Nephroprotective Effect of Mesenchymal Stem Cell-Based Therapy of Kidney Disease Induced by Toxicants
Abstract
Background. Renal damage caused by drug toxicity is becoming increasingly common in the clinic. Preventing and treating kidney damage caused by drug toxicity are essential to maintain patient health and reduce the social and economic burden. In this study, we performed a meta-analysis to assess the nephroprotective effect of mesenchymal stem cells (MSCs) in the treatment of kidney disease induced by toxicants. Methods. The Cochrane Library, Embase, ISI Web of Science, and PubMed databases were searched up to December 31, 2019, to identify studies and extract data to assess the efficacy of MSCs treatment of kidney disease induced by toxicants using Cochrane Review Manager Version 5.3. A total of 27 studies were eligible and selected for this meta-analysis. Results. The results showed that a difference in serum creatinine levels between the MSC treatment group and control group was observed for 2, 4, 5, 6-8, 10-15, 28-30, and ≥42 days (2 days: WMD = −0.88, 95% CI: -1.34, -0.42, P = 0.0002; 4 days: WMD = −0.74, 95% CI: -0.95, -0.54, P < 0.00001; 5 days: WMD = −0.46, 95% CI: -0.67, -0.25, P < 0.0001; 6-8 days: WMD = −0.55, 95% CI: -0.84, -0.26, P = 0.0002; 10-15 days: WMD = −0.37, 95% CI: -0.53, -0.20, P < 0.0001; 28-30 days: WMD = −0.53, 95% CI: -1.04, -0.02, P = 0.04; ≥42 days: WMD = −0.22, 95% CI: -0.39, -0.06, P = 0.007). Furthermore, a difference in blood urea nitrogen levels between the MSC treatment group and control group was observed for 2-3, 4-5, 6-8, and ≥28 days. The results also indicate that MSC treatment alleviated inflammatory cells, necrotic tubules, regenerative tubules, and renal interstitial fibrosis in kidney disease induced by toxicants. Conclusion. MSCs may be a promising therapeutic agent for kidney disease induced by toxicants.
1. Introduction
Kidney injury occurs during acute kidney injury (AKI) and chronic kidney disease (CKD), and it is a common condition associated with the morbidity and mortality of patients. A total of 80% of patients who survive an AKI episode completely recover kidney function, and recovered AKI patients present an almost 9-fold increase in risk for CKD development [1]. Toxicant-induced kidney injury is one of the most common causes of kidney disease, causing substantial morbidity and hampering drug development [2]. At present, renal damage caused by drug toxicity is becoming increasingly common in the clinic. Preventing and treating kidney damage caused by drug toxicity is essential to maintain patient health and reduce the social and economic burden.
Mesenchymal stem cells (MSCs), which are multipotent mesenchymal cells present in various tissues, have multilineage differentiation ability under appropriate conditions and are easy to obtain. They are a promising therapeutic option for some diseases because of their unique property of releasing some important bioactive factors [3–5]. Drug toxicity can induce renal tubular epithelial cell damage or death and can lead to renal interstitial inflammation, which eventually develops into renal interstitial fibrosis and renal loss. Previous studies have shown that MSCs can play a protective role against injury of renal tubular epithelial cells and prevent renal interstitial fibrosis [6–10]. Before clinical application, animal experiments in vivo are generally required to confirm the effectiveness of MSCs. Furthermore, there are few clinical trials of MSCs on kidney disease induced by toxicants. Therefore, in this study, we performed a meta-analysis to assess the nephroprotective effect of MSCs in the treatment of kidney disease induced by toxicants in animals.
2. Materials and Methods
2.1. Search Strategy
We searched databases (Cochrane Library, Embase, ISI Web of Science, and PubMed) up to Dec 31, 2019, using the following search terms: (mesenchymal stem cells OR MSC OR MSCs OR multipotent stromal cells OR mesenchymal stromal cells OR mesenchymal progenitor cells OR stem cells) AND (gentamicin OR aristolochic acid OR cisplatin OR adriamycin OR cadmium chloride OR methotrexate OR rifampicin OR glycerol OR streptozocin) AND (kidney injury OR renal failure OR kidney disease). The search was confined to English-language literature. An additional search was conducted among the eligible manual references of the cited articles.
2.2. Inclusion and Exclusion Criteria
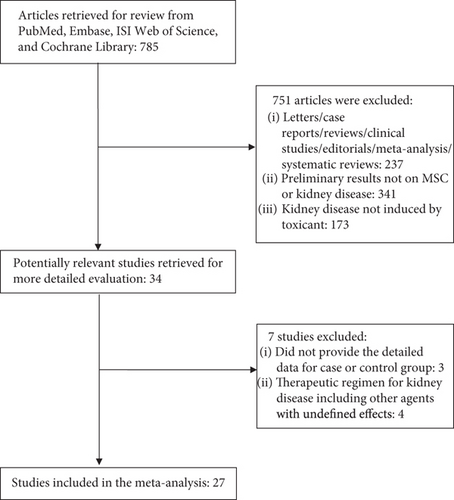
Our meta-analysis included studies analyzing the efficacy of MSC treatment in mice or rats with kidney disease. The following studies were excluded from the analysis: (1) letters, case reports, reviews, clinical studies, editorials, meta-analysis, and systematic reviews; (2) studies lacking the targeted indicators or number of case or control groups and were conducted in humans; (3) studies of kidney disease that was not induced by toxicants; and (4) studies with therapeutic regimen for kidney disease that included other agents with undefined effects.
2.3. Outcome Measures
We filtered the following outcomes associated with the efficacy of MSC treatment from the recruited studies: serum creatinine (Scr), blood urea nitrogen (BUN), urinary albumin excretion (UAE), malondialdehyde (MDA), L-glutathione (GSH), superoxide dismutase (SOD), and renal pathology. In addition, we conducted a mutual consensus when met with disagreements.
2.4. Quality Assessment
Two investigators independently evaluated the methodological quality using the Cochrane Handbook for Interventions. We assessed the following sections of every investigation: selection bias, attrition bias, performance bias, detection bias, reporting bias, and other bias. Each item was classified as unclear, high risk, or low risk.
2.5. Statistical Analysis
Review Manager Version 5.3 was applied to explore whether MSC treatment achieved a good efficacy in kidney disease induced by toxicants, and STATA 12.0 was used to test the publication bias. Heterogeneity of variation among individual studies was quantified and described using I2. The fixed effects model was used if the P value of the heterogeneity test was ≥ 0.1. Otherwise, the random effects model was applied to pool the outcomes. In addition, to compute continuous variables, we analyzed weighted mean differences (WMDs) for the mean values. We also calculated 95% confidence intervals (95% CI) for the included studies using the Mantel-Haenszel (M-H) method. Additionally, we evaluated the publication bias using Begg’s rank correlation test as well as Egger’s linear regression method among the studies. A P value < 0.05 was considered of statistical significance.
3. Results
3.1. Search Results
The databases mentioned above were searched, and only studies in mice or rats that evaluated the therapeutic efficacy of MSC treatment on kidney disease induced by toxicants were selected. Twenty-seven studies [11–37] were eligible and selected for this meta-analysis, and a flowchart of inclusion of studies is presented in Figure 1. Study characteristics are shown in Table 1.
| Author, year | n | Type of animal | Type of injury | MSC type | Number of MSC | Route of delivery | Endpoints for this meta-analysis |
|---|---|---|---|---|---|---|---|
| Herrera 2004 | 24 | Mice | Glycerol-induced | BM-MSCs | 1 × 106 | Intravenous injection | Scr |
| Bi 2007 | 12 | Mice | Cisplatin-induced | BM-MSCs | 2 × 105 | Intravenous injection or intraperitoneal injection | Scr, BUN |
| Sun 2008 | 40 | Rat | Glycerol-induced | BM-MSCs | 2 × 106 | Abdominal aorta injection | Scr, BUN |
| Qian 2008 | 6 | Rat | Glycerol-induced | BM-MSCs | 1 × 104 | Intravenous injection | Scr |
| Magnasco 2008 | 22 | Rat | Adriamycin-induced | BM-MSCs | 3 × 106 | Intravenous injection | Scr, BUN, UAE, renal damage score |
| Bruno 2009 | 16 | Mice | Glycerol-induced | BM-MSCs | — | Intravenous injection | Scr, BUN, MDA, GSH, SOD, renal damage score |
| Eliopoulos 2010 | 10 | Mice | Cisplatin-induced | BM-MSCs | 5 × 106 | Intraperitoneal injection | Scr, BUN |
| Kim 2012 | 17 | Rat | Cisplatin-induced | AD-MSCs | 5 × 105 | Intravenous injection | Scr, BUN |
| Zickri 2012 | 30 | Rat | Adriamycin-induced | hUC-MSCs | 5 × 105 | Intravenous injection | Scr |
| Sarhan 2014 | 19 | Rat | Adriamycin-induced | BM-MSCs | 4 × 106 | Intravenous injection | Scr, BUN, UAE, renal pathology, MDA, GSH |
| Moustafa 2016 | 80 | Rat | Cisplatin-induced | BM-MSCs | 5 × 106 | Intravenous, intraarterial or kidney subcapsular injection | Scr, MDA, GSH, SOD |
| Elhusseini 2016 | 40 | Rat | Cisplatin-induced | AD-MSCs | 5 × 106 | Intravenous injection | Scr, BUN, Ccr, renal pathology, MDA, GSH, SOD |
| Anan 2016 | 13 | Rat | Adriamycin-induced | BM-MSCs | 1 × 106 | Intravenous injection | Scr, BUN, SOD |
| Gad 2017 | 24 | Rat | Methotrexate-induced | BM-MSCs | 2 × 106 | Intraperitoneal injection | Scr, BUN, MDA, GSH |
| Rashed 2018 | 20 | Rat | Streptozotocin -induced | BM-MSCs | 1 × 106 | Intravenous injection | Scr, BUN, UAE, Ccr |
| Elbaghdady 2018 | 20 | Rat | Cadmium chloride-induced | BM-MSCs | 2 × 106 | Intravenous injection | Scr |
| Danjuma 2018 | 16 | Rat | Rifampicin-induced | BM-MSCs | 2.5 × 105 | Intravenous injection | Scr, BUN |
| Putra 2019 | 10 | Rat | Gentamicin induced | hUC-MSCs | 1 × 106 | Intraperitoneal injection | Scr, BUN, renal pathology |
| Cetinkaya 2019 | 17 | Rat | Aristolochic acid induced | hAMSC | 6 × 105 | Intravenous injection | Scr, BUN |
| Selim 2019 | 70 | Rat | Cisplatin-induced | AD-MSCs; BM-MSCs | 4 × 106 | Intravenous injection | Scr, BUN |
| Mata-Miranda 2019 | 10 | Mice | Cisplatin-induced | mESCs | 1 × 106 | Intraperitoneal injection | Scr |
| Vazquez-Zapien 2019 | 19 | Mice | Cisplatin-induced | mESCs | 1 × 106 | Intraperitoneal injection | Scr |
| Minocha 2019 | 3 | Rat | Cisplatin-induced | AFSC | 2 × 106 | Intravenous injection | Scr, BUN |
| Sun B 2019 | 10 | Rat | Cisplatin-induced | USCs | 2 × 106 | Intravenous injection | Scr |
| Sun 2019 | 6 | Rat | Cisplatin-induced | BM-MSCs | — | Renal parenchyma injection | Scr, BUN |
| Zhang 2020 | 9 | Rat | Cisplatin-induced | USCs | 5 × 106 | Subcutaneous injection | Scr, Ccr, renal pathology |
| Foroutan 2020 | 6 | Rat | Cisplatin-induced | BM-MSCs | — | Intraperitoneal injection | Scr, BUN |
- Note: BM-MSCs: bone marrow mesenchymal stem cells; hAMSCs: human amnion-derived mesenchymal stem cells; hUC-MSCs: human umbilical cord-mesenchymal stem cells; AD-MSCs: adipose tissue-derived mesenchymal stem cells; mESCs: mouse embryonic stem cells; AFSCs: amniotic fluid stem cells; USCs: urine-derived stem cells; Scr: serum creatinine; BUN: blood urea nitrogen; UAE: urinary albumin excretion; Ccr: creatinine clearance rate; MDA: malondialdehyde; GSH: L-glutathione; SOD: superoxide dismutase.
3.2. Quality Assessment of Included Studies
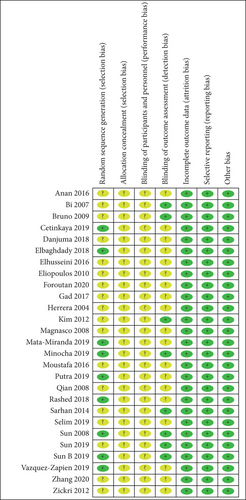
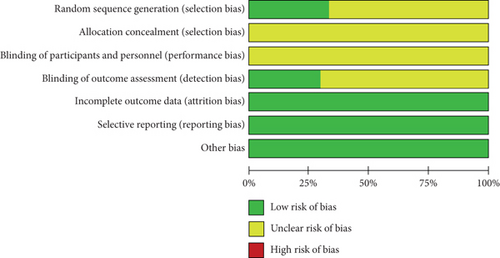
The methodological quality of the selected studies was considered acceptable because most study domains were ranked as unclear risk or low risk of bias. Unclear risk of bias was mostly detected in performance and selection bias. Low risk of bias mostly occurred in detection, reporting, and attrition bias. Figure 2 shows a summary of the risk of biases of the selected studies.
3.3. Scr
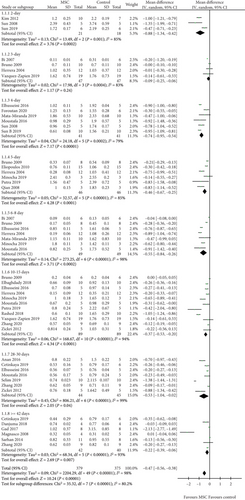
A total of 27 studies [11–37] were selected to assess the effect of MSCs on Scr, and the results show that a difference between the MSC treatment and control groups was observed for 2, 4, 5, 6-8, 10-15, 28-30 days, and ≥42 days (2 days: WMD = −0.88, 95% CI: -1.34, -0.42, P = 0.0002; 4 days: WMD = −0.74, 95% CI: -0.95, -0.54, P < 0.00001; 5 days: WMD = −0.46, 95% CI: -0.67, -0.25, P < 0.0001; 6-8 days: WMD = −0.55, 95% CI: -0.84, -0.26, P = 0.0002; 10-15 days: WMD = −0.37, 95% CI: -0.53, -0.20, P < 0.0001; 28-30 days: WMD = −0.53, 95% CI: -1.04, -0.02, P = 0.04; ≥42 days: WMD = −0.22, 95% CI: -0.39, -0.06, P = 0.007; Figure 3 and Table 2). However, no difference was observed between the MSC treatment and control groups for 3 days (3 days: WMD = −0.09, 95% CI: -0.25, -0.06, P = 0.24; Figure 3 and Table 2).
| Indicators | Time point | Studies | Q test | Model | OR/WMD | P |
|---|---|---|---|---|---|---|
| number | P value | selected | (95% CI) | |||
| Scr | 2 days | 3 | 0.001 | Random | -0.88 (-1.34, -0.42) | 0.0002 |
| 3 days | 4 | 0.0004 | Random | -0.09 (-0.25, 0.06) | 0.24 | |
| 4 days | 6 | 0.0002 | Random | -0.74 (-0.95, -0.54) | <0.00001 | |
| 5 days | 6 | <0.00001 | Random | -0.46 (-0.67, -0.25) | <0.0001 | |
| 6-8 days | 7 | <0.00001 | Random | -0.55 (-0.84, -0.26) | 0.0002 | |
| 10-15 days | 11 | <0.00001 | Random | -0.37 (-0.53, -0.20) | <0.0001 | |
| 28-30 days | 7 | <0.00001 | Random | -0.53 (-1.04, -0.02) | 0.04 | |
| ≥42 days | 6 | <0.00001 | Random | -0.22 (-0.39, -0.06) | 0.007 | |
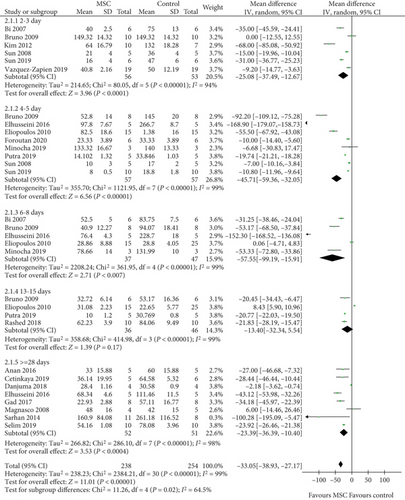
| BUN | 2-3 days | 6 | <0.00001 | Random | -25.08 (-37.49, -12.67) | <0.0001 |
| 4-5 days | 8 | <0.00001 | Random | -45.71 (-59.36, -32.05) | <0.00001 | |
| 6-8 days | 5 | <0.00001 | Random | -57.55 (-99.19, -15.91) | 0.007 | |
| 13-15 days | 4 | <0.00001 | Random | -13.40 (-32.34, 5.54) | 0.17 | |
| ≥28 days | 8 | <0.00001 | Random | -23.39 (-36.39, -10.40) | 0.0004 | |
| UAE | — | 3 | 0.72 | Fixed | -22.66 (-26.41, -18.90) | <0.00001 |
| MDA | — | 4 | 0.41 | Fixed | -17.21 (-20.38, -14.04) | <0.00001 |
| GSH | — | 4 | <0.00001 | Random | 4.62 (2.74, 6.50) | <0.00001 |
| SOD | — | 3 | <0.00001 | Random | 5.42 (2.92, 7.93) | <0.0001 |
| Renal pathology | ||||||
| Inflammatory cells | — | 4 | <0.00001 | Random | -2.66 (-3.83, -1.49) | <0.00001 |
| Necrotic tubule | — | 2 | <0.00001 | Random | -2.58 (-4.75, -0.40) | 0.02 |
| Regenerative tubules | — | 2 | — | Fixed | 6.00 (3.45, 8.55) | <0.00001 |
| Renal interstitial fibrosis | — | 3 | <0.00001 | Random | -5.82 (-7.41, -4.23) | <0.00001 |
- Note: Scr: serum creatinine; BUN: blood urea nitrogen; UAE: urinary albumin excretion; Ccr: creatinine clearance rate; MDA: malondialdehyde; GSH: L-glutathione; SOD: superoxide dismutase.
3.4. BUN
A total of 18 studies [11–15, 17–19, 21, 22, 24, 26–29, 32–34, 36, 37] were selected to assess the effect of MSCs on BUN, and the results indicate that the difference between the MSC treatment and control groups was observed for 2-3, 4-5, 6-8, and ≥28 days (2-3 days: WMD = −25.08, 95% CI: -37.49, -12.67, P < 0.0001; 4-5 days: WMD = −45.71, 95% CI: -59.36, -32.05, P < 0.00001; 6-8 days: WMD = −57.55, 95% CI: -99.19, -15.91, P = 0.007; ≥28 days: WMD = −23.39, 95% CI: -36.39, -10.40, P = 0.0004; Figure 4 and Table 2). However, no difference was observed between the MSC treatment and control groups for 13-15 days (WMD = −13.40, 95% CI: -32.34, 5.54, P = 0.17; Figure 4 and Table 2).
3.5. Urinary Albumin Excretion
Three studies [22, 26, 27] were selected in the meta-analysis for the assessment of MSCs on UAE. The results show that the MSC group had a lower UAE than the control group (WMD = −22.66, 95% CI: -26.41, -18.90, P < 0.00001; Table 2).
3.6. Oxidative Stress
Four studies [17, 19, 23, 27] were selected for the assessment of MDA, four [17, 19, 23, 27] for GSH, and three [11, 17, 23] for SOD. The results indicate that a difference between the MSC treatment and control groups was observed for MDA, GSH, and SOD (MDA: WMD = −17.21, 95% CI: -20.38, -14.04, P < 0.00001; GSH: WMD = 4.62, 95% CI: 2.74, 6.50, P < 0.00001; SOD: WMD = 5.42, 95% CI: 2.92, 7.93, P < 0.0001; Table 2).
3.7. Assessment of Renal Pathology
Four studies [17, 24, 27, 35] for inflammatory cells, two studies [17, 27] for necrotic tubules, two studies [17, 27] for regenerative tubules, and three studies [17, 27, 35] for renal interstitial fibrosis were included in this meta-analysis. The results indicate that the difference in inflammatory cells, necrotic tubules, regenerative tubules, and renal interstitial fibrosis between the MSC treatment and control groups was significant (inflammatory cells: WMD = −2.66, 95% CI: -3.83, -1.49, P < 0.00001; necrotic tubules: WMD = −2.58, 95% CI: -4.75, -0.40, P = 0.02; regenerative tubules: WMD = 6.00, 95% CI: 3.45, 8.55, P < 0.00001; renal interstitial fibrosis: WMD = −5.82, 95% CI: -7.41, -4.23, P < 0.00001; Table 2).
3.8. Publication Bias
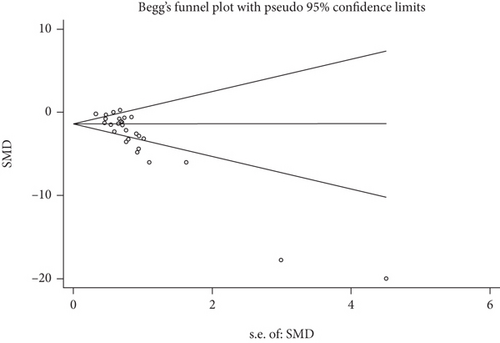
Publication bias was tested in this meta-analysis, and a funnel plot generated using STATA 12.0 for the primary outcome. Begg’s test and Egger’s test results suggest that publication bias was present (P ≤ 0.01 and P ≤ 0.01, respectively; Figure 5).
4. Discussion
We reviewed all the selected studies and evaluated the Scr, BUN, UAE, oxidative stress, and renal pathology results to assess the nephroprotective effect of MSCs in the treatment of kidney disease induced by toxicants. We found that MSC treatment reduced Scr levels at 2, 4, 5, 6-8, 10-15, 28-30, and ≥42 days and reduced BUN levels at 2-3, 4-5, 6-8, and ≥28 days. We also found that the MSC group had a lower UAE than the control group. It has been previously shown that MSC treatment reduces the levels of Scr, BUN, and proteinuria in lupus nephritis in mice [38]. Chen et al. [39] found that MSC ameliorates ischemia/reperfusion injury-induced acute kidney injury in rats and reduces Scr levels. Xiu et al. [40] found that MSC transplantation significantly reduces the concentration of BUN and Scr, prevents tissue injury, and reduces mortality after lipopolysaccharide-induced acute kidney injury. Clinical trials also supported that MSC injection decreases rejection after transplantation. Tan et al. [41] found that the therapy with MSCs achieve better renal function and lower incidence of acute rejection at 1 year compared with the anti-IL-2 receptor antibody induction. Vanikar et al. [42] demonstrated that infusion of MSCs as well as hematopoietic stem cells eases immunosuppression in living donor renal transplantation. Our previous meta-analysis also found that MSCs reduce Scr levels, BUN levels, and proteinuria, as well as alleviate renal damage in animal models of AKI [43]. Lower proteinuria was also found in patients with SLE after MSC therapy [44].
The MSC treatment group had a higher level of GSH, SOD, and a lower level of MDA when compared with the control group. El-Metwaly et al. [45] found that MSCs increase GSH levels and reduce MDA levels in lung tissue of rats subjected to acute lung injury. Li et al. [46] reported that MSCs can restore the levels of GSH and MDA in rats with chronic alcoholism, and its effects on repairing sciatic nerve were obvious. Liu et al. [47] reported that MSCs significantly increase the activity of glutathione (GSH) and reduce the levels of MDA in rats induced by unilateral ureteral obstruction.
The mechanism by which MSCs repair injured kidneys may be complex. After kidney injury, VCAM-1, GFP, SDF -1/CXCR4, and CD44 are upregulated in the injured tissue, which may play important roles in the migration of MSCs to the damaged area. These substances may be partly secreted by the MSCs themselves [20, 48, 49]. The presence of MSCs may limit the injury and repair the ischemic tubular damage to maintain the glomerular filtration rate and downregulate BUN [50]. In addition, MSCs lower the expression of several proinflammatory cytokines such as TNF-α, IL-1β, and IFN-γ as well as increase anti-inflammatory cytokines such as IL-1, IL-10, Bcl-2, TNF-α, bFGF, and prostaglandin E2 [49, 51]. Another possibility is that MSCs may restore damaged cells and prevent apoptosis by secreting microvesicles, which contain microRNAs, mRNAs, or proteins [49]. To conclude, MSCs can migrate to the damaged tissue, promote the recovery of renal function, enhance proliferation, and reduce fibrosis and inflammation.
Furthermore, our study indicates that MSC treatment can alleviate inflammatory cells, necrotic tubules, regenerative tubules, and renal interstitial fibrosis in kidney disease induced by toxicants. Some previous studies indicated that MSC treatment can alleviate renal pathological changes in unilateral ureteral obstruction rat or mice [9, 10, 52].
However, this meta-analysis also has some limitations. First, a small sample size was found for the recruited studies. The administered dose and the type of MSCs were not exactly the same. Publication bias was found in this meta-analysis, and the results should be reassessed in the future. Furthermore, the studies frequently had different animal models (mouse or rat), toxin doses, and administration routes for renal injury. These limitations may affect the robustness of our results.
5. Conclusions
The MSC treatment reduced Scr levels after 2, 4, 5, 6-8, 10-15, 28-30, and ≥42 days and reduced BUN levels after 2-3, 4-5, 6-8, and ≥28 days. The results also indicate that MSC treatment alleviated the inflammatory cells, necrotic tubules, regenerative tubules, and renal interstitial fibrosis in kidney disease induced by toxicants.
Abbreviations
-
- MSCs:
-
- Mesenchymal stem cells
-
- Scr:
-
- Serum creatinine
-
- BUN:
-
- Blood urea nitrogen
-
- UAE:
-
- Urinary albumin excretion
-
- MDA:
-
- Malondialdehyde
-
- GSH:
-
- L-glutathione
-
- SOD:
-
- Superoxide dismutase
-
- WMDs:
-
- Weighted mean differences
-
- CI:
-
- Confidence intervals
-
- M-H:
-
- Mantel-Haenszel.
Consent
There are no human subjects in this article and informed consent is not applicable.
Conflicts of Interest
The authors declare that they have no competing interests.
Authors’ Contributions
TBZ contributed to the conception and design of the study. TBZ and SJL were responsible for collection of data and performing the statistical analysis and manuscript preparation. WSL and CLL were responsible for checking the data. All authors were responsible for drafting the manuscript, read, and approved the final version.
Open Research
Data Availability
The data supporting this meta-analysis are from previously reported studies and datasets, which have been cited. The processed data are available from the corresponding author upon request.




