, and a rejuvenated foreland basin since the Neogene [51–53].
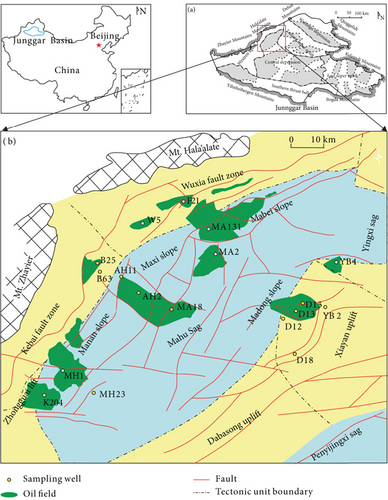
Several structural units occur in the Junggar Basin (Figure 1(a)), and the Mahu Sag is one of the largest depressions in the basin (Figure 1(a)) [54]. The slope of the Mahu Sag is the most favorable hydrocarbon accumulation area, where nearly one billion tons of oil have been discovered (Figure 1(b)). To the north, the slope area is bounded by the Wuxia (Wuerhe-Xiazijie) and Kebai (Karamay-Baikouquan) fault belts. The Yingxi Sag and Xiayan and Dabasong uplifts are located to the east of the Mahu Sag. According to drilling data, Neogene and Carboniferous strata are well developed in the Mahu Sag. Triassic strata unconformably overlie Permian strata. The source rocks in the Mahu Sag are mainly the lower Permian Jiamuhe and Fengcheng Formations [55, 56]. The Jiamuhe Formation has its hydrocarbon generation center in the west of the Mahu Sag and thins gradually to the east, north, and south. The Fengcheng Formation has its hydrocarbon generation center in the north of the Mahu Sag and thins to the east and west. Hydrocarbon accumulation in the Mahu Sag involved three stages, during the Middle-Late Permian, Late Triassic, and Early Cretaceous [57, 58]. The Triassic strata were generally deposited in the transgressive system of a lake basin, with alluvial fan and shallow-water fan-delta sediments deposited at the northwestern margin of the sag. The Baikouquan Formation (T1b) consists mainly of red-brown and grey sandy conglomerates.
3. Samples and Methods
In this study, we collected 15 drill core samples of the Baikouquan Formation from 11 wells (MA18, MH23, AH2, AH11, B25, B63, D12, D13, D15, D18, and YB2) for mineralogical and geochemical studies. These samples are mainly grey or greyish green sandy conglomerates (Table 1). The locations of these wells are shown in Figure 1, and sample details are listed in Table 1. All samples were made into polished thin sections and powdered to <200 mesh (74 μm) in an agate mortar.
| Well | Depth (m) | Lithology | Core characteristics | Oil-bearing characteristics |
|---|---|---|---|---|
| B63 | 2452/2514 | Sandy conglomerate | Grey conglomerate, the pebble is mainly lithic fragment and quartz | Oil stain |
| B25 | 2318.1 | Sandy conglomerate | Grey conglomerate, the pebble is mainly lithic fragment and quartz | Oil stain |
| YB2 | 4110.2 | Sandy conglomerate | Greyish-green conglomerate, the pebble is mainly lithic fragment and quartz | Oil stain |
| AH2 | 3313.6 | Sandy conglomerate | Greyish-green conglomerate, the pebble is mainly lithic fragment and quartz | Oil stain |
| AH11 | 2824.5 | Sandy conglomerate | Greyish-green conglomerate, the pebble is mainly lithic fragment and quartz | Oil stain |
| D15 | 4245/4253.7 | Sandy conglomerate | Greyish-green conglomerate, the pebble is mainly lithic fragment and quartz | Oil stain |
| D12 | 4269.2/4267/4284 | Sandy conglomerate | Greyish-green conglomerate, the pebble is mainly lithic fragment and quartz | Oil stain |
| D18 | 4451.4 | Sandy conglomerate | Greyish-green conglomerate, the pebble is mainly lithic fragment and quartz | Oil stain |
| D13 | 4209 | Sandy conglomerate | Greyish-green conglomerate, the pebble is mainly lithic fragment and quartz | Oil stain |
| MH23 | 4078.9 | Sandy conglomerate | Greyish-green conglomerate, the pebble is mainly lithic fragment and quartz | Oil stain |
| MA18 | 3905.8 | Sandy conglomerate | Greyish-green conglomerate, the pebble is mainly lithic fragment and quartz | Oil stain |
The samples were examined under a polarizing microscope (Zeiss Axio Lab A1) at the China University of Petroleum (East China), Qingdao. Cathodoluminescence (CL) imaging was undertaken in the Beijing SHRIMP Center, using a Carl Zeiss Merlin Compact instrument coupled to a GATAN Mono CL4. Scanning electron microscopy (SEM) was undertaken at the Key Laboratory of Shale Hydrocarbons, Chinese Academy of Geological Sciences, Beijing, using a Zeiss Sigma 300 instrument. In situ microanalysis was conducted at Beijing Standard Energy Ltd. using a JEOL JXA-8230 electron microprobe (major elements) and GeolasPro 193 nm laser ablation (LA) system coupled to an Agilent 7900 inductively coupled plasma mass spectrometer (ICP-MS; trace elements). The error of the electron microprobe analyses is <2%, while that of the LA-ICP-MS analyses is <10%. Whole-rock analyses were undertaken at ALS Chemex Company Ltd., Guangzhou, China, where major elements were determined by X-ray fluorescence (XRF) spectrometry with an error < 5% and trace elements were determined by ICP-MS with an error < 10%. XRF mapping was conducted in the Key Laboratory of Deep Hydrocarbons, China University of Petroleum (East China), Qingdao, using a Bruker M4 Tornado instrument with a scanning spot size of 20 μm.
4. Results
4.1. Petrological Features of Quartz
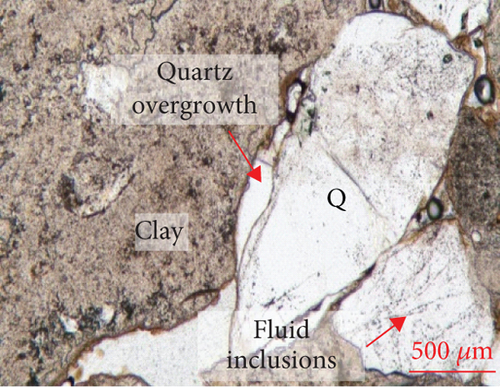
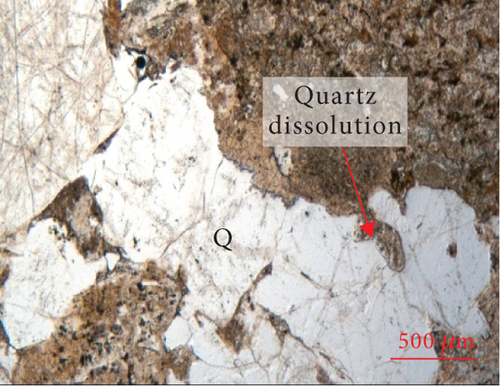
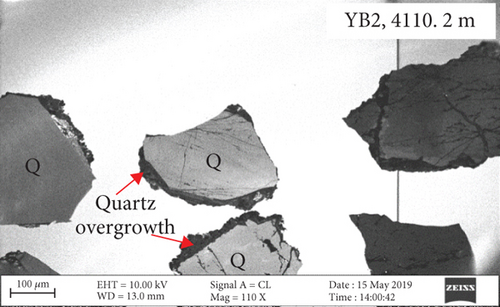
The edges of quartz grains exhibit curved dissolution profiles under the microscope (Figure 2), and parts of the quartz edges are replaced or infilled with clay minerals. The surface of the quartz is relatively rough, and a large number of fluid inclusions (typically <10 μm in size) are distributed along the fractures. Quartz overgrowths are developed along the edges of some grains. Most feldspar and lithic fragments in the sandy conglomerate have been altered, with the replacement products being mainly clay.

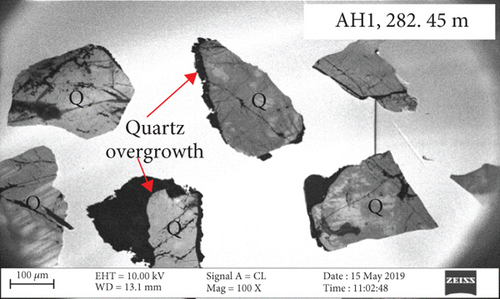
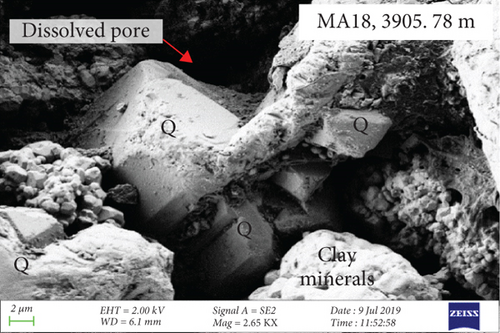
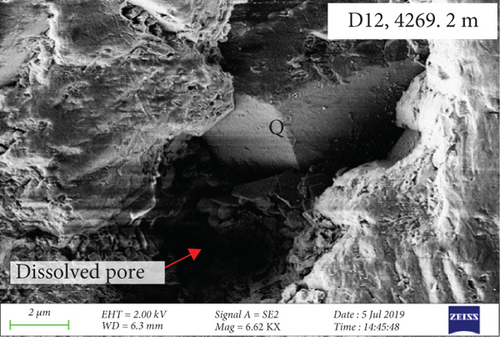
CL and SEM imaging was used to examine the interiors and edges of quartz grains. The quartz has obvious multiphase growth regions with different optical characteristics (Figures 3(a) and 3(b)). The secondary quartz is CL-dark and corresponds to quartz overgrowths. The quartz overgrowths are hexagonal and prism-shaped in SEM images (Figures 3(c) and 3(d)) and intergrown with clay minerals. Dissolution pores also occur near the edges of quartz grains.
4.2. In Situ Geochemistry of Quartz
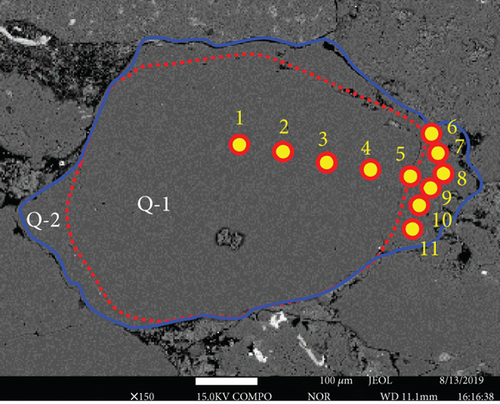
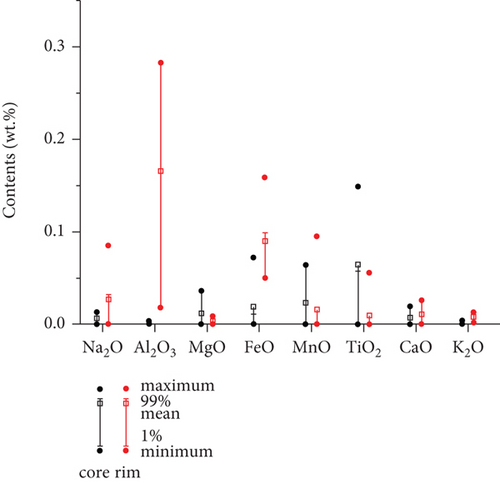
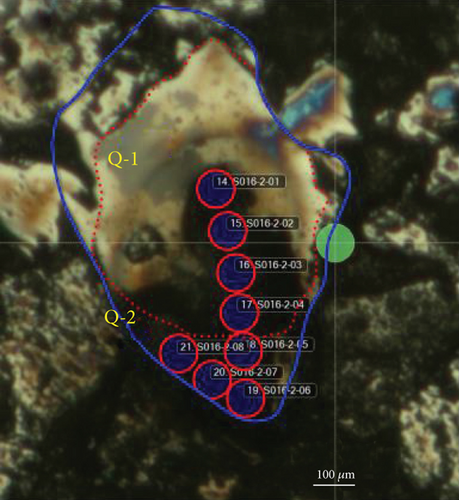
Backscattered electron images also reveal the original quartz cores and overgrowths, and they are separated with dark and curved layers, similar to the results of petrological observations (Figures 4(a) and 4(b)). Major element contents were analyzed from the core to the rim in selected quartz grains. SiO2 contents are all >98 wt.%. The distribution of the other major elements (Figures 4(a) and 4(b), Table 2) is variable, and most elements have significantly lower contents in the cores (Q-1) than in the overgrowths (Q-2), particularly for Fe and Al.
| Point | Na2O | Al2O3 | MgO | SiO2 | FeO | MnO | TiO2 | CaO | K2O | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.002 | bdl | bdl | 98.98 | 0.011 | 0.006 | bdl | bdl | bdl | 99.00 |
| 2 | 0.013 | bdl | bdl | 99.45 | 0.011 | bdl | 0.038 | 0.019 | 0.004 | 99.53 |
| 3 | 0.011 | bdl | 0.023 | 99.29 | bdl | 0.064 | bdl | bdl | bdl | 99.39 |
| 4 | 0.006 | bdl | bdl | 98.68 | bdl | bdl | 0.149 | 0.012 | bdl | 98.85 |
| 5 | bdl | 0.003 | 0.036 | 99.74 | 0.072 | 0.045 | 0.038 | 0.004 | bdl | 99.94 |
| 6 | 0.032 | 0.211 | bdl | 99.06 | 0.088 | bdl | 0.056 | 0.013 | 0.007 | 99.46 |
| 7 | 0.015 | 0.246 | 0.009 | 98.03 | 0.066 | bdl | bdl | bdl | 0.004 | 98.37 |
| 8 | 0.085 | 0.283 | 0.004 | 98.19 | 0.159 | 0.095 | bdl | 0.026 | 0.013 | 98.85 |
| 9 | bdl | 0.2 | bdl | 98.15 | 0.05 | bdl | bdl | 0.015 | 0.009 | 98.42 |
| 10 | bdl | 0.036 | 0.006 | 99.53 | 0.099 | bdl | bdl | 0.011 | 0.002 | 99.68 |
| 11 | 0.027 | 0.018 | bdl | 99.03 | 0.077 | bdl | bdl | bdl | 0.005 | 99.16 |
- Note: bdl denotes below detection limit.
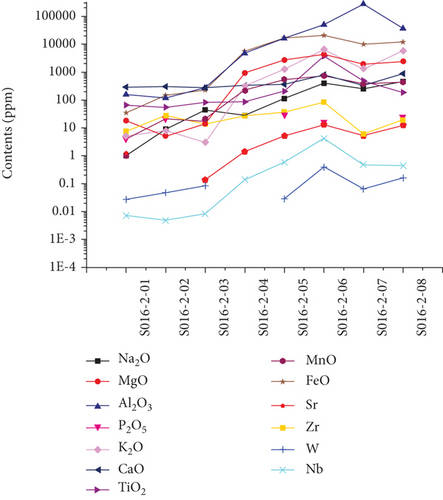
LA-ICP-MS was also used to analyze the major and trace element contents. The core analyses (S016-2-01–04) have average SiO2 = 98.94 wt.%, Al2O3 = 0.133 wt.%, MnO = 0.006 wt.%, and FeO = 0.148 wt.%. The cores also have average Co = 1.78 ppm, Ni = 3.91 ppm, Sr = 0.66 ppm, and W = 0.04 ppm. The average SiO2, Al2O3, MnO, and FeO contents of the rims (S016-2-05–08) are 98.85, 3.261, 0.052, and 1.482 wt.%, respectively. The rims have average Co = 2.47 ppm, Ni = 25.92 ppm, Sr = 8.98 ppm, and W = 0.16 ppm. The cores and rims separated by a thin, dark, and clay layer exhibit the largest compositional differences (Figures 4(c) and 4(d), Table 3). Some trace (e.g., Sr and W) and major element (e.g., Mn, Fe, and Al) contents are higher in the rims than in the cores. The differences can be by two or three orders of magnitude. However, some relatively immobile elements show no apparent concentration differences between the cores and rims (e.g., Zr).
| Element | Unit | S016-2-01 | S016-2-02 | S016-2-03 | S016-2-04 | S016-2-05 | S016-2-06 | S016-2-07 | S016-2-08 |
|---|---|---|---|---|---|---|---|---|---|
| Na2O | wt.% | 0.0001 | 0.0009 | 0.0044 | 0.0028 | 0.0114 | 0.0403 | 0.0248 | 0.0456 |
| MgO | wt.% | 0.0018 | 0.0005 | 0.0014 | 0.0924 | 0.2689 | 0.4281 | 0.1951 | 0.238 |
| Al2O3 | wt.% | 0.0157 | 0.0117 | 0.027 | 0.4778 | 1.5872 | 5.0255 | 27.8972 | 3.6433 |
| SiO2 | wt.% | 98.8128 | 99.3119 | 99.1262 | 98.5171 | 99.5735 | 99.4796 | 97.9821 | 98.3665 |
| P2O5 | wt.% | 0.0004 | 0.0021 | 0.0017 | bdl | 0.0029 | 0.0016 | bdl | 0.0025 |
| K2O | wt.% | 0.0005 | 0.0008 | 0.0003 | 0.0315 | 0.1263 | 0.6335 | 0.1276 | 0.5843 |
| CaO | wt.% | 0.0052 | 0.0023 | 0.0089 | 0.0106 | 0.0128 | 0.0484 | 0.0331 | 0.0502 |
| CaO | wt.% | 0.0286 | 0.0299 | 0.0275 | 0.0337 | 0.0359 | 0.0803 | 0.0321 | 0.0873 |
| TiO2 | wt.% | 0.0065 | 0.0054 | 0.0081 | 0.0085 | 0.0206 | 0.3722 | 0.0481 | 0.0186 |
| MnO | wt.% | 0.0001 | bdl | 0.0021 | 0.0219 | 0.0542 | 0.0746 | 0.0376 | 0.0429 |
| FeO | wt.% | 0.0035 | 0.0146 | 0.0227 | 0.5517 | 1.6852 | 2.0688 | 0.9899 | 1.1847 |
| Li | ppm | 2.0965 | bdl | 3.1423 | 7.8239 | 16.3127 | 23.3518 | 47.5405 | 47.7116 |
| Be | ppm | bdl | 0.0059 | 0.0268 | 0.1594 | 0.1286 | 0.5496 | 0.2767 | 0.5168 |
| B | ppm | 0.3452 | 0.9822 | 1.7401 | 0.3358 | 3.3413 | 15.6646 | 2.9438 | 5.3068 |
| Sc | ppm | 1.5332 | 1.5815 | 1.7752 | 2.4409 | 2.8277 | 5.2015 | 4.5676 | 3.5416 |
| V | ppm | 0.0768 | 0.141 | 0.1456 | 6.2744 | 18.6091 | 38.5048 | 44.2837 | 20.8029 |
| Cr | ppm | 0.9691 | 0.8712 | 1.1591 | 2.8601 | 5.3181 | 8.6423 | 8.2257 | 4.4051 |
| Co | ppm | 0.0849 | 3.4376 | 2.4933 | 1.1143 | 2.4508 | 3.1264 | 2.5711 | 1.737 |
| Ni | ppm | 0.1548 | 0.446 | bdl | 15.0496 | 18.0809 | 16.3241 | 60.7456 | 8.5679 |
| Cu | ppm | 4.6485 | 1.583 | 0.4035 | 10.0872 | 3.4157 | 8.3014 | 15.9753 | 10.3209 |
| Zn | ppm | 4.53 | 7.0383 | 3.4491 | 10.9982 | 15.4474 | 20.3231 | 8.3061 | 250.1787 |
| Ga | ppm | 0.0236 | 0.0091 | 0.0352 | 0.7123 | 2.3717 | 4.8447 | 5.7437 | 3.5102 |
| Ge | ppm | 1.1098 | 1.0578 | 0.917 | 1.2367 | 1.2775 | 1.584 | 1.1088 | 1.2653 |
| Rb | ppm | 0.0121 | 0.1982 | bdl | 0.7515 | 2.4961 | 21.3532 | 2.597 | 15.8376 |
| Sr | ppm | 1.1306 | bdl | 0.1347 | 1.3932 | 5.1935 | 13.4053 | 5.3072 | 12.018 |
| Y | ppm | 0.0789 | bdl | 0.3099 | 3.7087 | 4.4541 | 8.788 | 0.6344 | 3.0661 |
| Zr | ppm | 7.4731 | 27.9566 | 13.5468 | 27.0276 | 36.3953 | 83.4228 | 6.0641 | 19.4439 |
| Nb | ppm | 0.0072 | 0.0048 | 0.0083 | 0.1382 | 0.5832 | 4.2074 | 0.483 | 0.4359 |
| Mo | ppm | 0.0139 | 3.3567 | 0.8704 | bdl | 0.3764 | 0.1611 | 0.025 | 0.0231 |
| Cs | ppm | 0.0247 | 0.0419 | 0.146 | 0.0547 | 0.2173 | 1.947 | 0.1248 | 0.4007 |
| Ba | ppm | 0.0359 | 0.0357 | 1.0805 | 5.7055 | 20.774 | 92.4082 | 16.6073 | 125.0756 |
| La | ppm | 0.0032 | bdl | 0.0037 | 0.0462 | 0.2222 | 1.2463 | 0.3332 | 7.1036 |
| Ce | ppm | 0.1066 | 0.003 | 0.0191 | 0.1468 | 0.6328 | 1.9927 | 1.2214 | 12.4724 |
| Pr | ppm | 0.0059 | 0.0093 | bdl | 0.0183 | 0.079 | 0.1865 | 0.0786 | 1.1895 |
| Nd | ppm | 0.0064 | 0.0191 | 0.0074 | 0.107 | 0.4143 | 0.7609 | 0.2295 | 3.6637 |
| Sm | ppm | bdl | bdl | 0.0168 | 0.0664 | 0.2537 | 0.3327 | 0.045 | 0.4885 |
| Eu | ppm | 0.0185 | 0.0593 | 0.0309 | 0.0137 | 0.0202 | 0.1079 | 0.0064 | 0.1828 |
| Gd | ppm | bdl | bdl | 0.0078 | 0.1565 | 0.3665 | 0.4613 | 0.0312 | 0.3494 |
| Tb | ppm | bdl | bdl | 0.0038 | 0.0543 | 0.1052 | 0.2078 | 0.0237 | 0.0699 |
| Dy | ppm | bdl | 0.0307 | bdl | 0.6531 | 0.746 | 1.4292 | 0.1024 | 0.4987 |
| Ho | ppm | bdl | 0.0085 | 0.0136 | 0.1708 | 0.157 | 0.3226 | 0.0166 | 0.1081 |
| Er | ppm | 0.0164 | 0.418 | 0.0189 | 0.3973 | 0.4846 | 0.8769 | 0.0594 | 0.2808 |
| Tm | ppm | 0.0049 | bdl | 0.0056 | 0.1347 | 0.0878 | 0.1604 | 0.0075 | 0.046 |
| Yb | ppm | 0.0041 | 0.0041 | 0.0048 | 0.4731 | 0.493 | 1.0755 | 0.0836 | 0.3126 |
| Lu | ppm | 0.001 | 0.001 | 0.007 | 0.0804 | 0.0829 | 0.15 | 0.0078 | 0.0509 |
| Hf | ppm | bdl | bdl | 0.0036 | 0.6129 | 0.8206 | 2.2239 | 0.195 | 0.5997 |
| Ta | ppm | 0.0032 | bdl | bdl | 0.0082 | 0.0538 | 0.4038 | 0.1343 | 0.0415 |
| W | ppm | 0.0274 | 0.0471 | 0.0823 | bdl | 0.029 | 0.4034 | 0.0648 | 0.1617 |
| Hg | ppm | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| Tl | ppm | 0.6772 | bdl | 0.0362 | bdl | 0.0633 | 0.0847 | 0.0332 | 0.0451 |
| Pb | ppm | 6.8722 | 0.4472 | 1.3452 | 0.0906 | 0.3238 | 3.8856 | 0.7726 | 0.4805 |
| Th | ppm | 0.0192 | bdl | 0.0225 | 2.0437 | 1.3406 | 9.2599 | 0.8271 | 8.162 |
| U | ppm | 0.0017 | 0.0051 | 0.0778 | 0.2246 | 0.2462 | 0.9181 | 0.165 | 0.8694 |
- Note: bdl denotes below detection limit.
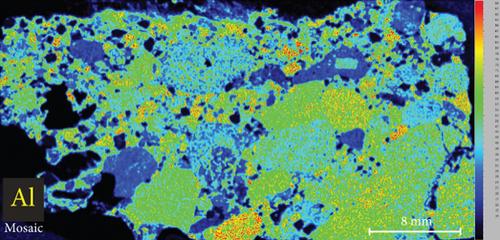
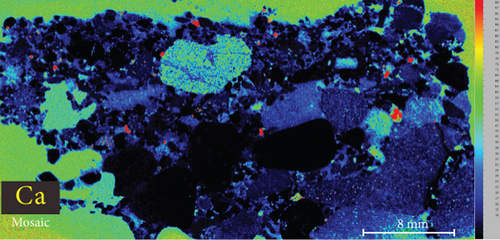
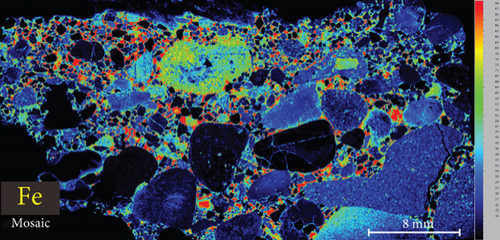
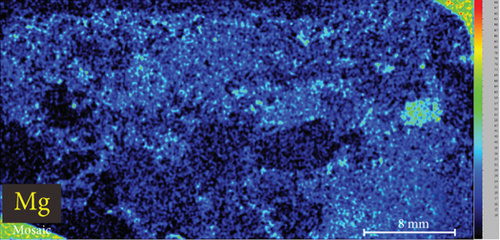
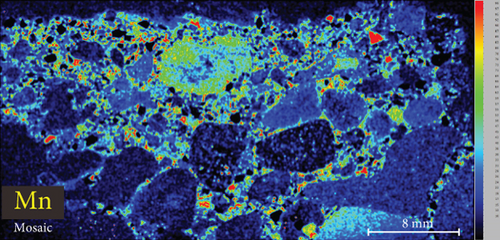
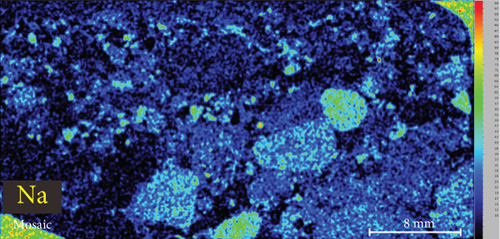
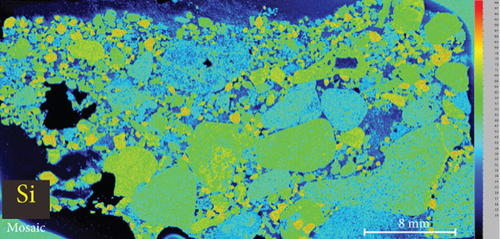
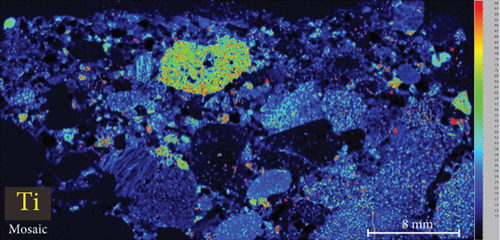
4.3. XRF Element Maps of Quartz
The elemental map showed that the distribution of elements in the rock thin sections is heterogeneous (Figure 5). Elements mobile in hydrothermal fluids, such as Mn and Fe, are significantly concentrated at the edges of quartz grains.

4.4. Whole-Rock Geochemistry
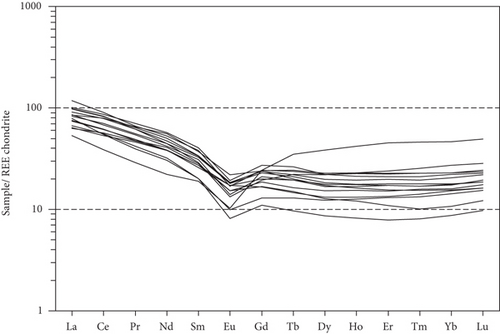
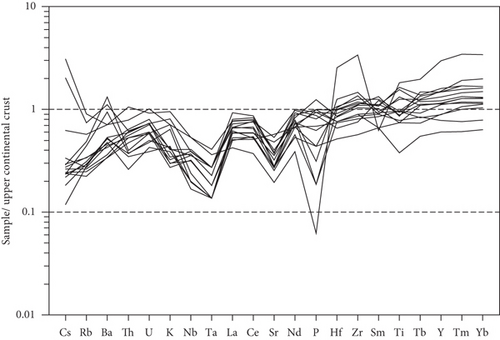
Whole-rock geochemical data for 15 samples from 11 wells are listed in Table 4. The samples have variable major element compositions, with SiO2 = 57.92–78.98 wt.%, MgO = 0.52–2.04 wt.%, TFe2O3 = 1.55–10.25 wt.%, Al2O3 = 9.69–19.66 wt.%, CaO = 0.25–1.40 wt.%, and MnO = 0.05–0.20 wt.%. Total rare earth element (REE) concentrations vary from 81.50 to 140.85 ppm. The chondrite-normalized REE patterns of these samples show apparent fractionation of light REEs relative to heavy REEs (Figure 6(a)), with LaN/YbN = 1.15–9.03. The Eu/Eu∗ values of the sandy conglomerates vary from 0.48 to 0.83, reflecting negative Eu anomalies. In trace element patterns (Figure 6(b)), the large-ion lithophile elements (e.g., Cs, Rb, K, and Ba) exhibit variable contents. Some high-field-strength elements exhibit obvious negative anomalies, such as Nb and Ta.
| Well | B63 | B63 | B25 | YB2 | AH2 | AH11 | D15 | D15 | D12 | D12 | D12 | D18 | D13 | MH23 | MA18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Depth (m) | 2514 | 2452 | 2318 | 4110 | 3314 | 2825 | 4245 | 4254 | 4269 | 4267 | 4284 | 4451 | 4209 | 4079 | 3906 |
| Al2O3 | 19.66 | 17.44 | 13.12 | 14.9 | 9.69 | 13.15 | 10.83 | 12.42 | 10.62 | 11.29 | 10.65 | 12.78 | 11.04 | 11.1 | 13.46 |
| BaO | 0.03 | 0.06 | 0.06 | 0.03 | 0.01 | 0.04 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.02 | 0.02 |
| CaO | 0.64 | 0.66 | 0.59 | 0.47 | 0.48 | 0.4 | 0.64 | 0.55 | 0.25 | 0.42 | 0.4 | 1.04 | 1.4 | 0.38 | 1.3 |
| Cr2O3 | 0.02 | 0.05 | 0.03 | <0.01 | 0.03 | 0.02 | 0.02 | 0.02 | 0.01 | 0.03 | <0.01 | <0.01 | <0.01 | <0.01 | 0.01 |
| TFe2O3 | 8.94 | 10.25 | 4.33 | 1.68 | 2.19 | 3.12 | 2.78 | 3.99 | 4.16 | 3.84 | 2.66 | 4.45 | 3.45 | 1.55 | 3.73 |
| K2O | 3.2 | 2.73 | 2.39 | 2.4 | 1.31 | 2.16 | 1.16 | 1.37 | 1.01 | 1 | 1.39 | 1.09 | 0.92 | 1.48 | 1.44 |
| MgO | 0.95 | 2.04 | 1.3 | 0.66 | 0.75 | 0.9 | 0.76 | 0.73 | 0.88 | 0.9 | 0.62 | 1.33 | 0.97 | 0.52 | 1 |
| MnO | 0.08 | 0.11 | 0.09 | 0.05 | 0.06 | 0.06 | 0.07 | 0.09 | 0.15 | 0.13 | 0.08 | 0.18 | 0.16 | 0.08 | 0.2 |
| Na2O | 1.23 | 1.33 | 2.85 | 2.4 | 2.95 | 3.83 | 3.05 | 3.3 | 2.68 | 2.99 | 2.61 | 3.12 | 2.96 | 2.75 | 3.84 |
| P2O5 | 0.01 | 0.03 | 0.16 | 0.05 | 0.07 | 0.11 | 0.15 | 0.13 | 0.03 | 0.16 | 0.1 | 0.2 | 0.15 | 0.07 | 0.14 |
| SiO2 | 57.92 | 58.04 | 70.95 | 72.4 | 78.91 | 73.28 | 77.35 | 73.81 | 77.68 | 76.59 | 78.93 | 70.36 | 75.04 | 78.98 | 70.47 |
| SO3 | <0.01 | 0.02 | 0.02 | 0.02 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.01 | <0.01 | <0.01 | 0.01 | <0.01 | 0.01 |
| SrO | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 |
| TiO2 | 0.91 | 0.82 | 0.66 | 0.63 | 0.39 | 0.48 | 0.43 | 0.48 | 0.37 | 0.47 | 0.37 | 0.78 | 0.48 | 0.19 | 0.44 |
| LOI | 6.14 | 6.36 | 2.92 | 3.37 | 2.49 | 2.04 | 2.37 | 2.6 | 1.95 | 2.17 | 1.9 | 3.97 | 3.53 | 2.26 | 3.56 |
| La | 12.7 | 19.7 | 15.2 | 23.3 | 14.9 | 17.6 | 24 | 27.9 | 15.9 | 23.1 | 20.3 | 21.7 | 17.8 | 18.6 | 20.1 |
| Ce | 23.7 | 43.2 | 32.7 | 51 | 34.8 | 37.7 | 53.1 | 55.3 | 34.3 | 50.6 | 41.7 | 48.1 | 38 | 33.7 | 48.9 |
| Pr | 2.68 | 5.16 | 4.28 | 5.75 | 4.41 | 4.53 | 6.52 | 6.13 | 3.89 | 5.92 | 5.06 | 5.72 | 4.44 | 3.65 | 6.03 |
| Nd | 10.1 | 20.4 | 17.5 | 20.4 | 17.6 | 17.6 | 26.1 | 24.1 | 14.7 | 23 | 19.2 | 21.6 | 18.6 | 13.9 | 25.5 |
| Sm | 2.81 | 4.82 | 4.02 | 4.28 | 4.02 | 3.82 | 5.98 | 5.65 | 2.99 | 5 | 4.59 | 4.98 | 4.16 | 2.97 | 5.52 |
| Eu | 0.58 | 1.02 | 0.97 | 0.75 | 0.86 | 0.97 | 1.09 | 0.95 | 0.56 | 1.04 | 0.79 | 1.01 | 0.86 | 0.46 | 1.24 |
| Gd | 4.84 | 4.79 | 3.98 | 3.87 | 3.35 | 3.31 | 5.42 | 4.86 | 2.59 | 4.72 | 4.18 | 4.58 | 3.71 | 2.2 | 4.75 |
| Tb | 1.26 | 0.88 | 0.7 | 0.8 | 0.54 | 0.53 | 0.95 | 0.86 | 0.47 | 0.79 | 0.7 | 0.76 | 0.59 | 0.35 | 0.72 |
| Dy | 9.43 | 5.57 | 4.37 | 5.38 | 3.14 | 3.24 | 5.47 | 5.47 | 3.04 | 4.85 | 4.14 | 4.51 | 3.76 | 2.12 | 4.24 |
| Ho | 2.29 | 1.25 | 0.96 | 1.25 | 0.66 | 0.72 | 1.16 | 1.23 | 0.69 | 1.06 | 0.92 | 0.97 | 0.84 | 0.45 | 0.89 |
| Er | 7.28 | 3.65 | 2.76 | 3.84 | 1.74 | 2.15 | 3.37 | 3.58 | 2.09 | 3.18 | 2.76 | 2.85 | 2.43 | 1.26 | 2.48 |
| Tm | 1.14 | 0.56 | 0.42 | 0.63 | 0.25 | 0.35 | 0.52 | 0.56 | 0.33 | 0.48 | 0.42 | 0.44 | 0.39 | 0.2 | 0.38 |
| Yb | 7.52 | 3.68 | 2.81 | 4.37 | 1.73 | 2.47 | 3.55 | 3.68 | 2.29 | 3.29 | 2.84 | 2.87 | 2.57 | 1.4 | 2.51 |
| Lu | 1.22 | 0.6 | 0.48 | 0.7 | 0.3 | 0.4 | 0.56 | 0.58 | 0.38 | 0.54 | 0.46 | 0.46 | 0.43 | 0.24 | 0.4 |
| Y | 65.7 | 35.3 | 25.1 | 33.1 | 17.2 | 19.6 | 32.4 | 32.6 | 19.8 | 29.1 | 25.3 | 26.6 | 24.4 | 13.2 | 24.9 |
| Cs | 11.35 | 7.47 | 1.07 | 2.31 | 0.68 | 0.87 | 0.87 | 1.09 | 0.9 | 0.97 | 1.02 | 1.24 | 0.88 | 0.44 | 0.81 |
| Rb | 101 | 83.1 | 54.5 | 64.3 | 33.1 | 49.3 | 32.4 | 37.7 | 27.7 | 29.4 | 38.2 | 30.4 | 25.1 | 31.4 | 34.7 |
| Ba | 392 | 612 | 724 | 390 | 263 | 521 | 293 | 253 | 186.5 | 200 | 235 | 245 | 187 | 290 | 283 |
| Th | 11.3 | 6.6 | 4.25 | 8.46 | 2.78 | 4 | 6.34 | 6.83 | 5.08 | 6.51 | 5.99 | 5.68 | 5.68 | 3.71 | 3.97 |
| U | 2.57 | 2.04 | 1.65 | 2.82 | 1.19 | 1.34 | 2.06 | 2.24 | 1.67 | 2.04 | 1.99 | 1.68 | 1.62 | 1.09 | 1.4 |
| Nb | 13.3 | 9.7 | 6 | 13.4 | 5 | 4.9 | 9.2 | 10.2 | 7.9 | 8.9 | 8.9 | 9.8 | 8 | 4.2 | 5 |
| Ta | 0.8 | 0.6 | 0.3 | 0.9 | 0.3 | 0.3 | 0.5 | 0.6 | 0.4 | 0.6 | 0.6 | 0.6 | 0.4 | 0.3 | 0.3 |
| Cr | 120 | 210 | 130 | 20 | 240 | 140 | 10 | 30 | 10 | 10 | 20 | 40 | 40 | 30 | 80 |
| Ga | 23.2 | 19.6 | 14.5 | 17.5 | 10.3 | 14.4 | 12.3 | 15.4 | 11.8 | 13.2 | 12.9 | 13.8 | 10.3 | 13.8 | 14.4 |
| Hf | 14.8 | 5.8 | 4.4 | 7.3 | 6 | 4.3 | 5.3 | 6.2 | 4.4 | 5.7 | 4.9 | 5 | 4.4 | 3 | 3.8 |
| Sr | 67.9 | 112 | 201 | 140 | 94.4 | 168.5 | 130.5 | 130.5 | 89 | 98.1 | 95.9 | 133.5 | 124.5 | 96.3 | 187 |
| V | 79 | 103 | 80 | 73 | 73 | 61 | 60 | 51 | 62 | 73 | 51 | 104 | 65 | 37 | 64 |
| Zr | 641 | 224 | 163 | 279 | 258 | 154 | 211 | 238 | 170 | 217 | 197 | 208 | 170 | 108 | 143 |
| MnO/Zr | 1.25 | 4.91 | 5.52 | 1.79 | 2.33 | 3.9 | 3.32 | 3.78 | 8.82 | 5.99 | 4.06 | 8.65 | 9.41 | 7.41 | 13.99 |
| Y/Ho | 28.69 | 28.24 | 26.15 | 26.48 | 26.06 | 27.22 | 27.93 | 26.5 | 28.7 | 27.45 | 27.5 | 27.42 | 29.05 | 29.33 | 27.98 |
| LaN/YbN | 1.15 | 3.64 | 3.67 | 3.62 | 5.85 | 4.84 | 4.59 | 5.15 | 4.72 | 4.77 | 4.86 | 5.14 | 4.71 | 9.03 | 5.44 |
| δEu | 0.48 | 0.65 | 0.74 | 0.56 | 0.71 | 0.83 | 0.58 | 0.55 | 0.61 | 0.65 | 0.55 | 0.64 | 0.67 | 0.55 | 0.74 |
| ΣREE | 87.55 | 115.3 | 91.15 | 126.3 | 88.3 | 95.39 | 137.8 | 140.9 | 84.22 | 127.6 | 108.1 | 120.6 | 98.58 | 81.5 | 123.7 |
5. Discussion
5.1. Fluid-Rock Interactions Recorded by Quartz
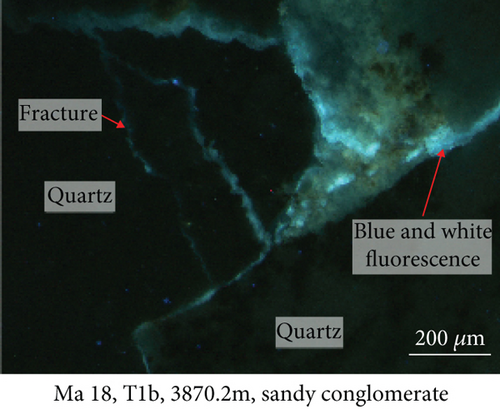
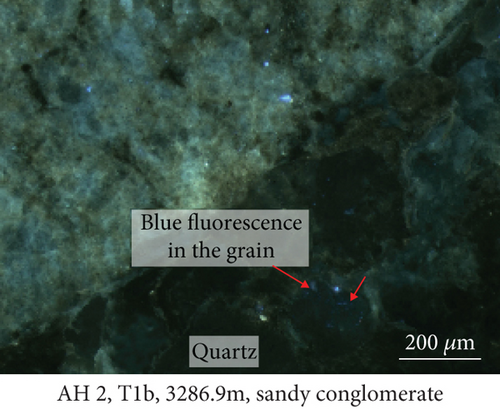
Fluid-rock interactions occur widely in reservoirs, and these can cause migration of chemically active elements, dissolution of minerals, or modification of primary minerals [34, 59, 60]. The sandy conglomerates of the Baikouquan Formation exhibit dissolution and overgrowths at the edges of quartz grains, suggesting that these samples experienced strong fluid-rock interactions (Figures 2 and 3). The quartz grains developed numerous microcracks, and SiO2 from the hydrothermal fluids infilled these microcracks to form new quartz. In addition, abundant fluid inclusions were captured during this process. These samples exhibit obvious blue and white fluorescence around the quartz grains (Figure 7(a)) and even some strong fluorescence at the edges of the grains (Figure 7(b)), implying that hydrocarbons migrated with the hydrothermal fluids involved in the fluid-rock interactions.
The geochemical data indicate that the chemically active elements, such as Fe, Mn, Al, Sr, and W, have higher contents in the rims than in the cores of quartz (Figures 4 and 5), implying that these elements migrated with the fluids and were deposited in the overgrowths.
During the process of hydrocarbon generation, a large amount of organic acids will be discharged along with hydrocarbons, which can promote the dissolution of feldspar and carbonates and thus form dissolution pores [63–65]. This is of significance in the formation and transformation of reservoirs and is also a common model for the formation of feldspar-rich reservoirs in lacustrine basins in eastern China (e.g., the Bohai Bay Basin [39]). However, in some areas of western China, such as the Sichuan and Junggar Basins [66–68], alkaline fluids are considered to have caused the dissolution of quartz during burial. The edges of quartz grains in the Baikouquan Formation have been dissolved and replaced by clay minerals (Figure 2). Most of these quartz grains exhibit features of multiphase growth and contain abundant fluid inclusions, which were related to late fluid activity (Figure 2).
Quartz is relatively stable in acidic and neutral environments but is more easily dissolved in alkaline solutions, especially at higher pH values [69, 70]. Experimental studies have shown that when the pH exceeds 6–7, the dissolution rate of quartz increases rapidly, thus forming curved quartz edges [71]. At higher temperatures and pressures, quartz is also more readily dissolved [72]. Clastic sediments experience the syngenetic, early diagenetic, and late diagenetic stages from the onset of sedimentation through to the earliest metamorphism [72]. Due to the effects of organic matter evolution and fluid-rock reaction in each stage, the fluid pH will increase [73, 74]. Therefore, alkaline fluids form because of burial diagenesis in clastic rocks [69–72]. Acid-labile minerals such as calcite and dolomite can be formed and preserved as cements in most clastic rock reservoirs, which also demonstrates that alkaline formation waters existed. Early and late carbonate cements in clastic rock reservoirs possibly reflect multiphase activity of alkaline fluids. It is known that the Mahu Sag was an alkaline lake basin in the Permian [68], which provided favorable conditions for the dissolution of quartz and formation of quartz overgrowths.
5.2. Implications for Hydrocarbon Migration
Given that a large number of elements are mobile in fluids, when fluids interact with rocks, the elements in the fluids can be partially incorporated into quartz overgrowths, thus resulting in elemental enrichment. Therefore, the distribution and enrichment of elements in authigenic minerals can indicate whether fluids have migrated through rocks and can also be used to infer the direction of hydrocarbon migration.
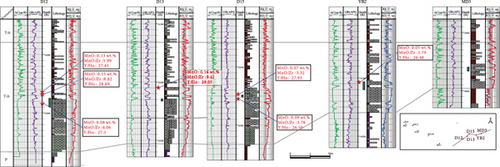
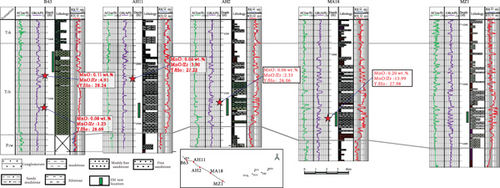
Based on our in situ geochemical data for quartz, we selected several proxies, including MnO contents and MnO/Zr and Y/Ho ratios, to examine the migration of hydrocarbon-bearing fluids in the Mahu Sag. The MnO contents tend to decrease from the center to the outer margin of the sag (Figure 8(a)). Those MnO contents to the south of wells MA18 and D18 are high and gradually decrease to the slope regions in the north, west, and east. The MnO contents on the western slope are slightly higher than those on the eastern slope. The reason for these differences is that Mn tends to concentrate in the center of a sedimentary basin, and MnO is then likely to be migrated away by hydrothermal fluids [47, 48]. Therefore, the direction in which the MnO contents decrease might have been a fluid migration pathway. Basin-scale pressure coefficient contours show that the distribution of MnO is consistent with the pressure coefficients, particularly near wells D12-D13-D15, MA18-AH2, and MA22. This reveals a trend of hydrocarbon migration towards the (N)NE and NW, which is also consistent with the distribution of the Triassic hydrocarbon reservoir (Figure 8(a)). For the trace elements, the chemically active elements also show a gradual change from the center to the margin of the sag. For example, Sr contents also increase to the NW and NE (Figure 8(b)), similar to MnO.
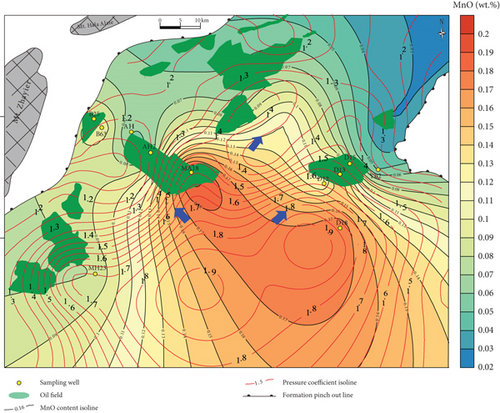
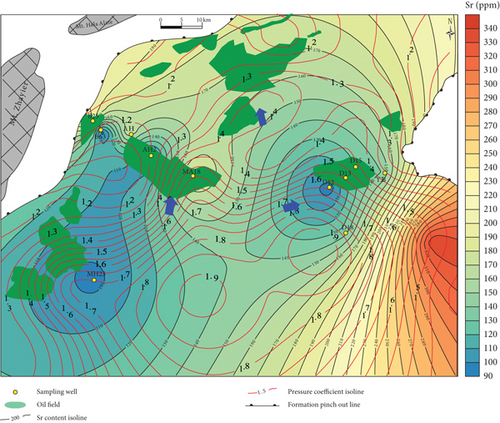
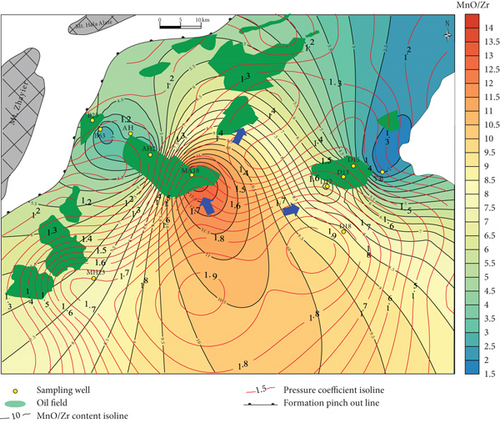
Inorganic geochemical proxies were used to investigate the fluid migration according to the distribution of these chemically active elements. For example, the MnO/Zr ratio was used as Zr which is a fluid-immobile element [62], which is also supported by the in situ geochemical data (Figures 4(c) and 4(d)), whereas Mn was fluid-mobile (Figures 4 and 5). MnO/Zr values decrease gradually from the center to the margin of the sag, and changes also occurred near wells DA12-DA13-DA15, MA18-AH2, and MA22 (Figure 8(c)), similar to the MnO contents. MnO/Zr values have a similar distribution to the pressure coefficients and also reveal fluid migration to the (N)NE and NW.
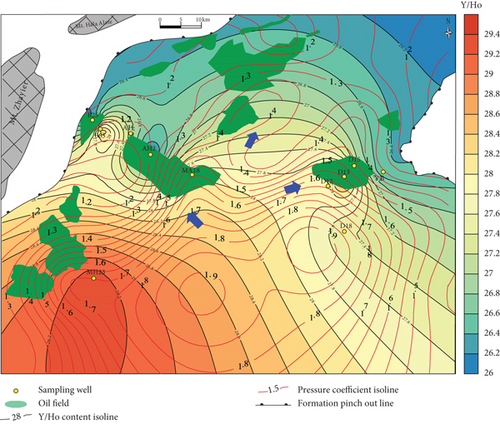
Yttrium and Ho typically exist exclusively as trivalent elements, with similar ionic radii of Y3+ = 0.900 Å and Ho3+ = 0.901 Å [75]. Therefore, Y and Ho often exhibit identical geochemical behavior in geological environments [76]. Previous studies have shown that partial melting and fractional crystallization cannot fractionate Y and Ho, and the Y/Ho ratio in igneous rocks is ~28 [77]. However, these two elements may be significantly fractionated in aqueous solutions [76], such as in seawater, river water, and hydrothermal fluids. The Y/Ho ratios of the studied samples vary widely (Table 4), suggesting that the samples experienced strong fluid-rock interactions. The Y/Ho ratios tend to decrease toward the margin of the sag (Figure 8(d)). As such, Y/Ho ratios also reveal a migration direction to the (N)NE and NW.
Well correlations also reflect the trends of hydrocarbon migration. On the east slope of the Mahu Sag (Figure 9(a)), MnO, MnO/Zr, and Y/Ho decrease gradually from the center to the margin of the sag, suggesting that rocks in the wells on the hydrocarbon migration pathways were subjected to strong fluid-rock interactions. For rocks in wells on the west slope (Figure 9(b)), MnO, MnO/Zr, and Y/Ho tend to decrease.
Our results are also consistent with the hydrocarbon migration inferred from organic geochemistry proxies, such as diasterane/regular sterane and C30 moretane/C30 hopane ratios, and oil exploration results (Figure 8), which show that the western slope is the preferred area for hydrocarbon exploration.
Organic matter in sediments becomes buried and forms source rocks and hydrocarbons. Most studies have used organic geochemistry proxies to infer hydrocarbon migration pathways [19, 20]. However, organic geochemistry proxies are affected by heat and biodegradation effects in geological environments, and these indicators may not be robust in complex geological settings. Previous studies have found that Mn-rich calcite veins are efficient in tracing possible fluid migration pathways in fault zones of the Junggar Basin, and these processes are consistent with the formation of Mn-depleted and Mn-enriched pore waters [47, 48]. This demonstrates that inorganic geochemical methods are another way to investigate hydrocarbon migration. Our study also shows that the distribution of elements can be recorded by quartz overgrowths.
Hydrocarbon migration from source rocks to reservoirs is usually accompanied by the migration of hydrothermal fluids, which can also form hydrocarbon and brine inclusions. Hydrothermal fluids can infill cracks, faults, and unconformities with vein minerals and also enter pores and chemically react with minerals causing mineral dissolution and the formation of authigenic minerals. Based on our inorganic geochemical study of quartz in the Baikouquan Formation in the Mahu Sag, samples on the migration pathway of hydrocarbon-bearing fluids formed authigenic minerals through fluid-rock interactions, which resulted in the exchange and enrichment of certain elements. The fluids evolved with migration distance, with different element enrichments, showing that inorganic elements are affected by the migration of hydrocarbon-bearing fluids. Therefore, our methodology provides a new means for tracing hydrocarbon-bearing fluid migration.
6. Summary and Conclusions
We conducted a petrological and geochemical study of reservoir rocks of the Triassic Baikouquan Formation from the slope of the Mahu Sag, Junggar Basin. Our results show that inorganic geochemical data can track the migration of hydrocarbon-bearing fluids.
The sandy conglomerate samples on the hydrocarbon-bearing fluid migration pathways underwent strong fluid-rock interactions, with formation of clay minerals, and quartz dissolution and overgrowths.
Chemically active elements, such as Mn, Fe, Al, Sr, and W, have higher contents in quartz overgrowths than in quartz cores, implying that these elements were supplied by hydrocarbon-bearing hydrothermal fluids.
MnO contents and Mn/Zr and Y/Ho ratios can be used to reconstruct the hydrocarbon migration and accumulation pathways. The direction in which these contents and ratios decrease can indicate the potential direction of hydrocarbon-bearing fluid migration. More studies from different settings would refine the results and understandings of the present study.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
We would like to express our sincere thanks to PetroChina Xinjiang Oilfield Company for providing core samples and data such as formation pressure and density of crude oil. Besides, our heartfelt thanks are also expressed to the Major National Science and Technology Project (Grant Nos. 2017ZX05001 and 2016ZX03003) and National Natural Science Foundation of China (Grant No. 41702138) which provide funding for the research work.
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.




