Kochiae Fructus, the Fruit of Common Potherb Kochia scoparia (L.) Schrad: A Review on Phytochemistry, Pharmacology, Toxicology, Quality Control, and Pharmacokinetics
Abstract
Kochiae Fructus (KF) is the fruit of an annual potherb Kochia scoparia (Linn.) Schrad and has been traditionally used for the treatment of diseases in the skin, eyes, and urinary tract for thousands of years in China. Recent studies have showed its anti-inflammatory, antifungal, antiallergic, and antipruritogenic effects to clarify the mechanisms of these actions. Meanwhile, its other effects, such as anticancer, hypoglycemic, and hepatoprotective effects, also have been reported. The achievement of these therapeutic effects is contributed by its chemical constituents. A total of 153 compounds have been identified in KF, mainly including triterpenoids, flavonoids, carbohydrates, amino acids, organic acids, and essential oils. Momordin Ic is the representative triterpene glycoside compound, which is used as a phytochemical marker for the quality control of Kochiae Fructus. The research on toxicity is insufficient, and only one article reported that the LD50 was 7.15 ± 0.03 g/kg for water extract of KF after oral administration in KM mice. In addition, the pharmacokinetic study was carried out on momordin Ic with linear pharmacokinetic characteristics. Above all, this review provides comprehensive information about Kochiae Fructus and may provide the theoretic foundation of its clinical application and further development.
1. Introduction
Kochia scoparia (Linn.) Schrad (shown in Figure 1(a)), also called Bassia scoparia (L.) A.J. Scott, is a large annual potherb in the family Chenopodiaceae widely distributed in Europe and Asia and naturalized in Africa, Australia, and North and South America [1]. Kochia Fructus (KF, shown in Figure 1(b)) is the fruit of Kochia scoparia, which is a spheroidal pentagram with a diameter of 1 to 3 mm [2]. It was first recorded in “Shennong Ben Cao Jing” as a “top grade” medicinal material. Up to now, KF has been used in traditional Chinese and Japanese medicine more than 2000 years for the treatment of diseases of the skin, eyes, and urinary tract [3]. With the deepening and development of pharmacology research, it has attracted attention particularly because of its antibacterial, anti-inflammatory, antiallergic, antigastric mucosal damage, hypoglycemic, and immunity enhancing effects [4]. Recently, researchers demonstrated that KF mainly contains terpenoids, flavonoids, essential oils, trace elements, and other ingredients. Although there were many researches on the chemical constituents, pharmacological activities of KF, a systematic and updated review is unavailable. Therefore, the aim of this review is to extensively summarize the phytochemistry, pharmacology, quality control, toxicology, and pharmacokinetics of KF, as well as providing novel insights for clinical uses and further researches.

2. Phytochemistry
With the advancement of analysis technologies such as liquid chromatograph-mass spectrometer (LC-MS), nuclear magnetic resonance-mass spectrometer (NMR-MS), and gas chromatography-mass spectrometer (GC-MS), identification of various components in traditional Chinese medicine has been simplified. To date, 153 compounds within KF, including 25 triterpenoids, 13 flavonoids, 22 carbohydrates (primarily mono- and disaccharides), 21 amino acids, 9 organic acid, 49 essential oils, and 14 heterocyclics, have been identified (Table 1 and Figure 2). Most of investigations indicated that triterpenoids are the main active ingredient within KF. They were characterized with tetracyclic or pentacyclic rings by the polymerization of isoprene. Among them, momordin Ic is a representative triterpenoid saponin with anti-inflammatory effect [5]. Flavonoids were another major component within KF [12]. Most of them are derivatives of flavonol aglycones including quercetin and isorhamnetin. The carbohydrates of flavonoid glycosides are glucopyranose, rhamnose, and galactose. Besides, other flavonoids such as 5,7,4′-trihydroxy-6,3′-dimethoxyflavone and 5,7,4′-dihydroxy-6-methoxyflavone were characterized by LC-MS [6]. KF contains many kinds of amino acid, and current research suggests that certain functional amino acids can play a pharmacological role through the gut-microbiome-immune axis [17]. The essential oil within KF is high fatty acid ester. Yang et al. used a supercritical CO2 extraction method combined with a gas chromatography-mass spectrometer (GC-MS) method to qualify these essential oil components [14]. Eighteen compounds were isolated and identified, most of which were fatty acid esters and aromatic compounds. The level of the higher fatty acid ester is high, and the relative amount of 9,12-octadecadienoic acid is the highest in oil, followed by 9-octadecenoic acid. Wen et al. used the GC-MS method to qualitatively analyze the essential oil within KF [15]. Compared with the standard mass spectrum, 36 components were identified. Among them, the relative level of high fatty acid esters is the highest, and the amount of terpenoids is small.
| No. | Compounds | Formula | Ref. |
|---|---|---|---|
| Triterpenoids | |||
| 1 | 20-Hydroxyecdysone | C27H44O7 | [5] |
| 2 | β-Sitosterol | C29H50O | [6] |
| 3 | Momordin Ic | C41H64O13 | [7] |
| 4 | Oleanic-acid-3-O-β-D-xylopyranosly(1⟶3)-β-D-glucopyranoside | C41H65O12 | [7] |
| 5 | Momordin I | C41H64O13 | [7] |
| 6 | Oleanolic acid | C30H48O3 | [7] |
| 7 | Oleanolic acid 3-O-glucuronide | C36H56O9 | [7] |
| 8 | Oleanolic acid 3-O-β-D-glucopyranoside | C36H56O8 | [7] |
| 9 | 28-O-Deglucosyl-chikusetsusaponin V | C42H66O14 | [7] |
| 10 | 2′-O-Glucopyranosyl-momordin Ic | C48H76O19 | [7] |
| 11 | Chikusetsusaponin V | C48H76O19 | [7] |
| 12 | Momordin IIc | C47H74O18 | [7] |
| 13 | Kochianoside I | C36H56O10 | [8] |
| 14 | Kochianoside II | C47H74O18 | [8] |
| 15 | Kochianoside III | C41H64O14 | [8] |
| 16 | Kochianoside IV | C41H64O13 | [8] |
| 17 | Daucosterol | C35H60O6 | [6] |
| 18 | Oleanolic acid 3-O-β-D-xylopyranosyl(1⟶3)-β-D-glucopyranosiduronic acid 6-methyl ester | C42H66O13 | [6] |
| 19 | 3-O-β-D-Glucuronopyranosy-28-O-β-D-glucuronopyranosyl-oleanic-acid | C42H66O14 | [6] |
| 20 | Oleanolic acid 3-β-D-glucopyranosiduronic acid 6-methyl ester | C37H58O9 | [9] |
| 21 | Stigmasterol-3-O-β-D-glucopyranoside | C35H58O6 | [8] |
| 22 | Oleanic-acid-3-O-[β-D-glucopyranosyl(1⟶2)-β-D-xylopyranosly(1⟶3)]-β-D-glucopyranosiduronic acid | C47H74O18 | [10] |
| 23 | 6′-Methyl ester of momordin Ic | C42H66O13 | [11] |
| 24 | 2′-O-β-D-Glucopyranosyl momordin Ic | C47H74O18 | [11] |
| 25 | 2′-O-β-D-Glucopyranosyl momordin IIc | C53H84O23 | [11] |
| Flavonoids | |||
| 26 | Quercetin 3-O-β-D-apiofuranosyl-(1⟶2)-β-D-galactopyranosyl-7-O-β-D-glucopyranoside | C32H38O21 | [12] |
| 27 | Quercetin 3-O-α-L-rhamnopyranosyl-(1⟶6)-β-D-galactopyranosyl-7-O- β-D-sophoroside | C39H50O26 | [12] |
| 28 | Quercetin 7-O-β-D-glucopyranoside | C21H20O12 | [12] |
| 29 | Quercetin 3-O-β-D-sophoroside | C27H30O17 | [12] |
| 30 | Quercetin 3-O-β-D-galactopyranosyl-7-O-β-D-glucopyranoside | C27H30O17 | [12] |
| 31 | Quercetin 3-O-β-D-apiofuranosyl-(1⟶2)- β-D-galactopyranoside | C26H28O16 | [12] |
| 32 | Isorhamnetin | C16H12O7 | [6] |
| 33 | Quercetin | C15H10O7 | [6] |
| 34 | Rutin | C27H30O16 | [6] |
| 35 | Isorhamnetin-3-O-glucoside | C22H22O12 | [6] |
| 36 | 5,7,4′-Trihydroxy-6,3′-dimethoxyflavone | C17H14O7 | [6] |
| 37 | 5,7,4′-Dihydroxy-6-methoxyflavone | C16H12O6 | [6] |
| 38 | Hyperoside | C21H20O12 | [10] |
| Carbohydrates | |||
| 39 | Sorbitol | C6H14O6 | [13] |
| 40 | Glucose | C6H12O6 | [13] |
| 41 | Galactopyranose | C6H12O6 | [13] |
| 42 | Fructose | C6H12O6 | [13] |
| 43 | Inositol-scyllo | [13] | |
| 44 | Trehalose | C12H22O11 | [13] |
| 45 | Sucrose | C12H22O11 | [13] |
| 46 | N-Acetyl glucosamine | C8H15NO6 | [13] |
| 47 | Mannitol | C6H14O6 | [13] |
| 48 | Mannose | C6H12O6 | [13] |
| 49 | Glucosamine | C6H13NO5 | [13] |
| 50 | Glycerophosphoric acid | C3H9O6P | [13] |
| 51 | Rhamnose | C6H12O5 | [13] |
| 52 | Ribitol | C5H12O5 | [13] |
| 53 | Xylulose | C5H10O5 | [13] |
| 54 | Erythritol | C4H10O4 | [13] |
| 55 | 2-O-Glycerol-α-D-galactopyranoside | C9H18O8 | [13] |
| 56 | Maltitol | C12H24O11 | [13] |
| 57 | Melibiose | C12H22O11 | [13] |
| 58 | Xylose | C5H10O5 | [13] |
| 59 | Ribose | C5H10O5 | [13] |
| 60 | Arabinose | C5H10O5 | [13] |
| Amino acids | |||
| 61 | Tryptophan | C11H12N2O2 | [13] |
| 62 | Tyrosine | C9H11NO3 | [13] |
| 63 | Phenylalanine | C9H11NO2 | [13] |
| 64 | Methionine | C9H11NO2 | [13] |
| 65 | Glutamic acid | C5H9NO4 | [13] |
| 66 | Lysine | C6H14N2O2 | [13] |
| 67 | Glutamine | C5H10N2O3 | [13] |
| 68 | Aspartic acid | C4H7NO4 | [13] |
| 69 | Asparagine | C4H8N2O3 | [13] |
| 70 | Leucine | C6H13NO2 | [13] |
| 71 | Isoleucine | C6H13NO2 | [13] |
| 72 | Pyroglutamic acid | C5H7NO3 | [13] |
| 73 | Threonine | C4H9NO3 | [13] |
| 74 | Valine | C5H11NO2 | [13] |
| 75 | Proline | C5H9NO2 | [13] |
| 76 | Serine | C3H7NO3 | [13] |
| 77 | Alanine | C3H7NO2 | [13] |
| 78 | Glycine | C2H5NO2 | [13] |
| 79 | Ornithine | C5H12N2O2 | [13] |
| 80 | Cysteine | C3H7NO2S | [13] |
| 81 | β-Alanine | C3H7NO2 | [13] |
| Organic acid | |||
| 82 | Glucaric acid | C6H10O8 | [13] |
| 83 | Gluconic acid | C6H12O7 | [13] |
| 84 | Glucuronic acid | C6H10O7 | [13] |
| 85 | Citric acid | C6H8O7 | [13] |
| 86 | Gluconic acid-1,5-lactone | C6H10O6 | [13] |
| 87 | Threonic acid | C4H8O5 | [13] |
| 88 | Malic acid | C4H6O5 | [13] |
| 89 | Malonic acid | C3H4O4 | [13] |
| 90 | Oxalic acid | C2H2O4 | [13] |
| Essential oils | |||
| 91 | α-Pinene | C10H16 | [14] |
| 92 | 2-Camphene | C10H16 | [14] |
| 93 | 9-Hexadecenoic acid | C16H30O2 | [14] |
| 94 | Hexadecenoic acid | C16H30O2 | [14] |
| 95 | Ethyl hexadecenoic acid | C18H34O2 | [14] |
| 96 | 9,12-Octadecadienoic acid | C18H32O2 | [14] |
| 97 | Ethyl 9,12-octadecadienoic acid | C20H36O2 | [14] |
| 98 | 9-Ethyl octadecanoate | C20H38O2 | [14] |
| 99 | Stearic acid | C18H36O2 | [14] |
| 100 | Methyl hexadecanoate | C17H34O2 | [14] |
| 101 | Methyl hexadecenoic acid | C17H32O2 | [14] |
| 102 | Octadeca-9,12,15-trienoic acid | C18H30O2 | [14] |
| 103 | Methyl 9-octadecenoic acid | C19H36O2 | [14] |
| 104 | Methyl 10-octadecenoic acid | C19H36O2 | [14] |
| 105 | Octadecenoic acid | C19H34O2 | [14] |
| 106 | Methyl 11-eicosenoic acid | C21H40O2 | [14] |
| 107 | Methyl eicosenoic acid | C21H42O2 | [14] |
| 108 | Benzene, 1-methyl-2-propyl- | C10H14 | [15] |
| 109 | Cyclohexene, 1-methyl-4-(1-methylethenyl)- | C10H10 | [15] |
| 110 | (E)-3,7-Dimethyl-2,6-octadienal | C10H16O | [15] |
| 111 | Methyl 2-methyl-octanate | C10H20O2 | [15] |
| 112 | Benzene, 1-methoxy-4-(1-propenyl)- | C10H12O | [15] |
| 113 | Methyl nonanoate | C10H20O | [15] |
| 114 | 1-Undecyne | C11H20 | [15] |
| 115 | Ethyl nonanoate | C11H22O2 | [15] |
| 116 | Naphthalene | C11H10 | [15] |
| 117 | Bicyclo(4,3,1)decan-10-one | C10H16O | [15] |
| 118 | 5-Ethyl-2-nonanol | C11H24O | [15] |
| 119 | 4,8-Dimethyl-1-nonanol | C11H24O | [15] |
| 120 | (Z)-β-Farnesene | C15H24 | [15] |
| 121 | trans-β-Farnesene | C15H24 | [15] |
| 122 | 2,6-Di-T-butyl-1,4-benzoquinone | C14H20O | [15] |
| 123 | β-Ionone | C13H20O | [15] |
| 124 | Naphthalene, 1,2,3,5,6,7,8a-octahydro-1,8a-demethyl-7-(1-methyethyl)-[1R-(1α,7β,8aα)]- | C15H24 | [15] |
| 125 | β-Chamigrene | C15H24 | [15] |
| 126 | Ethyl dodecanoate | C14H28O2 | [15] |
| 127 | Hexadecane | C16H34 | [15] |
| 128 | Methyl tetradecanoate | C15H30O2 | [15] |
| 129 | Benzene, 1,1’-(1,2-ethynediyl)bis- | C14H10 | [15] |
| 130 | Octadecane | C18H38 | [15] |
| 131 | 2-Pentadecanone, 6,10,14-trimethyl- | C18H36O | [15] |
| 132 | Ethyl pentadecanoate | C17H34O2 | [15] |
| 133 | Ethyl hexadecanoate | C18H36O2 | [15] |
| 134 | Eicosane | C20H42 | [15] |
| 135 | Methyl octadecadienoate | C19H34O2 | [15] |
| 136 | Heneicosane | C21H44 | [15] |
| 137 | Ethyl-octadecadienoate | C20H36O2 | [15] |
| 138 | Ethyl octadecanoate | C20H40O2 | [15] |
| 139 | Docosane | C22H46 | [15] |
| Heterocyclics | |||
| 140 | Oleamide | C18H35NO | [13] |
| 141 | Ethanolamine | C2H7NO | [13] |
| 142 | Uridine | C9H12N2O6 | [13] |
| 143 | Uric acid | C5H4N4O3 | [13] |
| 144 | Guanine | C5H5N5O | [13] |
| 145 | Adenine | C5H5N5 | [13] |
| 146 | Spermidine | C7H19N3 | [13] |
| 147 | Putrescine | C4H12N2 | [13] |
| 148 | Ethylene glycol | C2H6O2 | [13] |
| 149 | Urea | CH4N2O | [13] |
| 150 | Allantoin | C4H6N4O3 | [13] |
| 151 | Thymine | C5H6N2O2 | [13] |
| 152 | 2-(4-Hydroxy-3-methoxyphenyl)-ethanol | C9H12O3 | [13] |
| 153 | Dopamine | C8H11NO2 | [16] |
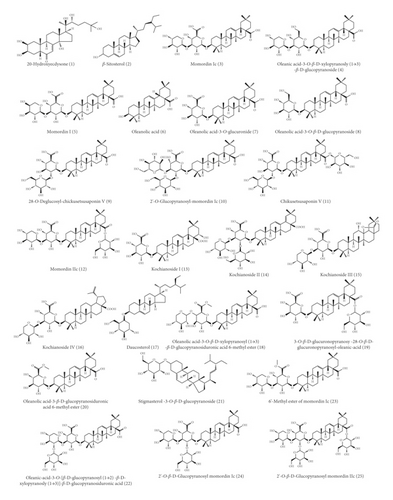
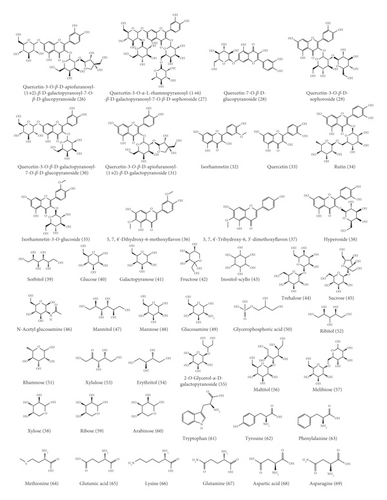
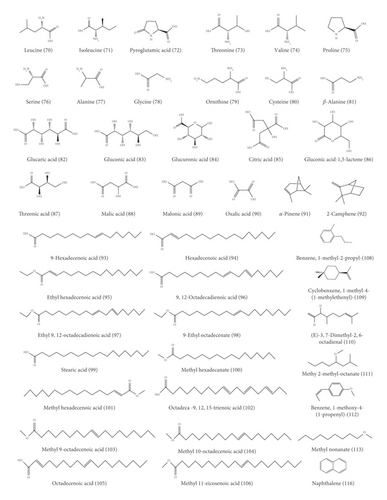
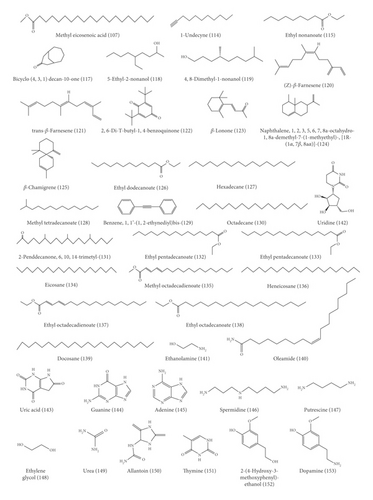
3. Pharmacology
Traditionally, according to records of “Shennong Ben Cao Jing,” KF was used with the therapeutic effects of diuresis and benefiting pneuma. Compendium of Materia Medica described that the KF could be used in the treatment of red eyes, hemntodiarrhoea, pregnancy combined with gonorrhea, and urinary stoppage [18]. Many other books also depicted the traditional use of KF, which are summarized in Table 2. Modern investigations have proved that KF has anti-inflammatory, hypoglycemic, anticancer, antifungal, antipruritogenic, and antinociceptive effects, as well as antiallergic, antiedema, and hepatoprotective activities. We have enlisted an overview of the pharmacological studies in the following sections (Table 3).
| Traditional uses | Reference |
|---|---|
| KF is used to treat frequent urination, urinary incontinence, and abnormal leucorrhea and has an effect of strengthening ears | Ben Cao Qiu Yuan |
| KF is used to treat frequent urination and urinary incontinence and has an effect of strengthening ears | Ben Cao Qiu Zhen |
| KF has an effect of heat-clearing | Ben Cao Shu |
| KF is used to treat skin itching and eczema and has effects of reducing swelling and improving eyesight and ears | Yao Jian |
| KF is good for urinating and has effects of improving eyesight and ears and antiaging | Ben Jing |
| KF is used to treat eczema | Bie Lu |
| KF has an effect for urinating | Ben Cao Zheng Yi |
| KF is used to treat male impotence | Yao Xing Lun |
| KF has an effect of reducing swelling | Ri Hua Zi Ben Cao |
| KF is used to improve urination problems, treat rubella, and abnormal vaginal discharge | Dian Nan Ben Cao |
| KF is used to treat itchy skin | Ben Cao Yuan Shi |
| KF can help urination | Ben Cao Bei Yao |
| KF is used to treat swelling and pain of the head and eyes, back pain, blood in the stool, and malignant sores | Yu Qiu Yao Jie |
| Pharmacological effect | Model | Administration | Minimal active concentration | Reference |
|---|---|---|---|---|
| Anti-inflammatory effect | The ddY mice in an acetic acid-induced vascular permeability |
|
200 mg/kg | [19] |
| The ddY mice in a carrageenin-induced edema |
|
200 mg/kg | [19] | |
| The ddY mice in a compound 48/80-induced edema |
|
500 mg/kg | [19] | |
| The ddY mice in a chemical mediator-induced edema |
|
200 mg/kg | [19] | |
| The isolated ileum of guinea pig in a histamine-induced contraction | Tested drug: 70% ethanol extract of KF at the doses of 10, 50, 100, and 300 μg/mL | 220 μg/mL | [19] | |
| The ddY mice in an arachidonic acid-induced edema |
|
500 mg/kg | [19] | |
| A picryl chloride-induced ear inflammatory model in ICR mice |
|
500 mg/kg | [20] | |
| A dinitrochlorobenzene-induced allergic contact dermatitis model in rats |
|
100 mg/kg | [21] | |
| A DNFB-induced contact dermatitis model in mice |
|
100 μg/ear | [22] | |
| The ddY mice in a carrageenin-induced edema |
|
20 mg/kg | [19] | |
| LPS-stimulated RAW 264.7 cell line |
|
6.25 μM | [5] | |
| Hypoglycemic effect | ||||
| Normal, alloxan-induced hyperglycemic and insulin-induced hypoglycemic mice | Tested drug: n-butanol extract of KF at the doses of 25 and 50 mg/kg, p.o. | 25 mg/kg | [23] | |
| Examining the activity of α-glucosidase in rat intestine in vitro |
|
125 μg/mL | [23] | |
| Examining the ability of glucose absorption in rat intestine in vitro | Tested drug: n-butanol extract of KF at the doses of 0, 100, 200, 400, and 800 μg/mL | 100 μg/mL | [23] | |
| Testing the propulsive function of small intestine in normal, fenfluramine-treated, dopamine-treated, acetic acid-treated, and Nω-nitro-L-arginine-treated rats | Tested drug: n-butanol extract of KF at the doses of 25 and 50 mg/kg, p.o. | 50 mg/kg | [24] | |
| Testing the gastric emptying in rats |
|
25 and 12.5 mg/kg, respectively | [25] | |
| Testing the glucose uptake in rat small intestine in vitro |
|
50 and 5 μM, respectively | [25] | |
| Gastric emptying test on 1.5% carboxymethyl cellulose sodium salt test meal-loaded mice, 40% glucose test meal-loaded mice, milk test meal-loaded mice, and 60% ethanol test meal-loaded mice | Tested drug: momordin Ic in the dose range of 12.5–50 mg/kg, p.o. | 50 mg/kg | [3] | |
| Anticancer effects | ||||
| Antiliver cancer | Inducing apoptosis of human hepatocyte carcinoma HepG2 cells | Tested drug: momordin Ic in the concentration range of 10–30 μM | 15 μM | [26] |
| Inducing autophagy of human hepatocyte carcinoma HepG2 cells | Tested drug: momordin Ic in the dose range of 5–20 μM | 10 μM | [27] | |
| Inducing apoptosis of human hepatocyte carcinoma HepG2 cells | Tested drug: momordin Ic in the dose range of 5, 10, and 15 μM | 5 μM | [28] | |
| Suppressing invasion of human hepatocyte carcinoma HepG2 cells | Tested drug: momordin Ic at the dose of 10 μM | 10 μM | [29] | |
| Inhibiting migration and invasion of human hepatocyte carcinoma HepG2 cells | Tested drug: momordin Ic in the dose range of 1–10 μM | 5 μM | [30] | |
| Antiprostate cancer | VEGF-induced angiogenesis in human umbilical vein endothelial cells and proliferation in prostate cancer cells | Tested drug: methanol extract of KF in the dose range of 10–20 μg/mL and 10–250 μg/mL, respectively | 20 μg/mL and 100 μg/mL, respectively | [31] |
| Testing the SUMO-specific protease 1 in prostate cancer cells and a xenograft PC3 tumor mouse model | Tested drug: momordin Ic at the dose of 6.25, 12.5, and 25 μM and 10 mg/kg/day, i.p. For 20 days, respectively | IC50 was 15.37 μM and 10 mg/kg/day | [32] | |
| Antifungal effect | ||||
| In vitro for Trichophyton mentagrophytes, Trichophyton rubrum, Microsporum canis, Trichophyton violaceum, and Trichophyton schoenleinii | Tested drug: water extract of KF in the concentration range of 0.04%–25% | 3.12% | [33] | |
| Inhibiting the growing of Fusarium graminearum, Fusarium oxysporum, Monilia cinerea, Physalos porapiricola, Alternaria alternata, and Valsa mali | Tested drug: water, petroleum ether, chloroform, ethylacetate, and methanol extract of KF with the concentration of dose 1 mg/15 mL | 1 mg/15 mL | [34] | |
| In vitro for Microsporum ferrugineum, Microsporum gypseum, Trichophyton schoenleini, Trichophyton mentagrophytes, Trichophyton violaceum, Trichophyton rubrum, Epidermophyton floccosum, Aspergillus fumigatus, Candida albicans, Cryptococcus neoformans | Tested drug: the saponin extract, flavone extract I (40% alcohol eluent), flavone extract II (80% alcohol eluent), and lipid extract with no concentration mentioned | All samples have the antifungal effect on Microsporum ferrugineum and Trichophyton rubrum, but the concentration was not mentioned | [35] | |
| Antipruritogenic effect | ||||
| A compound 48/80-induced pruritogenic model in male ddY mice | Tested drug: 70% ethanol extract of KF at the dose of 200 and 500 mg/kg, p.o. | 200 mg/kg | [36] | |
| Tested drug: methanol extract of KF at the dose of 50, 200, and 500 mg/kg, p.o. | 500 mg/kg | |||
|
50 mg/kg | |||
| Itching guinea pig model caused by histamine itching mice model |
|
0.15 g/mL | [33] | |
| Antinociceptive effect | ||||
| The ddY mice in an acetic acid-induced writhing test | Tested drug: 70% ethanol extract of KF at the dose of 50, 200, and 500 mg/kg, p.o. | 500 mg/kg | [19] | |
|
20 mg/kg | |||
| Inhibition effect on hypersensitivity | ||||
| DTH models upon challenge with SRBC or PC |
|
100 mg/kg | [20] | |
|
200 mg/kg | |||
| Myocardial protection | ||||
| A furazolidone-induced dilated cardiomyopathy model in Wistar rats |
|
20 mg/g | [37] | |
| Hepatoprotective effect | ||||
| Carbon tetrachloride-induced liver damage in rats |
|
30 mg/kg/day for 14 days | [38] | |
| Protecting gastric mucosal lesions | ||||
| Ethanol-induced gastric mucosal lesions in rats and indomethacin-induced gastric mucosal lesions in rats |
|
5 mg/kg | [39] | |
| Suppressing osteoclastogenesis | ||||
| A cocultured system and a RANKL-induced osteoclast precursor system | Tested drug: momordin Ic in the dose range of 0.1–5 μM | 0.5 μM | [40] | |
| Antibacteria effect | ||||
| Minimum inhibitory concentration (MIC) test on Escherichia coli | Tested drug: oleanolic acid in the dose range of 15.6–4000 μg/mL | 31.3 μg/mL | [41] | |
3.1. Anti-Inflammatory Effect
Pharmacological studies showed that anti-inflammatory is a very significant pharmacological activity of KF. In six different animal models (the ddY mice in an acetic acid-induced vascular permeability, the ddY mice in a carrageenin-induced edema, the ddY mice in a compound 48/80-induced edema, the ddY mice in a chemical mediator-induced edema, the ddY mice in an arachidonic acid-induced edema, a picryl chloride-induced ear inflammatory model in ICR mice), the 70% alcohol extract of KF has been proved with obvious inhibition effect on the development of inflammation [19, 20]. The methanol extract of KF was used as a candidate drug for the treatment of inflammatory skin diseases due to its eutherapeutic effect on 1-fluoro-2,4-dinitrofluorobenzene-induced contact dermatitis mice model. The mechanism might be involved in inhibiting the skewing reaction of T helper cell type 1 [22]. The total flavonoids of the KF have shown an anti-inflammatory effect on the dinitrochlorobenzene-induced allergic contact dermatitis rats, and the most likely mechanism of this action involves regulating pERK1/2/TLR4-NF-κB pathway activation [42]. The anti-inflammatory effect of KF was coincident with its traditional use for inflammations in vagina and skin.
Three triterpenoid saponins, namely, 20-hydroxyecdysone, momordin Ic, and oleanolic acid from KF have also been investigated on LPS-stimulated murine macrophage RAW 264.7 cell line. 20-Hydroxyecdysone performed significant inhibitory action on prostaglandin E2 (PGE2) generation at the dose of 12.5 μM, while momordin Ic and oleanolic acid showed the anti-inflammatory effect at the dose of 6.25 μM. In addition, momordin Ic significantly reduced productions of tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) at the concentration of 12.5 μM [5].
3.2. Hypoglycemic Effect
KF has shown a potential hypoglycemic effect. Dai et al. illustrated that n-butanol fraction of KF could markedly inhibit gastric emptying in normal mice and could more potently inhibit gastric emptying in hyperglycemic and hypoglycemic mice at the dose of 25 mg/kg. The hypoglycemic mechanism is probably related to transportation and transformation of sugar in the digestive tract and absorption of glucose via the membrane of the small intestine [23]. Subsequently, a research on the function of small intestine was implemented and it found that the n-butanol fraction with a dose of 50 mg/kg could improve the propulsive function of small intestine, and the mechanism of this action probably involves cholinergic nerve and nitric oxide [24].
Matsuda et al. found that momordin Ic inhibited gastric emptying in rats and inhibited glucose uptake in the small intestine in vitro, which contributed to the hypoglycemic action of momordin Ic [25]. Further study showed that momordin Ic inhibits gastric emptying in normal mice, hyperglycemic (including diabetic) and hypoglycemic mice, nonnutrient meal-loaded mice, and nutrient meal-loaded mice [3]. When gastric emptying is slow, the postprandial absorption of food will prolong. Hence, the inhibition of gastric emptying induced by momordin Ic may be useful for the prevention and treatment of diabetes and the morbid obesity with accelerated gastric emptying.
3.3. Anticancer Effect
3.3.1. Antiliver Cancer Effect
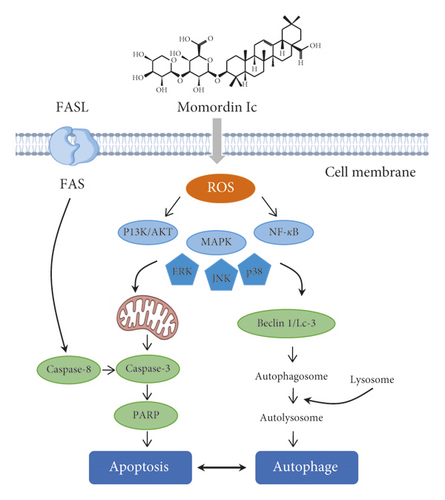
Momordin Ic was the main triterpenoid saponins within KF and has showed an antiliver cancer effect. Wang et al. have carried out a series of researches and found that HepG2 cells were sensitive to the cytotoxic effect of momordin Ic. Momordin Ic could induce apoptosis through oxidative stress-regulated mitochondrial dysfunction involving MAPK and PI3K-mediated iNOS and HO-1 pathways [26]. Based on these results, Wang et al. investigated the MAPK and PI3K pathways and their downstream proteins, such as PPARg and COX-2. Then, they provided the evidence that momordin Ic-induced HepG2 cell apoptosis was associated with PI3K and MAPK pathway-mediated PPARg activation [28]. In addition, Mi et al. showed that the underlying mechanisms of the cross-talk between apoptosis and autophagy involved ROS-related PI3K/Akt, MAPK, and NF-κB signaling pathways, and momordin Ic simultaneously induced apoptosis and autophagy by activating these intersecting signaling pathways [27]. The summarized signal pathway is presented in Figure 3. On the contrary, momordin Ic showed a good anti-invasive activity by altering E-cadherin, VCAM-1, ICAM-1, and MMP-9, and the underlying mechanism involved PPARγ activation and COX-2 inhibition [30].
3.3.2. Antiprostate Cancer Effect
The MeOH extract of KF has shown inhibition effects on human umbilical vein endothelial cell angiogenesis and human prostate cancer cell proliferation [31]. As a member of the de-SUMOylation protease family, SUMO-specific protease 1 (SENP1) is elevated in prostate cancer (PCa) cells and is involved in PCa pathogenesis [43–46]. Momordin Ic as a novel SENP1 inhibitor could inhibit proliferation of prostate cancer cells in vitro and in vivo by inducing cell cycle arrest and apoptosis [32]. The possible mechanism is that momordin Ic could increase the sub-G1 phase cell population, increase numbers of annexin-V positive cells, increase active caspase-3, caspase-8, and PARP1 cleavage, and reduce cyclin B and CDK1 levels. Thus, it was considered that SENP1 may play an important role in momordin Ic-induced cell death in prostate cancer cells, even though the downstream effectors of SENP1 that mediate momordin Ic-induced apoptosis are currently unknown.
3.4. Antifungal Effect
In vitro, the water extract of KF showed a strong inhibition on common dermatophytes. Its minimum inhibitory concentration (MIC) on Trichophyton mentagrophytes was 3.12% and on Trichophyton rubrum, Microsporum canis, Trichophyton violaceum, and Trichophyton schoenleinii were 0.78% [33]. Wu et al. tested six KF extracts against Fusarium graminearum, Fusarium oxysporum, Monilia cinerea, Physalos porapiricola, Alternaria alternata, and Valsa mali. As a result, the water extract had the strongest inhibition effect on all six plant pathogenic bacteria with antifungal activities of more than 74.34%, and the water, petroleum ether, chloroform, ethylacetate, and methanol extracts showed stronger antifungal activities against Monilia cinerea and Valsa mali than the others [34]. In addition, the saponin extract, flavone extract I (40% alcohol eluent), flavone extract II (80% alcohol eluent), and lipid extract from KF were tested against Microsporum ferrugineum, Microsporum gypseum, Trichophyton schoenleini, Trichophyton mentagrophytes, Trichophyton violaceum, Trichophyton rubrum, Epidermophyton floccosum, Aspergillus fumigatus, Candida albicans, and Cryptococcus neoformans. The lipid extract showed a good antifungal effect on Microsporum ferrugineum, Microsporum gypseum, Trichophyton mentagrophytes, and Trichophyton rubrum; the saponin extract gave inhibition effects on Microsporum ferrugineum, Microsporum gypseum, Trichophyton schoenleini, Trichophyton mentagrophytes, and Trichophyton rubrum; the flavone extract I (40% alcohol eluent) exerted inhibition effects on Microsporum ferrugineum, Trichophyton rubrum, and Epidermophyton floccosum, whereas the flavone extract II (80% alcohol eluent) play an inhibitory role on Microsporum ferrugineum, Microsporum gypseum, and Trichophyton rubrum. Unfortunately, the MIC was not mentioned in this article [35]. Above all, this effect of KF supports its traditional use in gynecological infection.
3.5. Antipruritogenic Effect
The 70% ethanol extract (200 mg/kg) and methanol extract of KF (500 mg/kg) have been proved to inhibit the scratching behavior on a compound 48/80-induced pruritogenic model in male ddY mice [36]. Momordin Ic isolated from KF also exhibited an inhibition effect at a dose of 50 mg/kg. Meanwhile, in an itching guinea pig model and an itching mice model, the water extract of KF at the concentration of 0.15 g/mL could significantly decrease the number of itching and total time of itching within 30 minutes, indicating that KF could be used as an antipruritogenic agent [33]. These results agree with the traditional use of KF for itch.
3.6. Others
The inhibition effect of hypersensitivity of 70% ethanol and total saponin extracts from KF has been tested on DTH models upon challenge with SRBC or PC. As a result, the 70% ethanol extract produced a concentration-dependent reduction on immediate and delayed-type hypersensitivity, while total saponins extract showed an inhibitory tendency at 200 mg/kg [20]. This effect might have a close relationship with its stabilization of mast cell membrane, reduction of release of anaphylactic mediators, and anti-inflammatory activities [20]. The water extract of KF at the dose of 20 mg/g could reverse the imbalance of Th1/Th2 cell in rat model with dilated cardiomyopathy, which reduced the damage of immune response to myocardium and protected the heart function. Momordin Ic gave a protective effect on gastric mucosal lesions [37]. Kim et al. discovered that momordin Ic has a hepatoprotective effect against CCl4-induced liver damage because it could enhance the hepatic antioxidant defense system [38]. Meanwhile, as an AP-1 inhibitor, momordin Ic could downregulate NF-κB activation as well as AP-1 activation, which plays a key role in osteoclast differentiation, by inhibiting IκB degradation and c-Fos expression, respectively. Therefore, momordin Ic has high potential to be a good candidate for controlling bone disorders in the future [40]. In addition, the extracts of KF, including total flavonoid, saponin, and phenolic, were proved to have a good antioxidant activity by measuring free radical scavenging activities with ABTS, DPPH, or FARP assay [47–50]. Scavenging free radicals play a key role in aging and inflammation [51]. Therefore, its antioxidant effects need to be further studied on these aspects.
The studies on toxicity of KF are scarce. Although KF is almost nontoxic in traditional use, the animal death was found when a large dosage is used. According to a toxicological study, when KM mice were orally administrated with water extract of KF in a dose range from 4.5 mg/kg to 9.4 mg/kg, the median lethal dose (LD50) was 7.15 ± 0.03 g/kg [35]. Therefore, the toxic and side effects of KF should be paid more attention in its clinical application.
4. Quality Control
The quality of herbal medicines is the key for its clinical efficacy and safety, and hence, establishing a quality control system is the premise of its clinical application. The quality control of KF was focused on quantitative analysis of components by a series of analytical methods, such as ultraviolet-visible detector (UV), gas chromatography-mass spectrometry (GC-MS), and high-performance liquid chromatography-evaporative light scattering detector (HPLC-ELSD). Since the main component of KF is triterpenoid saponin, whose UV absorption is poor, researchers incline to use ELSD. Xia et al. developed a method to determine the content of momordin Ic in KF and the content of momordin Ic was 0.83%–0.21% in four kinds of marketed KF [52]. Moreover, they used HPLC-ELSD and colorimetric methods to determine the content of momordin Ic and total saponins, respectively, in KF at different collecting times or from eleven places in China [53, 54]. Except for saponin, there also established an HPLC method with good stability and reproducibility to simultaneously determine the content of rutin and quercetin in KF [55]. Currently, the fingerprint derived from HPLC has been an acknowledged method to control the quality of traditional Chinese medicine and botanical medicine. An HPLC fingerprint method was applied to 10 batches of KF purchased from Shandong, China. According to the cluster analysis, 19 common peaks of fingerprint were found, and 10 batches could be divided into 3 groups related to their origins [56]. The results showed that this method could differentiate samples from different geographical origins or processing methods. Nevertheless, there were few compounds quantified as mark compounds for the quality control of KF, and it is in urgent need of a comprehensive quantification method to further ensure the quality control.
5. Pharmacokinetics
Momordin Ic is a representative pharmacologically active ingredient and quality control marker of KF, and its pharmacokinetic property has attracted attentions. Yan et al. developed and validated a highly selective and sensitive method based on ultraperformance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) for routine analysis of momordin Ic in rat plasma. After intravenous administration of momordin Ic at 0.52, 1.56, and 4.67 mg/kg in rats, the AUClast (area under the concentration-time curve from time 0 to t hours postodose) values were 1864.17 ± 431.01, 5466.00 ± 889.86, and 16890.45 ± 3028.64 ng h/mL, respectively, which was consistent with linear pharmacokinetic characteristics. The elimination half-life (t1/2) values were 1.22 ± 0.39, 1.14 ± 0.10, and 1.83 ± 0.39 h, respectively [4]. However, there are few studies on the pharmacokinetic of other substances within KF and on the interaction between substances during their ADME in vivo.
6. Conclusion and Perspectives
Recent pharmacology studies showed anti-inflammatory, antifungal, antiallergic, and antipruritogenic effects of KF, which supports the traditional clinical applications including the treatment of diseases in the skin, eye, and urinary tract in China, Korea, and Japan. Interestingly, the anticancer, hypoglycemic, and hepatoprotective effects of KF were also tested. Besides, the potential mechanisms of some effects were also elucidated. However, there are few toxicology studies on KF, which may be necessary for its better application as a medicine or a food. A total of 25 triterpenoids, 13 flavonoids, 22 carbohydrates, 21 amino acids, 9 organic acid, 49 essential oils, and 14 heterocyclics within KF have been reported. Momordin Ic is a main substance, and it is usually used as a phytochemical marker for the quality control of KF. The pharmacological effects were achieved by the chemical constituents within KF. Hence, the interrelationship between compounds and pharmacological activities should be further studied. The pharmacokinetics of KF was lack, and a range of pharmacokinetic studies on its active compounds are needed to provide comprehensive data for clinical application. Altogether, this review extensively summarized phytochemistry, pharmacology, toxicity, quality control, and pharmacokinetic studies on KF to provide information for its further research and clinical applications.
Disclosure
The funding sponsors had no intervention in the design of the study, collection, analyses, and interpretation of data, writing the manuscript, and also the decision to publish the experiment results.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors’ Contributions
Wei Zou and Zhong Tang contributed equally.
Acknowledgments
This research was funded by the Hunan Provincial Science and Technology Department (Grant nos. 2018SK50501, 2019JJ30013, and 2020RC3065) and Hunan Administration of Traditional Chinese Medicine (Grant no. 202020).
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.




