The Impact of Media Coverage and Curfew on the Outbreak of Coronavirus Disease 2019 Model: Stability and Bifurcation
Abstract
In this study, the spreading of the pandemic coronavirus disease (COVID-19) is formulated mathematically. The objective of this study is to stop or slow the spread of COVID-19. In fact, to stop the spread of COVID-19, the vaccine of the disease is needed. However, in the absence of the vaccine, people must have to obey curfew and social distancing and follow the media alert coverage rule. In order to maintain these alternative factors, we must obey the modeling rule. Therefore, the impact of curfew, media alert coverage, and social distance between the individuals on the outbreak of disease is considered. Five ordinary differential equations of the first-order are used to represent the model. The solution properties of the system are discussed. The equilibria and the basic reproduction number are computed. The local and global stabilities are studied. The occurrence of local bifurcation near the disease-free equilibrium point is investigated. Numerical simulation is carried out in applying the model to the sample of the Iraqi population through solving the model using the Runge–Kutta fourth-order method with the help of Matlab. It is observed that the complete application of the curfew and social distance makes the basic reproduction number less than one and hence prevents the outbreak of disease. However, increasing the media alert coverage does not prevent the outbreak of disease completely, instead of that it reduces the spread, which means the disease is under control, by reducing the basic reproduction number and making it an approachable one.
1. Introduction
The most considerable species in habitat are viruses. Since they cannot reproduce themselves, they are known as parasites. Some of these viruses, when they are replicated, cause dangerous diseases in the host, whether humans or animals. One of these species is the coronavirus (COVID-19). It is a disease caused by a new coronavirus called SARS-CoV-2. An outbreak of pneumonia caused by a novel coronavirus (COVID-19) began on 31 December 2019, in Wuhan, China. This pandemic that rapidly spread over 215 countries as of March 2020 continues to hit heavy public health and socio-economic burden in many parts of the world, including in Iraq. From January 22, 2020, up to March 3, 2021, it was reported that over 115 million confirmed cases and about 2,565,310 deaths around the world [1].
The experience in China showed that the use of complete curfew and the speed diagnosis of the infected individuals have a strong impact on the dynamics of the epidemic [2]. It is well known that there is a long history of applying mathematical models in epidemiology to understand and control the outbreak of disease. Carcione et al. [3] implemented an SEIR model to compute the COVID-19 infected population and the number of casualties of this epidemic. Eikenberry et al. [4] developed a compartmental model for assessing the community-wide impact of masks used in the US states of New York and Washington by the general, asymptomatic public, a portion of which may be asymptomatically infectious. Maji [5] formulated a mathematical model for the COVID-19 outbreak by introducing a quarantine class with media-induced fear in the disease transmission rate to analyze the dynamic behavior of this epidemic. Asamoah et al. [6] developed a deterministic model to study the transmission dynamics of the disease with two categories of the susceptibles: Immigrant Susceptibles and Local Susceptible. Peter et al. [7], proposed a new mathematical model to investigate the recent outbreak of coronavirus disease (COVID-19) in Pakistan. Das et al. [8] formulated a mathematical model with comorbidity to study the transmission dynamics as well as an optimal control-based framework to diminish COVID-19. Musa et al. [9] employed an SEIR model to study the transmission dynamics of SARS-CoV-2 outbreaks in Nigeria; the model incorporates a different group of populations, namely, high and moderate risk populations. They used the model to investigate the influence of each population on the overall transmission dynamics. Recently, Paul et al. [10] proposed a simple population dynamics solution based on the incidence-fitness relationship in predicting that a plateau or steady-state of SARS-CoV-2 will be reached using the basic concept of geometry. Many other researchers have been proposed and studied mathematical models to describe the dynamic of COVID-19 (see [11–14] and the references therein).
In view of the above, in this paper, however, a mathematical model that describes the spreading of COVID-19 is proposed and studied. It is assumed that the population is divided into five compartments including susceptible, exposed, infected, hospitalized infected, and recovered. The impact of curfew, media alert coverage, and social distance on the dynamical behavior of the model is considered. The paper is organized as follows. Section 2 treats the formulation of the model with standard properties of the solution of the model. Section 3 interests in the existence of equilibria and computing the basic reproduction number. Section 4 studies local stability. While global stability is discussed in Section 5. The local bifurcation analysis is investigated in Section 6. However, Section 7 contained the numerical simulation for the model. Finally, Section 8 gives the discussion and conclusion of this study.
2. Formulation of the Model
All the parameters are nonnegative real numbers, and their descriptions are explained in Table 1. Note that b(I) = (b1 − b2I/m + I) is the contact rate after the media alert (see [15]). The choice of this function to describe the media alert is due to the following reasons. Most human beings will protect themselves from infection using all possible ways as soon as infected individuals are reported by media coverage. This reduces the transmission rate more or less. Accordingly, as the infected individuals increase, the susceptible individuals reduce their contact with other individuals to avoid the infection. Moreover, the term (b2I/m + I) refers to the reduced value of the contact (transmission) rate in the case of reporting of the infectious individuals. Clearly, as the infected individuals increase without bound, the term (b2I/m + I) approaches its maximum that is given by b2. However, the value of (b2I/m + I) equals half of the maximum b2 when the reported infective number arrives at m. Finally, since the media alert report cannot prevent the disease from spreading completely, it is assumed that b1 ≥ b2.
| Parameter | Description |
|---|---|
| A | The recruitment rate |
| c | The probability of individuals responding to the curfew |
| b1 | The direct contact rate between S and I in the absence of media alerts |
| b2 | The direct contact rate between S and I in the existence of media alerts |
| m | Nonresponse rate of the individuals to the media alerts |
| p | The rate of incubation termination and transition from uninfected to infectious individuals |
| q | The fraction of the exposed individuals move to the infected compartment, while the rest (1 − q) moves to the hospitalized infected population |
| r1 | The recovery rate due to natural immune response |
| r2 | The recovery rate due to treatment |
| v | The fraction of the nonrecovered individuals who start suffering severe symptoms move to the hospitalized infected population |
| d0 | The natural death rate |
| d1 | The death rate of disease in the infectious compartment |
| d2 | The death rate due to disease in the hospitalized infected population |
Recall that the interaction functions between the compartments given by the right-hand side of the system (1) are continuous and have continuous partial derivatives. Therefore, they are Lipschitz functions, and hence system (1) has a unique solution.
Theorem 1. All solutions of system (1) which initiate in are uniformly bounded.
Proof. Let (S(t), E(t), I(t), Ih(t), R(t)) be any solution of system (1) that initiates at the nonnegative value (S(0), E(0), I(0), Ih(0), R(0)).
Consider the function N(t) = S(t) + E(t) + I(t) + Ih(t) + R(t).
By taking the derivative of N(t) with respect to the time along with the solution of system (1), it is obtained that
Then, using direct computation gives that
Accordingly, it is obtained that
According to system (1), the feasible region of it can be written as follows:
3. Existence of the Equilibrium Points and Basic Reproduction Number
Since the basic reproduction number is computed at this equilibrium point, hence in the following the computation of this number is carried out, and then the endemic equilibrium point is determined.
4. Local Stability Analysis
Theorem 2. The disease-free equilibrium point (ρ0) of system (7) is locally asymptotically stable provided the following condition holds:
Proof. According to equation (18), the Jacobian matrix at the point (ρ0) is given by
Direct computations show that this Jacobian matrix has the following characteristic equation:
According to theorem (6), the basic reproduction number represents the vital value for controlling the outbreak of COVID-19, so that the disease will disappear provided that the basic reproduction number is less than one while the COVID-19 will outbreak when the basic reproduction number is greater than one.
Theorem 3. The endemic equilibrium point (ρ1) of system (7) is locally asymptotically stable provided that
Proof. Substituting the endemic equilibrium point (ρ1) in the Jacobian matrix given by equation (18) gives that
Hence, the characteristic equation of this Jacobian matrix can be written as
While Δ = A1A2 − A3 = A1[b11b22 + b22b33 − b23b32] − b11b33(b11 + b33), accordingly, by substituting the values of bij and then using condition (22) along with the existence condition (16), we obtain that A1, A3, and Δ are positive. Therefore, all the roots (eigenvalues) of the third-order polynomial equation in (24) have negative real parts. Furthermore, since the fourth eigenvalue λ4 = b44 = −B3 < 0, then the proof is complete.
From theorem (7), it concluded that the COVID-19 will be endemic and the disease is out of control if in addition to the basic reproduction number greater than one, condition (22) is satisfied.
5. Global Stability
In this section, the stability analysis of system (7) is studied using the Lyapunov function in order to obtain the global stability conditions or the basin of attraction for each equilibrium point. Therefore, the following theorem discusses the global stability of the free-disease equilibrium point.
Theorem 4. Assume that free-disease equilibrium point ρ0 = (A/d0, 0,0,0) is locally asymptotically stable, then it is global asymptotically stable.
Proof. Consider the following positive definite real valued function:
Clearly, , V0 (ρ0) = 0, and V0 (x) > 0 where x ≠ ρ0. Moreover, direct computation gives that
Clearly, we have dV0/dt ≤ 0 if and only if the local stability condition (19) for the free-disease equilibrium point holds. Hence, V0 is negative definite for any initial point that belongs to , and then the proof is complete.
Theorem 5. Assume that endemic equilibrium point is locally asymptotically stable, then it is a global asymptotically stable provided that the following conditions hold:
Proof. Consider the positive definite function
Clearly, , V1 (ρ1) = 0, and V1(x) > 0 where x ≠ ρ1. Moreover, direct computation gives that
Accordingly, by using conditions (27a)–(27e), we obtain that
Clearly, the derivative dV1/dt is negative definite for any initial point belonging to , and then the proof is complete.
6. The Local Bifurcation Analysis
Theorem 6. System (7) exhibits a transcritical bifurcation near the disease-free equilibrium point ρ0, where R0 = 1 or .
Proof. Straightforward computation shows that the Jacobian matrix of system (7) at (ρ0, b1 ∗) can be written as
Therefore, the eigenvalues of J0 can be written as
Accordingly, the disease-free equilibrium point becomes the nonhyperbolic point. Now, define as the eigenvector corresponding to the eigenvalue λ03 = 0. Direct computation gives that
Define as the eigenvector corresponding to the eigenvalue λ03 = 0 of the matrix . Direct computation gives that
Hence,
Then, it is obtained that . Furthermore, it is observed that
Therefore, . However, substituting the values of U1 in equation (32) instead of V and then computing give that
Clearly, . Hence, all the transcritical bifurcation conditions in the sense of Sotomayor’s theorem are satisfied. Therefore, the proof is complete.
It is observed that although system (7) becomes a nonhyperbolic near the endemic equilibrium point ρ1 as p = −b11 b22 b33/q(b13b21 − b11b23)(≡p∗) where bij, ij = 1,2,3,4 are the elements if J(ρ1) that is given in equation (23), it does not exhibit any types of local bifurcation in the sense of Sotomayor. This is can be verified directly by substituting the values of ρ1 and p∗ in the value of A3 that is given in equation (24) and then computing the eigenvectors corresponding to the resulting zero eigenvalues of J(ρ1, p∗) and their transpose, respectively.
It is obtained that the eigenvectors corresponding to the zero eigenvalue of J(ρ1, p∗) and their transpose are, respectively, given by
Accordingly, any type of the local bifurcations in the sense of Sotomayor cannot occur.
7. Numerical Simulation
To complete the analysis of the proposed system, it should be simulated numerically with real and reasonable parameter values and then the obtained results are validated with those obtained in the previous sections. It is well known that the model parameters affect the dynamical behavior of the model greatly. This occurs due to either error in their measurement or missing the parameter values. Accordingly, the impact of varying the model’s parameters on the behavior of the solution of the model is important. Therefore, the parameters that are having vital roles in the dynamics of the model will be given clear attention to understand their effects on the control of the spread of disease. Most of the parameter value ranges used in this section are taken from the World Health Organization reports and adopted by many references as stated in Table 2. Since the population of Iraq is nonhomogenous distributed, therefore, we will confine ourselves to apply the proposed model on a sample that exists in a specific region to get more accurate results.
| Parameter | Range | Value used | Source |
|---|---|---|---|
| A | 30–1000 day−1 | 50 | [6] |
| c | [0,1] | 0.5 | Assumed |
| b1 | 6.038 × 10−8–0.8158 day −1 | 0.001 | [6, 11, 12] |
| b2 | (0, b1) day−1 | 0.0001 | [15] |
| m | [0, ] | 10 | [15] |
| p | [1/14–1/3] day−1 | 0.1 | [4, 13, 14] |
| q | (0,1) | 0.5 | Assumed |
| r1 | [1/14–1/3] day−1 | 0.08 | [4, 13, 14] |
| r2 | 0.05–0.9 day−1 | 0.1 | [6] |
| v | 0.01–0.5 day−1 | 0.3 | Assumed |
| d0 | 0.000042–0.011 day−1 | 0.01 | [6, 11, 12] |
| d1 | 0.0044–0.1850 day−1 | 0.001 | [6, 11, 12] |
| d2 | 0.0044–0.1850 day−1 | 0.01 | [6, 11, 12] |
For the parameter values used in Table 2, system (1) is solved, and the trajectories are obtained as shown in Figure 1.
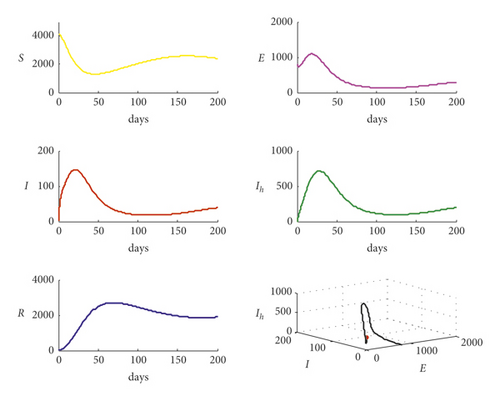
According to Figure 1, system (1) is settled at the endemic point when the basic reproduction number is R0 = 2.4771, which means that the disease is out of control. However, for the data used in Table 1 with different values of parameter m, that m = 0,100,200,300, respectively, the trajectories of system (1) are drawn in Figure 2.
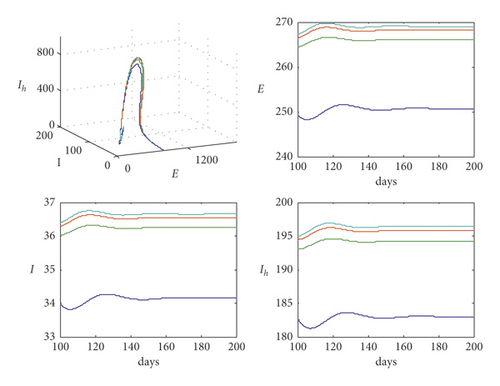
It is clear from Figure 2 that with the increase in the value of m, the trajectory still settled at endemic but the populations of exposed, infected, and hospitalized infected increased too.
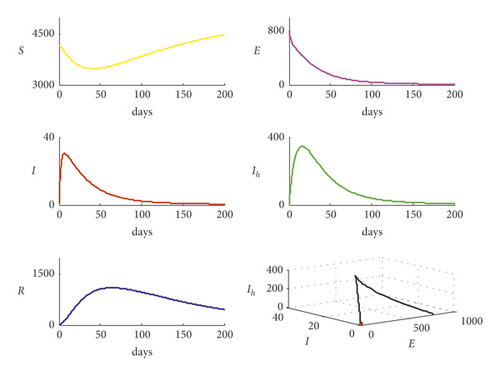
Now, for the data given in Table 2 with c = 0.9, it is observed that the basic reproduction number decreases to R0 = 0.6193 and the trajectory of system (1) is shown in Figure 3. It is clear from Figure 3 that when R0 < 1, system (1) approaches asymptotically to disease-free equilibrium point and that confirms the theoretical results in previous sections.
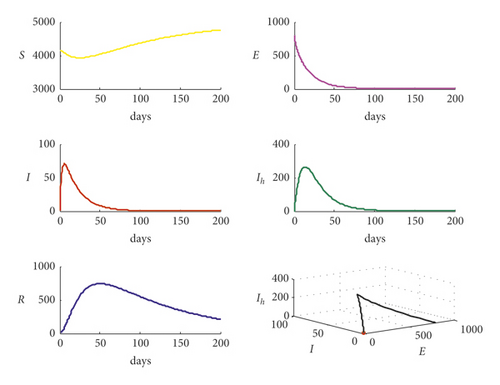
On the other hand, increasing the parameter b1 from b1 = 0.001 to b1 = 0.01 with data used in Figure 3 makes the trajectory of system (7) return back to settle at the endemic equilibrium point as shown in Figure 4.
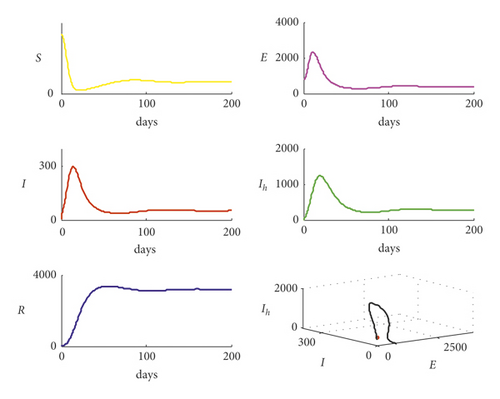
Clearly, from Figure 4, the bifurcation occurs in system (1) and the trajectory of system (1) transfers from disease-free point to endemic point as the bifurcation parameter b1 increases, passing through the value and then becomes b1 = 0.01. It is noted that the basic reproduction number increases from the value R0 = 0.6193 to the value R0 = 6.192717 passing the bifurcation value R0 = 1. Now, for the data given in Table 2 with reducing the value of q from the value q = 0.5 to the value q = 0.2, the basic reproduction number decreases from the value R0 = 2.4771 to the value R0 = 0.9908, and the trajectory of system (1) is shown in Figure 5.
Again, Figure 5 ensures the obtained results regarding the stability analysis of the disease-free equilibrium point. Finally, other parameters are also varying to study their effects on the dynamical behavior of system (1), and it is observed that quantitative effects exist rather than qualitative one so that the population size of the different compartments in the system changes slightly.
8. Discussion and Conclusion
In this paper, an epidemic model to describe the spread of COVID-19 is proposed using the first-order ordinary differential equations. The model takes into account the impact of the curfew and media coverage on the control of the spreading of disease. It is observed that model (1) has two equilibrium points: the disease-free point that is stable, which means the disease is under control from a biological point of view, when the basic reproduction number satisfies R0 < 1 and unstable for R0 > 1, and the endemic equilibrium point that is stable, which means the disease out of control from a biological point of view, whenever the disease-free becomes unstable. All the properties of the solution of the model are studied. The local bifurcation near the disease-free point is investigated too. An application of the proposed model on the sample of Iraqi population of size 5000 has been done using baseline values of the parameters of system (1) as suggested by World Health Organization reports and adopted by many researchers, and the following observations are obtained.
The model has only one type of attractor, approaches either to disease-free equilibrium point or to the endemic equilibrium point, which means either stop the outbreak of COVID-19 or spread the COVID-19, depending on R0 < 1 and R0 > 1, respectively. The model is solved numerically using the Runge–Kutta fourth-order method with the help of Matlab. The model is applied to a sample of the Iraqi population using the parameter value ranges as suggested by the World Health Organization reports and adopted by many references as stated in Table 2. It is observed that increasing the probability of an individual’s response to the curfew leads to reducing the basic reproduction number below one and then controlling the spread of COVID-19, and hence system (1) approaches asymptotically to the disease-free point. However, increasing the direct contact rate between S and I in the absence of media alerts or decreasing the social distance between the population individuals leads to an increase in the basic reproduction number above one, and then an increase in the outbreak of the disease occurred. Hence, the trajectory of system (1) approaches the endemic point. Although increasing the response rate of the individuals to the media alerts (that means decreasing m) cannot prevent the disease from spreading completely, it leads to reduce it up to a minimum rate. A similar result is observed by increasing the parameter b2, which increases the term (b2I/m + I) that reduces the value of the transmission rate when infectious individuals appear and are reported. Finally, decreasing the fraction of the exposed individuals moving to the infected compartment, which leads to increasing the number of individuals moving to hospitalized infected populations, can prevent the disease from spreading and the system approaches a disease-free equilibrium point.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Open Research
Data Availability
The data used to support the findings of this study are included within the article.