Establishment of the Causal Agent(s) of Spike Shedding and Stem Wilting of Pepper (Piper nigrum L.) in Morogoro District, Tanzania
Abstract
Pepper (Piper nigrum L.) serves as a source of income to smallholder spice farmers in Morogoro district of Tanzania. Recently, spike shedding and stem wilting of pepper plants were reported to cause about 30% yield loss of the crop. This study was undertaken to identify the causal agent(s) of the problems. Three pepper gardens in each of the four hamlets (Nyange, Doga, Tandai, and Lukenge) in Tandai village (Kinole ward) were surveyed, and plant tissue and soil samples were collected for laboratory analysis. An experiment was laid out in a randomized complete block design with three replications. Pepper cultivars, Babu kubwa, Babu ndogo, Babu kati, and Ismailia grown in the study area, were used as treatments for evaluation of the prevalence of spike shedding and stem wilting. Treatments mean separation was conducted based on Duncan’s multiple range test at p = 0.05. Regression (R2) and simple correlation (r) analyses between stem wilting (incidence and severity) and termite pest infestation were performed. The identified pathogen in most of the soil and plant tissue samples was Fusarium oxysporum. The nutrients, N, P, Mg, and Cu, concentration in soil and leaf samples was below the optimal ranges. Termite attacks were significantly high in some farmer’s gardens where up to 50.5% of the pepper plants was damaged. Hence, a significant positive correlation was observed between termite pest infestation in gardens and both incidence (r = 0.881, R2 = 0.74, p < 0.001) and severity of stem wilting (r = 0.918, R2 = 0.84, p < 0.001) of pepper plants. Most of the termite attacks and damages (70%) were observed on pepper plants supported on silver oak, unlike those trained on Jatropha. A higher percentage of undeveloped berries (36%) and intermediate-size berries (34%) was recorded on cultivars “Babu kati” and “Babu kubwa,” respectively. Multiple factors have been associated to be the causes of spike shedding and stem wilting of pepper, which could be used to develop appropriate control solutions useful to farmers. Confirmation of F. oxysporum pathogenicity is recommended. A comprehensive study is suggested on the repellent or lethal activities of Jatropha against F. oxysporum and termites. Furthermore, studies through field experiments are needed to generate site-specific soil nutrient improvement recommendations and design an integrated approach to control F. oxysporum and termites and to determine existing alternative host plants for these pests.
1. Introduction
Pepper (Piper nigrum L.) is one of the most important agricultural commodities and traded spices in the world [1]. It serves as a cash crop with a diverse industrial and domestic uses. Pepper is used in food and drinks for imparting agreeable flavor and aroma and as a preservative. It is also used in the production of essential oil for the pharmaceutical and perfumery industries [2]. Pepper is rich in aroma and pungency, which are attributed to the presence of an alkaloid called piperine [3, 4]. It is an important cash crop to small-scale farmers along the slopes of Mountain Uluguru in Morogoro district. Other spices produced in the district include clove, cinnamon, cardamom, ginger, turmeric, lemongrass, and vanilla [5]. In most part of the world, pepper cultivation is limited by a number of factors including lack of appropriate agronomic practices, continuous use of low yielding cultivars, unavailability of superior planting materials, and losses due to incidences of biotic and abiotic stress [4]. Nutrient imbalance between the soil and plant often predispose the pepper plants to diseases including spike shedding and stem wilting and leaves yellowing [6]. Besides diseases, pepper is also attacked by insect pests such as flea beetles, leaf gall thrips, mealybugs (Ferrisia spp.), and pollu beetles (Longitarsus spp.). In India, pollu beetles have been reported to cause spike shedding which accounts for 30–40% of yield losses. Adults and nymphs of mealybugs, stinkbugs (Euschistus spp.), and leaf-footed bugs (Leptoglossus spp.) tend to suck the plant sap, resulting in discoloration and wilting of plants [7].
Spike shedding of pepper includes but not limited to either drop of flowers or berries and improper filling of spikes when berries fail to develop into normal size. Stem wilting of pepper plants includes withering, drooping/limping, and the drying out of stems. In many areas producing pepper, spike shedding [6] and stem wilting [8] are common incidences. Spike shedding occurs at various stages of flower and fruit development. In India, stem wilting has led to crop loss of 40% or more [9]. Factors leading to shedding of berries include lack of pollination, prolonged drought, heavy rains, and sudden change in weather among seasons. Nutrition imbalance between the soil and plant predisposes the pepper plants to diseases such as fusarium wilt and slow wilt [6]. Apart from nutrition imbalances, pathological attacks of fungi and nematodes either singly or in their combinations have been associated with stem wilting [10, 11]. Two nematodes of taxa Radopholus spp. (burrowing nematodes) and Meloidogyne spp. (root-knot nematodes) are known to cause stem wilting in pepper. Varying degrees of foliar yellowing, defoliation, and damages on feeder roots caused by nematodes tend to be misinterpreted as symptoms of nutrient deficiency. Fungi species such as Fusarium and Phytophthora have been reported to cause yield losses of 30.5–64.6% in India [10].
The biocontrol bacterium (Curtobacterium luteum) applied in combination with the fungicide metalaxyl significantly reduced nematode population and foot rot incidence due to Phytophthora spp. and increased the growth and yield of pepper in India [12]. In Vietnam, a systemic fungicide (potassium phosphonate) as a soil drench (applied at rates of 50–100 g a.i/plant) significantly inhibited the colonization of P. capsici on excised leaf, stem, and root tissues [13]. Edward et al. [14] reported significant control (antifungal activity) of five endophytic bacteria isolates (EB1 to EB5) over both inhibition of mycelia growth and spore germination of F. oxysporum. Secretion of volatile and diffusible bioactive compounds was among the demonstrated antifungal activities. Enterobacter sp. and Bacillus sp. (B. megaterium and B. cereus) were suggested to be the closest identities of the bacterial isolates. Endophytic bacteria (Bacillus megaterium) applied as a biocontrol significantly inhibited nematodes in the soil (81.86% inhibition), colonization of pepper plant roots (by 73.11%), and reduced the nematode build-up rate to 0.23. B. megaterium also promoted plant growth and enzymatic activities (chitinase and protease) related to the biocontrol of Meloidogyne sp. [15]. Foliar sprays with a plant growth regulator, 1-naphthaleneacetic acid (NAA) at 50 ppm [16] and soil application of zinc sulphate at 2.5 kg ha−1 [17], reduced spike shedding and increased the yield and quality of pepper. These studies indicate that spike shedding and stem wilting of pepper can have various biotic causes that are amenable to mitigation using available conventional and biological control agents. Despite that yield loss, studies have not yet been conducted; crop losses of about 30% have been reported by pepper farmers along the slope of Mount Uluguru in Morogoro [18]. Still, information on the etiology/causes of the problem in the district is limited. Based on observations by Maerere and Van Noort [19], a comprehensive survey was needed to assess the extent and cause(s) of spike shedding and stem wilting of pepper in Morogoro district, Tanzania. It was also desirable to provide/establish appropriate control solutions, but this study sought to determine the causal agent(s) of the problem in a selected pepper-growing village located along the Uluguru Mountains in northeastern Tanzania, which in turn will allow the development of appropriate control measures.
2. Materials and Methods
2.1. Study Site
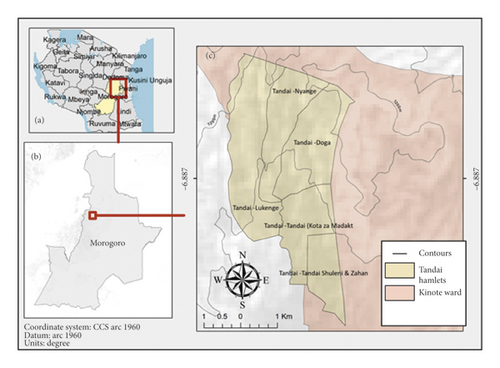
The study was conducted in Tandai village (Kinole ward) in Mkuyuni division, Morogoro district, between April and September 2019. The village is located at 6°54′S, 37°45′E. The area experiences a bimodal rainfall pattern with short rains between late October and the end of January and long rains between February and May, while a dry season is experienced from June to mid-October. The area receives monthly precipitation ranging from 6 to 66 mm and mean monthly minimum and maximum air temperature range of 17–20°C and 27–31°C, respectively [20]. Four hamlets (Figure 1), namely, Nyange, Doga, Tandai, and Lukenge located between 532 and 806 meters above sea level (masl), were selected for the study based on their importance in pepper production. Pepper is an introduced crop to this area and suitably grown due to the ecological requirements of relatively high rainfall, humidity, and warm temperature. Moreover, a multipurpose market exists in Kinole ward particularly at Tandai village where buyers rent space for collecting, drying, and storage of produce including spices [19]. Based on the number of plants and varieties grown, three pepper gardens were sampled in each of the selected hamlets for the establishment of the causes of spike shedding and stem wilting of pepper plants. The study utilized the participatory action approach where farmers were involved at every possible stage during the farm survey and sample collection [21].
2.2. Soil Sampling and Analysis
The method by Zu et al. [22] was used, whereby three composite soil samples were collected in each garden. Each of the three composite samples was made up of five subsamples collected on an S-shape sampling configuration at a plough depth of 0–20 cm in each garden. The composite soil samples were reduced by the quartering procedure to obtain a representative sample weighing 0.5 kg and then passed through a 2 mm aperture sieving before laboratory analysis. The soil samples were analyzed for physical and chemical properties in the Soil and Geological Science Laboratory at Sokoine University of Agriculture (SUA), Morogoro. Soil particle size distribution was determined using the hydrometer method [23]. Soil pH was determined potentiometrically in water (H2O) solution after 24 hours in soil suspension (soil/liquid = 1/2.5 w/v) [24]. Exchangeable bases were extracted with neutral ammonium acetate solution. Exchangeable bases, calcium (Ca) and magnesium (Mg), were determined by an atomic absorption spectrophotometer, while potassium (K) and sodium (Na) were determined by flame photometry [25]. Soil available phosphorus (P) was determined by the Bray II method [26], and soil organic carbon was determined by the Walkley–Black method [27]. Total nitrogen (N) was determined by the Kjeldahl digestion method [28]. Plant extractable Cu, Zn, Mn, and Fe were extracted by the DTPA and measured by an atomic absorption spectrometer [29].
2.3. Leaf Sampling and Analysis
Samples were collected according to the procedure described by Partelli [30]. The pepper plant leaves were sampled during the period of fast fruit growth (three months after flowering). The first adult complete leaves (blade + petiole) from the terminal bud of productive branches localized in the middle and external portions of the canopy were taken. Four leaves per plant were sampled from a minimum of 20 plants per garden. The leaf samples were washed to eliminate contamination from adhering particles such as dust and then sun-dried to brittleness for 48 hours [31]. The dried leaf samples were ground, and a portion (1 g of each sample) was turned into ashes in a muffle furnace at 550°C for 4 hours in preparation for laboratory analysis [31]. The leaf samples were analyzed for chemical properties in the Soil and Geological Science Laboratory at SUA. Levels of Ca, Mg, K, and Na in pepper plant leaves were determined by an atomic absorption spectrophotometer [25]. Level of P in leaves was determined by the acid wet digestion method (HCIO4/HNO3) using an AAS flame photometer [31]. Level of N in leaves was determined by the Kjeldahl digestion method [32]. Levels of Cu, Zn, Mn, and Fe in pepper plant leaves were extracted by the DTPA and measured by an atomic absorption spectrometer [29].
2.4. Incidence and Severity of Leaf Yellowing and Stem Wilting
Determination of the incidence and severity of leaf yellowing and stem wilting on each of the pepper cultivar under the study was conducted based on the method described by Abebe [33]. The number of plants with leaves yellowing and/or stem wilting out of 50 plants observed in the target farms was used to calculate the percentage incidence of leaves yellowing and stem wilting (equations (1) and (3)). Scoring for leaves yellowing severity was performed based on visual observation using a leaves yellowing severity index of the 0–3 scale, where 0 is no yellowing, 1 is up to 25%, 2 is 26–50%, and 3 is more than 50%. Scoring for stem wilting severity was also performed based on visual observation using stem wilting severity index of the 0–4 scale, where 0 is no wilting, 1 is 1–25%, 2 is 26–50%, 3 is 51–75%, and 4 is >75%. The percentage severity of leaves yellowing and stem wilting was calculated using equations (2) and (4), respectively. Stem of pepper plants was examined for wilting through visual observation. The number of wilted internodes was counted out of 60 plants with stem wilting observed in the selected farms. The percentage of affected plants was established with respect to the number of wilted internodes of plants stem summarized in equation (5).
2.5. Extraction of Plant Parasitic Nematodes from Soil and Pepper Plant Tissues
A total of three soil samples and three root samples were collected per garden on each of the pepper cultivar plants under the study showing symptoms of leaf yellowing and stem wilting. Collected samples were analyzed in the Plant Pathology Laboratory at SUA. The tray method modified from Baermann funnel and maceration and sieving methods [34] were used to extract nematodes from the soil and roots. The soil was placed on a sieve in a dish with water and left at room temperature for 48 hours. Roots were washed, cut into pieces of 1 cm length, macerated in a blender, and passed through a series of sieves with 200 mm, 160 mm, and 25 mm apertures. A stereo microscope at 40x–80x magnification was used to examine the presence of plant parasitic nematodes from the filtrate.
2.6. Isolation of Pathogenic Fungi from Soil and Pepper Plant Tissues Showing Leaf Yellowing and Stem Wilting
A total of three soil samples and three pepper plant tissue samples were collected per cultivar in each garden. Plant tissue samples (roots together with the collar of the stem) and soil samples were taken from the base of pepper plants showing typical leaf yellowing and stem wilting [11]. The plant tissues were washed under tap water, cut into small pieces, and surface sterilized with 1% sodium hypochlorite solution for one minute. The tissues were then rinsed using distilled water and dried on sterile paper towels under aseptic conditions. The tissues were transferred into Petri dishes containing 20 ml of molten V8 juice agar media. Soil sample weighing 1 g was added to 50 ml of sterile water, and then, the suspension was centrifuged for 5 minutes. About 1 ml of the supernatant was transferred into Petri dishes containing 20 ml of molten V8 juice agar media. All plates were incubated at 25°C for five days. Fungi were identified based on microscopic observation (mycelial and sporangial characters) of the colonies developed from the soils and plant tissues [35].
2.7. Assessment of Termite Pest Incidence on Pepper Gardens
2.8. Prevalence of Spike Shedding
2.9. Data Analysis
The collected data were subjected to the analysis of variance using GenStat 16th version software (VSN International). Treatments mean separation was conducted based on Duncan’s multiple range test at p = 0.05 as the level of significance. Regression (R2) and simple correlation (r) analysis between stem wilting (incidence and severity) and termite pest infestation in pepper gardens were calculated using Pearson product moment correlation coefficient and tested for the degree of significance of correlation coefficients in the MS office excel programme.
3. Results
3.1. Status of Soil Nutrients in Pepper Gardens
Most of the surveyed farms in Tandai village had soils with pH which was strongly acidic to slightly acidic (Table 1). Soils of surveyed farms in Nyange, Doga, Tandai, and Lukenge hamlets showed deficiency of potassium (K), calcium (Ca), and magnesium (Mg) and optimum levels for organic carbon (OC) content, manganese (Mn), and iron (Fe). Exceptions were for nitrogen (N) at Tandai and Lukenge hamlets, phosphorus (P) at Nyange, Doga, and Lukenge hamlets, and zinc (Zn) at Tandai hamlets which were low. In addition, copper (Cu) at Nyange was deficient. Most of the surveyed farms at Nyange hamlets had sandy clay loam soil, while farms in Doga, Tandai, and Lukenge hamlets had sandy clay soil. Table 1 in the Supplementary Material shows comprehensive table analysis.
| Soil property | Optimum value | Nyange hamlet | Doga hamlet | Tandai hamlet | Lukenge hamlet |
|---|---|---|---|---|---|
| Chemical properties | |||||
| pH | 4.75–6.25 | 5.15STA | 5.90MA | 5.60MA | 6.23SLA |
| OC | 2.0–7.5% | 3.03OP | 2.13OP | 2.15OP | 2.23OP |
| Tot. N | 0.2–0.5% | 0.23M | 0.22M | 0.17L | 0.19L |
| Bray P | 12–96 ppm | 10.45L | 10.16L | 12.49OP | 11.323L |
| Exch. K+ | 91–286 ppm | 0.18D | 0.20D | 0.80D | 0.82D |
| Exch. Ca2+ | 61–139 ppm | 1.03D | 0.37D | 0.20D | 0.70D |
| Exch. Mg2+ | 40–194 ppm | 2.35D | 0.97D | 1.04D | 1.30D |
| DTPA Fe | 12–65 ppm | 124.50H | 83.85H | 25.41OP | 38.11OP |
| DTPA Mn | 5–35 ppm | 11.80OP | 62.27H | 32.56OP | 56.57H |
| DTPA Zn | 2.1–7.0 ppm | 7.92OP | 4.33OP | 2.03L | 3.13 OP |
| DTPA Cu | 0.51–7.7 ppm | 0.0281D | 1.5712OP | 2.3427OP | 3.1143OP |
| Physical properties | |||||
| Soil particle size density (P.S.D) | % Clay | 30.2 | 39.2 | 37.2 | 39.22 |
| % Silt | 6.82 | 10.82 | 9.82 | 9.82 | |
| % Sand | 62.96 | 49.96 | 52.96 | 50.96 | |
| Texture class | Sandy clay loam | Sandy clay | Sandy clay | Sandy clay |
3.2. Concentration of Nutrient Elements in Mature Pepper Leaves
The results (Table 2) of pepper plant nutritional status assessment through leaf analysis indicate low N and P for Nyange and Doga hamlets. The analysis indicated optimal levels for all the other nutrients. Exceptions were for K at Doga and Mg and Cu at Doga, Tandai, and Lukenge hamlets which were low. Table 2 in the Supplementary Material shows comprehensive table analysis.
| Nutrient | Optimum value | Nyange hamlet | Doga hamlet | Tandai hamlet | Lukenge hamlet |
|---|---|---|---|---|---|
| N | 1.65–2.79% | 1.56L | 1.35L | 1.66OP | 2.15OP |
| P | 0.11–0.26% | 0.09L | 0.07L | 0.11OP | 0.12OP |
| K | 1.78–2.84% | 2.45OP | 0.76L | 2.73OP | 2.08OP |
| Ca | 1.42–3.33% | 2.25OP | 2.87OP | 1.55OP | 2.17OP |
| Mg | 0.40–0.69% | 0.56OP | 0.22L | 0.36L | 0.27L |
| Fe | 126–1145 ppm | 387.48OP | 215.97OP | 400.18OP | 686.03OP |
| Mn | 109–721 ppm | 197.38OP | 206.54OP | 406.98OP | 254.36OP |
| Zn | 21–67 ppm | 47.56OP | 21.91OP | 23.30OP | 32.31OP |
| Cu | 16–120 ppm | 16.70OP | 5.76L | 12.72L | 9.74L |
- L, low; D, deficient; OP, optimum [39].
3.3. Characteristics and Incidence of Stem Wilting on Pepper Plants
Results of visual observation of affected plant tissues showed unilateral necrosis of the vascular tissues that extended to leaf veins of apical twigs resulting in wilt and death of plants. Externally, diseased plants showed foliar yellowing, shedding of leaves, internodes necrosis, and lack of rootlets (Figures 2(a)–2(c)). The internodes showed necrotic lesions around the nodes of main branches resulting in unilateral necrosis of the internode (Figures 2(b) and 2(c)). Longitudinally dissected branches showed necrotic vessels immediately below the epidermis.

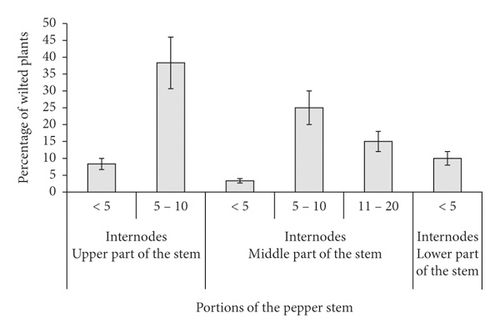
In the occasion where stem wilting occurred during the reproductive stage (spiking and fruit development stages), the spikes and fruits also wilt and shed-off. Most (38% and 25%) of the affected pepper plants had 5–10 wilted internodes on the upper and middle parts of the stem. About 10% of affected pepper plants had less than five wilted internodes on the lower part of the stem (Figure 3).
3.4. Nature of Pathogenic Species
Test results for establishment of the causative agent of stem wilting turned negative for plant parasitic nematodes. However, the study showed positive results for fungi on samples collected from all the four surveyed hamlets in Tandai village. Based on plant tissue, soil culture, microscopic observations, and characterization [35], the fungus was isolated, purified, and identified as Fusarium oxysporum (Table 3).
| Village | Hamlet | Incidence of leaf yellowing (%) | Severity of leaf yellowing (%) | Incidence of stem wilting (%) | Severity of stem wilting (%) | Fungi | Nematodes |
|---|---|---|---|---|---|---|---|
| Tandai (n = 24) | Nyange | 24.0 | 10.0 | 4.0 | 1.0 | Fusarium oxysporum | Negative |
| Doga | 36.0 | 16.7 | 20.0 | 4.7 | Fusarium oxysporum | Negative | |
| Tandai | 40.0 | 20.0 | 32.0 | 16.7 | Fusarium oxysporum | Negative | |
| Lukenge | 38.0 | 19.2 | 36.0 | 17.6 | Fusarium oxysporum | Negative |
- ∗ n, number of samples examined; altitudinal location of the hamlets: Nyange (806 m asl); Doga (650 m asl); Tandai (581 m asl); and Lukenge (532 m asl).
3.5. Cultural and Morphological Characteristics of Fungi
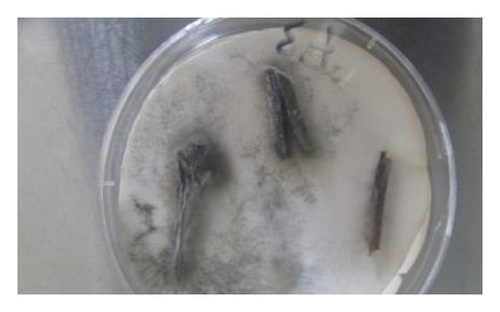
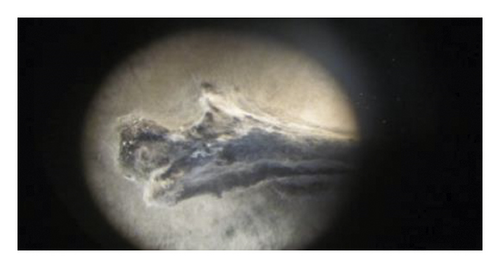
During the study, the pathogen (Fusarium oxysporum) was characterized by colons with the following features: sparse to abundant white mycelial growth covered part of the plant tissue and soil samples (Figures 4(a)–4(c)). Slimy masses of conidia were seen along the hyphae. Macroconidia were hyaline thin-walled, falcate to almost straight, with apical cells short and slightly hooked. Septum number varied from 3 to 7 with 3-4 septa spores being more frequent (Figures 4(d)–4(f)). Microconidia were 0 is septate or 1 is septate (mostly 1, septate) and were produced in abundance varying in shape from ovoid to elliptic (Figure 4(d)).

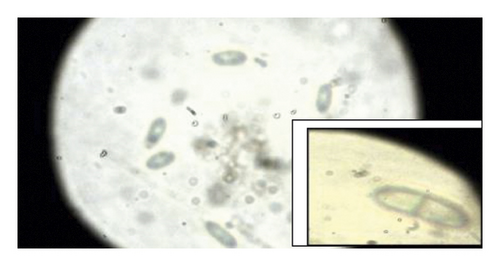
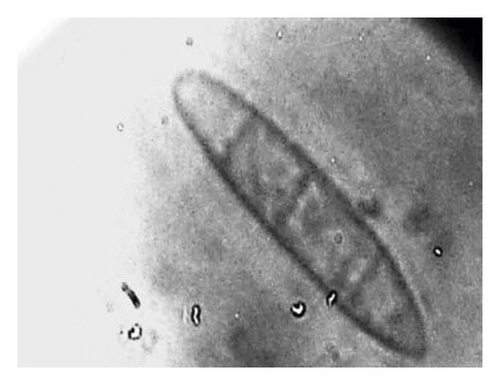
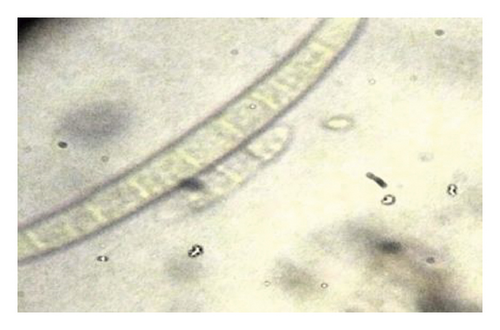
3.6. Termite Pest Infestation on Pepper Plants and on Gardens as Influenced by the Type of Standards
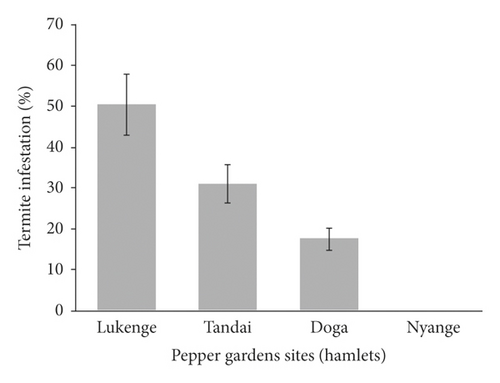
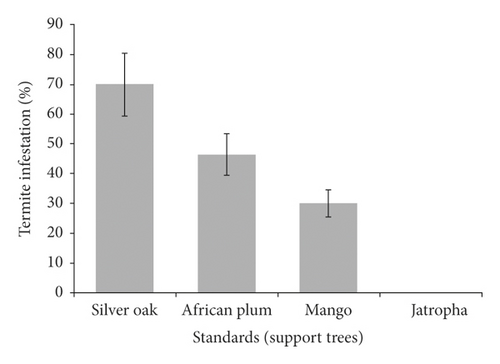
Termites infestation level was significantly different (p < 0.05) among pepper gardens. No signs of termite infestation were observed on pepper gardens at Nyange hamlet. However, 50.5%, 31%, and 17.5% of pepper plants in the gardens at Lukenge, Tandai, and Doga, respectively, were damaged by termites (Figure 5(a)). Termite infestation varied significantly (p < 0.05) with support plants (standards) used in the gardens. No signs of termite infestation were observed on Jatropha (Jatropha curcas) (Figure 5(b)). However, the highest termite infestation/damage was recorded on silver oak (Grevillea robusta (70%)), African plum (Vitex doniana (46.3%)), and mango (Mangifera indica (30%)) and the least (11.8%) on jackfruit (Artocarpus heterophyllus). Termite mounds in gardens (Figures 6(a) and 6(b)) and foraging on pepper stems covered with soil tunnels along the support plants (Figures 6(c) and 6(d)).

3.7. Relationship between Termite Pest Infestation and the Occurrence of Stem Wilting on Pepper Plants in Gardens at Tandai Village
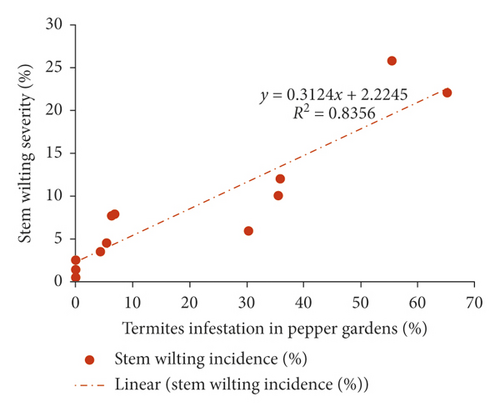
Termite infestation on pepper gardens in surveyed hamlets at Tandai village was significantly higher, indicating a positive correlation with the incidence (r = 0.881, R2 = 0.74, p < 0.001) and severity of stem wilting (r = 0.918, R2 = 0.84, p < 0.001) of pepper (Figures 7(a) and 7(b)).

3.8. Number of Individual Termites Species on Pepper Gardens
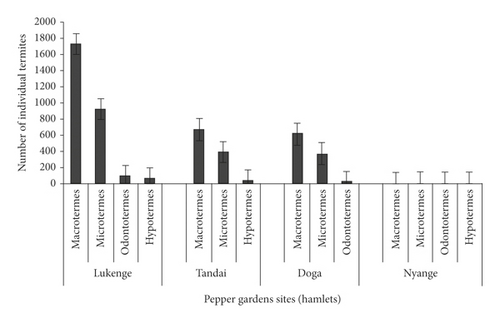
The number of individual termites in pepper farms varied significantly (p < 0.001) with termite species. The common termite pest species at Lukenge hamlet were Macrotermes (1732.8), Microtermes (922.2), Odontotermes (91.5), and Hypotermes (58). On the other hand, common termite pest species at Tandai hamlet were Macrotermes (668.8), Microtermes (392), and Hypotermes (38). At Doga hamlet’s pepper gardens, the dominant termite species were Macrotermes (614.5), Microtermes (371.2), and Odontotermes (19.5). Generally, the results (Figure 8) show that Macrotermes followed by Microtermes were the most frequent termite species recorded in pepper farms at Lukenge, Tandai, and Doga hamlets.
3.9. Prevalence of Spike Shedding in Pepper Plants Grown in the Study Area
3.9.1. Number of Spikes per 100 Leaves
Pepper cultivars differed significantly (p < 0.05) in terms of number of spikes per 100 leaves (Table 4). Cultivar “Babu kati” had a significantly higher number of spikes (76.4) followed by “Ismailia” (71.2), Babu kubwa” (65), and “Babu ndogo (37.7).
| No. of spikes/column at different heights | Percentage (%) of berry per spike | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cultivars | Spike length (cm) | No. of spikes/100 leaves | 1 m | 2 m | No. of flowers spike−1 | No. of well-developed berries spike−1 | Full-size (berry set) | Intermediate-size | Undeveloped berries |
| Babu kubwa | 12.4c | 65.0b | 86.6ab | 105.5a | 93.5b | 48.1ab | 51.3a | 34.0b | 12.7a |
| Babu ndogo | 5.9a | 37.7a | 73.6a | 127.5b | 55.4a | 35.9a | 65.0ab | 26.3ab | 6.7a |
| Babu kati | 10.6b | 76.5b | 141.3c | 183.7c | 90.7b | 40.2ab | 46.7a | 15.3a | 36.0b |
| Ismailia | 10.6b | 71.2b | 121.0bc | 254.4d | 67.6a | 58.0b | 86.0b | 10.7a | 1.3a |
| Mean | 9.9 | 62.6 | 105.6 | 167.8 | 76.8 | 45.6 | 62.2 | 21.6 | 14.2 |
| SE± | 0.31 | 9.72 | 16.85 | 7.92 | 6.41 | 7.09 | 9.81 | 6.70 | 6.40 |
| CV% | 4.7 | 5.1 | 2.4 | 1.3 | 5.0 | 4.7 | 19.3 | 38.0 | 55.3 |
| Fpr | <0.001 | 0.028 | 0.023 | <0.001 | 0.003 | 0.079 | 0.026 | 0.046 | 0.007 |
- Means bearing the same letter(s) within the column are insignificantly (p < 0.05) different according to Duncan’s multiple range test. Fpr, F distribution probability; CV, coefficient of variation; SE±, standard error.
3.9.2. Number of Spikes on the Vertical Side at 1 m Height from the Ground
Significant difference (p < 0.05) was recorded among pepper cultivars in terms of a number of spikes on the vertical side at 1 m height (Table 4). The highest number of spikes (141.3) was recorded on cultivar “Babu kati” than all other varieties; however, varieties “Babu kubwa” (86.6) and “Ismailia” (121) had higher number of spikes than “Babu ndogo” (73.6).
3.9.3. Number of Spikes on the Vertical Side at 2 m Height from the Ground
Number of spikes on the vertical side at 2 m height differed significantly (p < 0.001) with pepper cultivars (Table 4). Ismailia had the highest number of spikes (254.4) followed by “Babu kati” (183.7) and “Babu ndogo” (127.5) and the lowest (105.5) for “Babu kubwa.”
3.9.4. Number of Flowers Formed per Spike
The number of flowers per spike varied significantly (p < 0.05) with pepper cultivars (Table 4). Babu kubwa had significantly the highest number of flowers spike−1 (93.5) than all other cultivars, while varieties “Babu kati” (90.7) and “Ismailia” (67.6) had higher number of flowers spike−1 than “Babu ndogo” (55.4).
3.9.5. Percentage of Full-Size Berries
The results (Table 4) show that pepper cultivars differed significantly (p < 0.05) in terms of percentage of full-size berries (berry set). Cultivar “Ismailia” had significantly the highest (86%) full-size berries followed by “Babu ndogo” (65%), “Babu kubwa” (51.3%), and lowest (46.1%) for “Babu kati.” The berry set among pepper cultivars is illustrated in Figure 9.

3.9.6. Percentage of Intermediate-Size Berries
Percentage of intermediate-size berries varied significantly (p < 0.05) with pepper cultivars (Table 4). Babu kubwa had significantly the highest (34%) intermediate-size berries than all other cultivars, while “Babu ndogo” had higher (26.3%) intermediate-size berries than varieties “Babu kati” (15%) and “Ismailia” (10%).
3.9.7. Percentage of Undeveloped Berries
The results (Table 4) show that the percentage of undeveloped berries differed significantly (p < 0.05) with pepper cultivars. Cultivar “Babu kati” had significantly the highest (36%) undeveloped berries than all other cultivars, while “Babu kubwa” had higher (12.7%) undeveloped berries than varieties “Babu ndogo” (6.6%) and “Ismailia” (1.3%).
4. Discussion
4.1. Causal Agent(s) of Stem Wilting
The fungi, Fusarium oxysporum, isolated from soils and tissues of pepper plants showing leaf yellowing and stem wilting at Tandai village in Morogoro district of Tanzania also has been reported (in Brazil and Malaysia) to cause pepper stem wilt and leaf yellowing [40–42]. Studies by Duarte et al. [40, 41] reported Fusarium oxysporum (F. oxysporum Schl. f. sp. piperis) as the pathogen of pepper wilting in Brazil. On the other hand, studies by Shahnazi et al. [42], on the basis of morphology, DNA sequences and the pathogenicity test showed that another fungal isolate of Fusarium spp. (F. solani) was also a causal agent of leaf yellowing and stem wilting on pepper grown in Malaysia. In the relatively recent past, F. oxysporum Schl. f. sp. piperis was isolated from infected pepper plants grown in the central region of Sarawak in Malaysia [14] and Indonesia [43].
The fungi (F. oxysporum) can survive in the soil for at least 20 years as mycelium or as spores in the absence of its hosts [44]. Between crops, it survives in infected plant debris in the soil as mycelium and in all its spore forms but, most commonly in the cooler areas, as chlamydospores. The fungi spreads over short and long distances by means of water, contaminated farm equipment, and primarily in infected transplants or in the soil carried with them. Usually, once an area becomes infested with Fusarium, it remains so for an indefinite period. In the presence of its host, mycelium from germinating spores penetrate the roots and enter the vascular system (xylem) where it multiplies and causes the host to develop wilting symptoms. The spread of ascospores (sexual spores) from perithecia (sexual fruiting bodies) is also one of the main inoculum sources of the fusarium wilt disease on adjacent pepper plants [45, 46].
Although Fusarium spp. are known to cause stem wilting in pepper, leaf yellowing can be caused by a combination of biotic and abiotic factors including nutrient deficiency [6, 47]. Climatic and edaphic factors such as temperature (25–30°C) and pH of 5–7 [48, 49] favor the growth, sporulation, and survival of Fusarium species including F. oxysporum which is described as a warm climate pathogen mostly found in acidic and sandy soils [44]. Regardless of the plant diseases appearance (such as wilt, necrosis/discoloration of shoots, branches, and entire plants), the reduced plant growth and ability to produce is linked to plant diseases interference with various physiological functions of the plant including absorption and translocation of water and nutrients from the soil and photosynthesis [50].
Soil pH influences both the pathogen and the host as it affects the availability of plant nutrients [51]. Deficiency of some of the soil nutrients may be weakening the strength of plants cell walls, hence less resistance to fungi. Soils at Tandai village had pH indicating strongly acidic to slightly acidic and generally of low nutrient content likely to aggravate the effects of pathogens causing stem wilting. Paulitz and Schroeder [51] reported that soil acidity may affect the microbial populations that hold the Fusarium spp. in check. Fungi are generally more adapted to acidic soil conditions [44]. Soil acidity/extreme pH may lead to plant stress and reduced resistance to such pathogens as it may affect the composition of the root exudates which attract soilborne pathogens and affect fungal cell growth, development, and possibly pathogenicity [51].
This study established the incidence of termites on pepper plants at Tandai village. The findings suggest that termites can cause plant wilt through their direct damaging effect on the plant stems. Termites are also known to be involved in spreading pathogenic agents of stem wilting. Sharma and Anandaraj [52] reported the involvement of termites in spreading fungal pathogens and as passive carriers of soil inoculum on standards of pepper vines during the off-season. Some mites such as eriophyid/bud mite (Aceria mangiferae) can carry fungus (Fusarium mangiferae), which causes bud malformation disease in mango trees [50, 53]. The bud mite has the ability to carry F. mangiferae in its digestive tract, can also bear fungal conidia externally on its body and vector conidia between apical buds on the same infected tree, and hence assists in fungal penetration and colonization. Gamliel-Atinsky [53] found that fungal conidia were observed adhering to the mite’s body, and in bud bracts of mango, indicating that the mite vectored the conidia into the apical bud. However, no windborne bud mites bearing fungi conidia were found, implying that the bud mite did not play a role in aerial dissemination of fungal conidia between different mango trees.
Study by Oyetunji et al. [54] showed that the detrimental effect caused by fungi (Botryodiplodia theobromae and Trichoderma sp.) on rice plants was made possible by the infestation of termites (Macrotermes spp.) that damage the root and fill the stem with soil composed of the pathogenic fungi and consequently predisposed the root and stems to fungal attack.
Among termite species found in the study area (Tandai village), Macrotermes were more frequent than Microtermes, Odontotermes, and Hypotermes. Daba et al. [7] considered the Macrotermes species as minor pests infesting pepper gardens in major producing areas of Ethiopia due to their very low infestation level, although they had a wide distribution. However, under termites-fungi interactions, fungi might function as pathogens/lethal parasites to the termites [55]; they can also act as symbionts, attractants, antagonists, or even synergists to the termites [56, 57]. The ability of termites to survive and interact with soil fungi could be supported by the lower density of the fungi in soil (or concentration of fungal conidia, spores/mycelium) and the antifungal compounds (peptide termicin) released in termite saliva from the gut during hygienic and grooming activities. Thus, some soil fungal species might exist in a dormant state in such occasion [57].
Based on field observation, termites attack on pepper plants was prominent during the dry period. The influence of dry condition on termites damages has been reported in other crops such as coconut (transplanted seedlings), associated with shortage of food from plant debris compared to the rainy seasons where the attacks were low [36]. Termite attack is known to be prominent on perennial crops including spices during dry than wet seasons and on annual crops towards harvesting [58, 59]. The high magnitude of the incidences of termites attack on pepper plants trained on silver oak, mango, and jack fruit is possibly due to the susceptibility of these support plants to termites especially in the dry period. Low water content and an increased surface area may lead to a greater likelihood of gaps and fractures in the bark when a tree is either stressed, damaged, or grows older. These facilitate the termites to attack such support trees [60]. Absence of termites attack on pepper plants trained on Jatropha is possibly due to high bioactivity of Jatropha oil attributed to the presence of antifungal/pesticidal compounds (phorbol esters) in the stem, root, and bark [61].
4.2. Causative Agent(s) of Spike Shedding
Spike shedding indicated by the higher percentage of intermediate-size berries and undeveloped berries recorded in cultivar “Babu kubwa” and “Babu kati,” respectively, may be attributed to nutrients (N, P, Mg, and Cu) concentration below the optimal ranges. Previous studies [62–64] have also reported that increased competition for nutrients coupled with low soil moisture content and low availability of photosynthates affects fruit development in pepper. Moreover, environmental factors (temperature, light intensity, microhumidity, and rainfall intensity) during flowering, berry set, and development play a vital role in pepper productivity. Plenty of water and high amount nutrients are demanded by pepper plants [65]. Rainfall ranging between 2000 and 3000 mm year−1 with an average of 2300 mm year−1 without dry months during the reproductive stage is required for optimum growth of pepper plants. Its growth, however, is hampered when the monthly rainfall is less than 90 mm month−1 [66]. Significantly higher number of spikes at 2 m height from the ground in cultivar “Ismailia” grown in Morogoro district corroborated well as reported by Hussain [4] where the variety “Panniyur-1” of India exhibited less spike shedding, sustenance of relatively large number of spikes on highly developed productive laterals relative to high leaf area in the upper part of the canopy (at 2 m height) and higher photosynthetic rate during spike development.
Parthasarathy et al. [67] associated spike shedding with the failure of the berry set (improper filling of the spikes) or undeveloped ovules on pepper varieties with insufficient pollination, unfertilized flowers or imperfect fertilization, loss of stigma receptivity before pollination either singly or a combination of these factors. This suggests that cultivar “Ismailia” and “Babu ndogo,” which had low spike shedding indicated by the higher percentage of full-size berries (berry set per spike), possibly received sufficient pollination and high rate of fertilization of flowers compared to cultivar “Babu kubwa” and “Babu kati.” Genotypic characters, exposure to environment, distribution of rainfall, incidence of diseases, and spike shedding (when developing pepper flowers or berries are aborted from the spike) influence the performance of pepper varieties [68]. The variation in the number of spikes, flowers, and percentage berry set (yield components) among pepper cultivars may also be attributed to differences in genetic makeup and influence of environmental condition, as with long dry season which was experienced during the growth season in the Morogoro district. Krishnamurthy et al. [69] also reported decreasing trend of performance in pepper due to lower rainfall and higher temperature concurrently.
Krishnamurthy et al. [63] reported that most of pepper cultivars are monoecious and have bisexual flowers. About 50–150 minute flowers are borne in the spikes; however, the number of bisexual flowers affects the percentage of the fruit set. The higher the percentage of bisexual flowers, the higher the percentage of fruits [63]. Possibly, cultivars “Ismailia” and “Babu ndogo” had a higher percentage of hermaphrodite flowers so the spikes are full of fruits, hence no/less spike shedding. High percentage (98%) of productive bisexual flowers and berry setting percentage (91.5%) was also attributed to the very compactly arranged berries (133 berries spike−1) on the hybrid variety “Panniyur-7,” an open pollinated progeny of landrace “Kalluvally” of India [70]. Similar findings have been reported on the variety “Ciinten” from Indonesia, which is characterized with compact spikes and was associated to a higher percentage of hermaphrodite flowers [71]. Symptoms observed at Tandai village in Morogoro district in occasion where stem wilting occurred during the reproductive stage (spiking and fruit development stages) and the spikes and fruits also wilt and shed-off are much similar to the symptoms due to Fusarium spp. (F. solani and F. oxysporum) which cause damages on stems and roots of pepper plants, leading to detrimental effects to other parts including defoliation, wilting of stems, and spike shedding as discussed by Duarte [40, 41] and as discussed elsewhere [14, 42, 43].
5. Conclusion
Based on the characteristics of wilted plants, soil, and plant tissue analysis, stem wilting was attributed to the fungal pathogen Fusarium oxysporum. The high incidence of termites in gardens and plants showing stem wilting suggests that termites could be attributed to the disease symptoms through direct damage on plants and indirectly as agents for spreading the fungi. The nutrients (N, P, Mg, and Cu) concentrations in soil and leaf samples were below the optimal ranges suggesting that nutrient deficiency could be among contributing factors to the incidence of spike shedding. Support trees varied in the extent of termite-damaged pepper plants and the occurrence of stem wilting. Hence, the type of support trees likely influences stem wilting. Prevalence of spike shedding varied with pepper cultivars; hence, the type of cultivar may influence spike shedding. Confirmation of fungi pathogenicity is recommended. A comprehensive study is suggested on the repellent or lethal activities of Jatropha against F. oxysporum and termites. Further studies through field experiments are needed to generate site-specific fertilizer recommendations and design an integrated approach to control F. oxysporum, termites, and to determine existing alternative host plants for these pests.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors are grateful to pepper farmers at Tandai village in Kinole ward of Mkuyuni division in Morogoro district who facilitated this work in many ways to completion. The authors frankly thank the Sustainable Agriculture Tanzania (SAT) for providing financial support to undertake this study through the 4th Workshop for Participatory Research Design.
Open Research
Data Availability
The analysed data used to support the findings of this study are available from the corresponding author upon request.




