Optimal Strategies for Control of COVID-19: A Mathematical Perspective
Abstract
A deterministic ordinary differential equation model for SARS-CoV-2 is developed and analysed, taking into account the role of exposed, mildly symptomatic, and severely symptomatic persons in the spread of the disease. It is shown that in the absence of infective immigrants, the model has a locally asymptotically stable disease-free equilibrium whenever the basic reproduction number is below unity. In the absence of immigration of infective persons, the disease can be eradicated whenever ℛ0 < 1. Specifically, if the controls ui, i = 1,2,3,4, are implemented to 100% efficiency, the disease dies away easily. It is shown that border closure (or at least screening) is indispensable in the fight against the spread of SARS-CoV-2. Simulation of optimal control of the model suggests that the most cost-effective strategy to combat SARS-CoV-2 is to reduce contact through use of nose masks and physical distancing.
1. Introduction
Starting in November 2019, from the city of Wuhan, China, a disease caused by a novel coronavirus (SARS-CoV-2) has ravaged the entire world, causing the World Health Organization (WHO) to declare it as a pandemic of international concern. As of 8 June 2020, the virus had affected about 188 countries and regions, resulting in over 7 million infections and over 400,000 deaths globally [1]. During talking, coughing, or sneezing by infected persons, the virus is released through droplets and can be inhaled by susceptibles who are in close contact. The virus may also be picked up by susceptible persons from surfaces that got contaminated by droplets from infected persons and infection may result if the susceptible touches their face with the contaminated hands or objects. Symptoms of infection usually appear between 2 and 14 days and may include fever or chills, cough, shortness of breath or difficulty breathing, fatigue, muscle or body aches, headache, new loss of taste or smell, sore throat, congestion or runny nose, nausea or vomiting, and diarrhoea. When infected persons have trouble breathing, persistent pain or pressure in the chest, new confusion, inability to wake up or stay awake, and/or bluish lips or face, then it is time to seek emergency treatment. With no known proven vaccine and treatment drug so far, nonpharmaceutical interventions including use of nose masks, hand washing (sanitizing), and other safe health protocols are a major part in the fight against the infection. Mathematical modelling has proven to be very helpful in increasing the understanding of the spread and providing optimal strategies towards controlling infectious diseases (see [2] and reference therein). As a result, a number of mathematical models have been proposed to study the spread of SARS-CoV-2 and to provide direction towards control (see [3, 4] and the references therein). Specifically, [5] developed a mathematical model to study the impact of nonpharmaceutical interventions on the spread of COVID-19, concluding that the use of face masks and adhering to social distancing are key in the fight against the disease. The model in [6, 7] proposed an optimal control problem that sought to advise what governments could do to curb COVID-19 spread. We note that the majority of those infected with COVID-19 are asymptomatic, and if they are not tested, they may spread the virus without knowing. Thus, the role of symptomatic infected persons in the spread of COVID-19 needs to be studied properly. Use of nose masks and/or face shields, social/physical distancing, and disinfection of surfaces are some nonpharmaceutical interventions that have been proposed to help curb COVID-19. However, since the implementation of these controls comes at some cost, the need to determine the best combination of these control cannot be overemphasized. To the best of our knowledge, no work has been done considering optimal control of COVID-19 in the presence of infectious asymptomatic persons. With better strategies needed to curb the disease, an optimal control problem with four controls (namely, use of face masks and social distancing u1, avoidance of touching contact surfaces u2, prevention of surface contamination u3, and disinfection of environment u4) is proposed, and the cost-effectiveness of all sixteen possible combinations of these controls is computed.
2. The COVID-19 Model
Given a population of the time-dependent size of N(t) that is subdivided into susceptibles, S(t), asymptomatically infected, E(t), clinically infected (those with mild symptoms I1(t) and those with severe symptoms I1(t)), and the recovered, R(t), so that N = S + E + I1 + I2 + R, and denoting the concentration of coronavirus on surfaces by V(t), a mathematical model to describe the spread of coronavirus in such a population is constructed.
The parameters of the model are further summarized in Table 1.
| Par | Description | Value | Source |
|---|---|---|---|
| Λ | Recruitment rate | 1000 | Assumed |
| β | Probability of infection per contact | 8.073×10−3 | [5] |
| c | Average contacts of infectious person per time | 0.5297 | [9] |
| θ | Rate of loss of immunity after recovery | 0.0357 | — |
| ρ | Rate of recovery from COVID-19 | 1/10 | [5] |
| δ1 | Rate of progression from exposure to the symptomatic stage | 1/5.2 | [10] |
| δ2 | Rate of progression from mild to severe symptomatic stage | 1/5.8 | [10] |
| μ | Natural death rate in humans | 1.86×10−2 | — |
| μd | COVID-19-induced death rate in humans | 1.50×10−2 | [5] |
| μv | Death rate of coronaviruses on surfaces | 3.33×10−1 | Estimated |
| f1 | Proportion of immigrants who are exposed | 0.100 | — |
| f1 | Proportion of immigrants who are mildly symptomatic | 0.0100 | — |
| η1 | Coefficient of infectivity of exposed persons | 1.5 | Assumed |
| η2 | Coefficient of infectivity of severely symptomatic persons | 0.100 | Assumed |
| η3 | Coefficient of viral shedding of severely symptomatic persons | 0.001 | Assumed |
| q | Efficacy of quarantine to prevent transmission | 0.5 | Assumed |
| βv | Surface-to-human transmission probability | 0.001 | — |
| K | Coronavirus concentration on surfaces | 103 cells/m2 | — |
| ξ | Viral shedding rate of infected persons | 100 cells/day | — |
| ν | Rate of disinfection of the environment | — | — |
3. Main Results
3.1. Positivity and Boundedness of Model Solution
We state the result of the positivity and boundedness of solutions of model (1) in Lemma 1.
Lemma 1. Given , all solutions of (1) starting in Ω remain in Ω for all t ≥ 0. Also, the region is a positively invariant set for the model (1).
Proof. Let t1 = sup{t > 0|S ≥ 0, E ≥ 0, I1 ≥ 0, I2 ≥ 0, R ≥ 0, V ≥ 0} and λ(t) = λ1(t) + λ2(t).
The first equation of (1) yields
Then,
Hence,
Clearly, S(t1) ≥ 0.
Similar arguments can be used to show that E ≥ 0, I1 ≥ 0, I2 ≥ 0, R ≥ 0, and V ≥ 0, and thus, all solutions with nonnegative initial conditions are nonnegative.
Further, adding the first five subequations of (1) gives
Thus, N(t) ≤ N(0)e−μt + (Λ/μ)(1 − e−μ).
Therefore, if 0 ≤ N(0) ≤ (Λ/μ), then, ≤limsupt⟶∞N(t) ≤ (Λ/μ).
The last equation of (1) implies that
So if 0 ≤ V(0) ≤ (ξ(1 − u3)Λ/μ), then 0 ≤ V(t) ≤ (ξ(1 − u3)Λ/μ(u4ν + μv)). Thus, all solutions starting within Ω remain inside Ω. This completes the proof of the lemma.
3.2. Equilibria and Basic Reproduction Number
From the expression of ℛ0, it is noted that if the controls u1, u2, and u3 are implemented with 100% efficacy, the disease easily is eradicated. The following result follows from [11].
Theorem 1. In the absence of immigration of infective persons, the COVID-19 model (1) possesses a disease-free equilibrium (ε0) which is locally asymptotically stable whenever ℛ0 < 1 and unstable whenever ℛ0 > 1.
In the next section, the sensitivity of the basic reproduction number ℛ0 and the endemic equilibrium ε∗ to the model parameters is discussed.
3.3. Sensitivity Analysis
We note that the summary description herein of the technique of [12] makes implementation easier especially with computer algebra systems (CAS) since one only needs to define the model in the appropriate format for the CAS and identify the state variables and model parameters. The sensitivity indices of the parameters are presented in Table 2.
| Par. | Output variables | ||||||
|---|---|---|---|---|---|---|---|
| ℛ0 | S∗ | E∗ | R∗ | V∗ | |||
| Λ | 0.8946 | 0.9884 | 1.1660 | 1.1350 | 1.1530 | 1.1550 | 1.1570 |
| f1 | 0.0000 | −0.06107 | 0.8750 | 0.7072 | 0.8040 | 0.8158 | 0.8263 |
| f2 | 0.0000 | −0.005847 | 0.00946 | 0.1994 | 0.08979 | 0.07641 | 0.0646 |
| β | 0.1054 | −0.008432 | 0.1208 | 0.0976 | 0.111 | 0.1126 | 0.1141 |
| βv | 0.8946 | −0.01796 | 0.2573 | 0.2080 | 0.2365 | 0.2400 | 0.2430 |
| C | 0.1054 | −0.008432 | 0.1208 | 0.09764 | 0.111 | 0.1126 | 0.1141 |
| g | 0.106 | 0.001847 | −0.04294 | −0.843 | 0.1957 | −0.054 | −0.2747 |
| ρ | −0.7521 | 0.02509 | −0.4128 | −0.6773 | −1.273 | 0.1424 | −0.4903 |
| δ1 | −0.1164 | 0.004036 | −0.7009 | 0.2417 | 0.2748 | −0.09762 | −0.4268 |
| δ2 | 0.06281 | 0.001354 | −0.03147 | −0.6179 | 0.1435 | −0.03958 | −0.2013 |
| μ | −1.0340 | −1.0200 | −0.3479 | −0.3451 | −0.4859 | −0.7548 | −0.3472 |
| μd | −0.05436 | −0.004219 | −0.001719 | −0.001389 | −0.1139 | −0.05433 | −0.0017 |
| ν | −0.2066 | 0.001709 | −0.02448 | −0.01979 | −0.0225 | −0.02283 | −0.2541 |
| μv | −0.688 | 0.00569 | −0.08152 | −0.06589 | −0.07491 | −0.07601 | −0.846 |
| ξ | 0.8946 | −0.007399 | 0.106 | 0.08568 | 0.09741 | 0.09884 | 1.1000 |
| θ | 0.0000 | 0.02745 | 0.01066 | 0.008617 | 0.009797 | −0.6475 | 0.01007 |
| Q | 0.0000 | −0.0001883 | 0.002697 | 0.00218 | 0.002479 | 0.002515 | 0.0025 |
| η1 | 0.08128 | −0.00622 | 0.08911 | 0.07203 | 0.08189 | 0.08309 | 0.08415 |
| η2 | 0.006211 | −0.0005172 | 0.00741 | 0.005989 | 0.006809 | 0.006909 | 0.006997 |
| η3 | 4.775e − 4 | −6.545e − 6 | 9.377e − 5 | 7.579e − 5 | 8.616e − 5 | 8.743e − 5 | 9.731e − 4 |
| K | −0.8946 | 0.007399 | −0.106 | −0.08568 | −0.09741 | −0.09884 | −0.1001 |
It can be noted from Table 2 that increasing Λ, β, βv, c, ξ, η1, η2, and η3 increases ℛ0 (and consequently increases , and ) while increasing ρ, δ1, mu, μd, μv, and K decreases ℛ0 (and consequently decreases , and ). Therefore, efforts aimed at reducing Λ, β, βv, c, ξ, η1, η2, and η3 and increasing ρ, δ1, mu, μd, μv, and K should be made in order to keep ℛ0 (and consequently, , and ) small enough to contain or eradicate COVID-19.
3.4. Optimal Control of COVID-19 Spread
Theorem 2. Let be the solution associated with the tuple that minimizes J over . Then,
- (1)
There exists adjoint variables Mx such that
- (2)
The following transversality conditions hold:
- (3)
, where
Proof. Corollary 4.1 of [14] shows the existence of an optimal quadrupole due to the convexity of the integrand of J with the controls, a priori boundedness of the state solutions, and the Lipschitz property of the state system with respect to the state variables. The equations in (17) governing the adjoint variables are obtained by differentiation of the Hamiltonian with respect to the associated state variables evaluated at the optimal control.
The expressions for are obtained from dH/dui = 0, which hold at optimality. Using standard control arguments involving the bounds on the controls gives the characterisations for the controls in (19).
4. Numerical Experimentation
In this section, some numerical experiments are performed, first to study the impact of the various model parameters on the spread on COVID-19 and to illustrate the analytical results obtained and, second, via the optimal control, to determine the best strategy that can be used to combat COVID-19 spread. All simulations are done using the parameter values in Table 1.
4.1. Simulation with Constant Controls
Figure 1 compares results of the simulation for the case of no controls at all on the one hand (i.e., 0% implementation of the controls) and where all four controls are implemented to perfection (i.e., 100% implementation) on the other hand. It is observed that even though implementing the controls to perfection produces some desirable results as it leads to reduced (and stabilized) infections, the disease persists during the whole period of study even with ℛ0 < 1. This is attributable to the fact that immigrants are still allowed into the country.
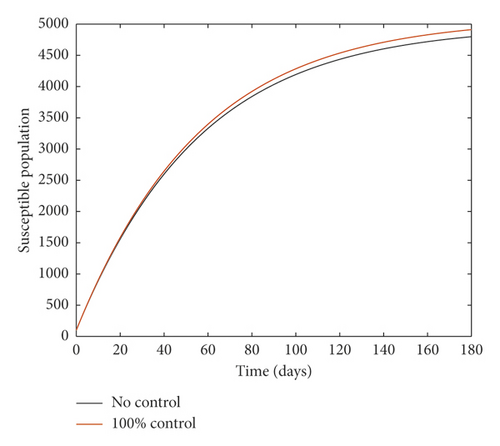
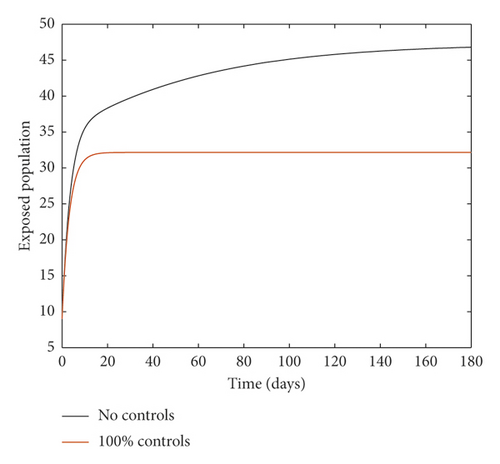
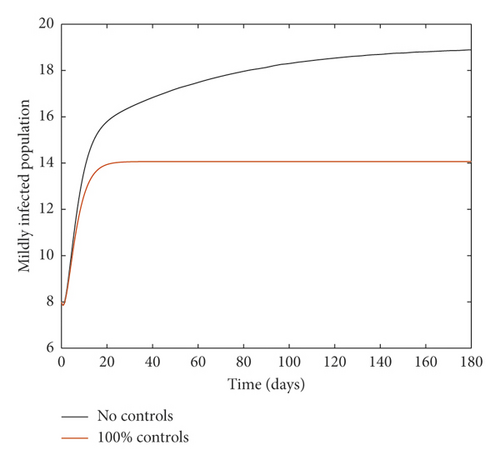
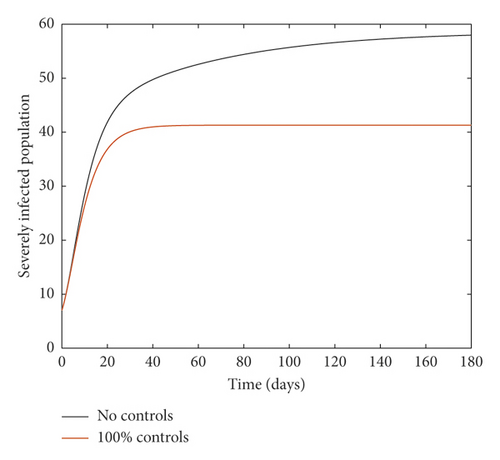
Figure 2 compares the simulation at 100% implementation of all controls with and without infective immigrants.
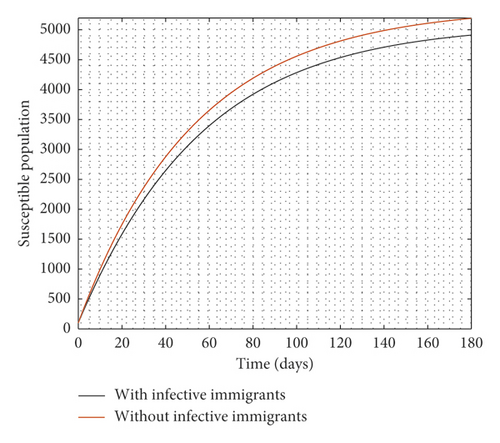
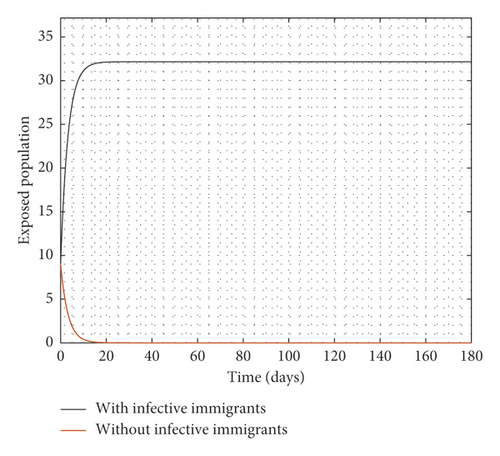
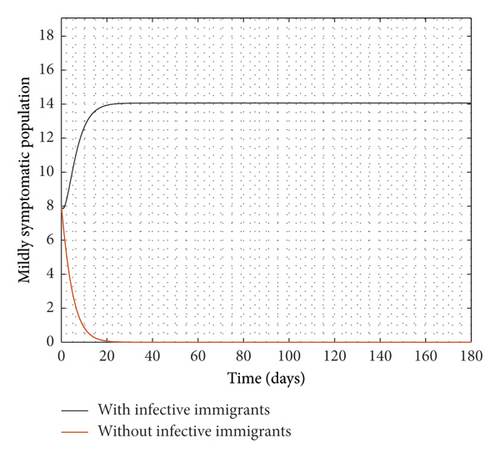
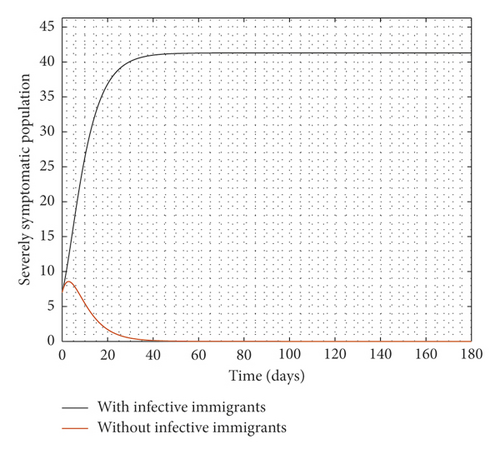
From Figure 2, it is observed that with effective border closure, if the necessary actions are taken to detect and treat exposed and symptomatic persons, COVID-19 can be eradicated within the first month of implementation of the strategies. It is also observed that a perfect implementation of all controls is not sufficient to stop the spread of COVID-19 unless there is a restriction on immigration. Therefore, the decision by governments all over the world to close their borders was in the right direction.
Experimenting with T = 30 days, the result of the simulation is presented in Figure 3.
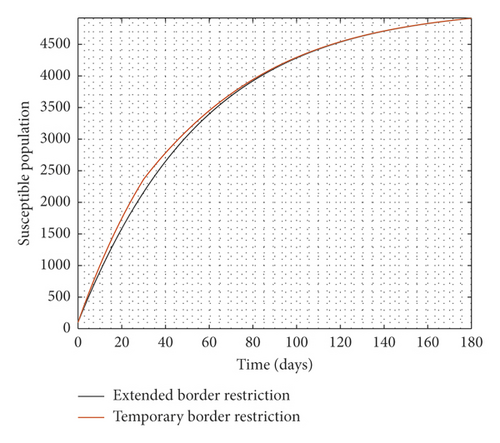
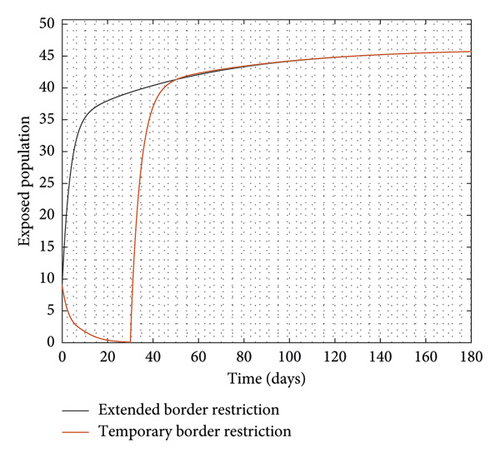
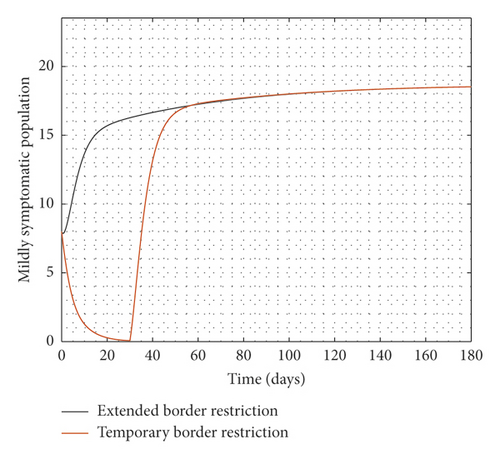
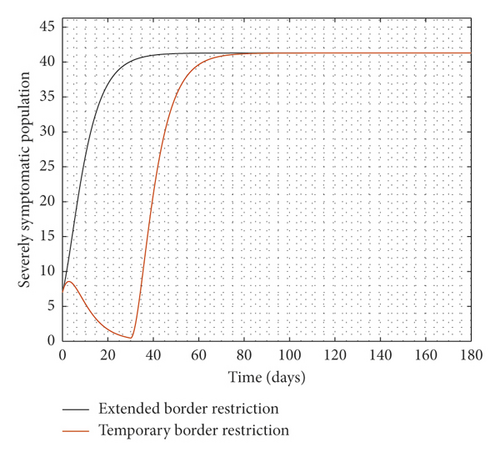
It is observed from Figure 3 that even after the disease is eradicated, a relaxation of the borders to allow immigration of all manner of persons will trigger a second wave of the disease. Therefore, the need to continue to close borders or screen immigrants in order to identify, quarantine, and treat infected persons cannot be overemphasized even after the community transmissions are eliminated.
4.2. Simulation of Optimal Control
The question of which combination of the four controls (u1, u2, u3, and u4) is most cost-effective in combatting the spread of SARS-CoV-2 is considered here. The optimal control problem (14) is solved for all possible combinations of the controls to determine which combination is most cost-effective. The optimal control problem (5) can be solved as a boundary value problem consisting of the state system (1) endowed with initial conditions and the adjoint system (17) endowed with final conditions (18). We make use of the bvp4c function in MATLAB to solve the boundary value problem for each of the fifteen (15) combinations of the controls (see Table 3) calculating (see Table 4) the total number averted of exposed persons E, mildly symptomatic I1, severely symptomatic I2, surface concentration of SARS-CoV-2 V, , the weighted sum , and the total cost incurred in implementing the control set. The total infections averted are the difference between the total infections without controls (ui = 0, ∀i) and the total infections with control.
| Strategy | Control combination |
|---|---|
| 1 | (u1 ≠ 0, u2 ≠ 0, u3 ≠ 0, u4 ≠ 0) |
| 2 | (u1 ≠ 0, u2 ≠ 0, u3 ≠ 0, u4 = 0) |
| 3 | (u1 ≠ 0, u2 ≠ 0, u3 = 0, u4 ≠ 0) |
| 4 | (u1 ≠ 0, u2 ≠ 0, u3 = 0, u4 = 0) |
| 5 | (u1 ≠ 0, u2 = 0, u3 ≠ 0, u4 ≠ 0) |
| 6 | (u1 ≠ 0, u2 = 0, u3 ≠ 0, u4 = 0) |
| 7 | (u1 ≠ 0, u2 = 0, u3 = 0, u4 ≠ 0) |
| 8 | (u1 ≠ 0, u2 = 0, u3 = 0, u4 = 0) |
| 9 | (u1 = 0, u2 ≠ 0, u3 ≠ 0, u4 ≠ 0) |
| 10 | (u1 = 0, u2 ≠ 0, u3 ≠ 0, u4 = 0) |
| 11 | (u1 = 0, u2 ≠ 0, u3 = 0, u4 ≠ 0) |
| 12 | (u1 = 0, u2 ≠ 0, u3 = 0, u4 = 0) |
| 13 | (u1 = 0, u2 = 0, u3 ≠ 0, u4 ≠ 0) |
| 14 | (u1 = 0, u2 = 0, u3 ≠ 0, u4 = 0) |
| 15 | (u1 = 0, u2 = 0, u3 = 0, u4 ≠ 0) |
| Strategy | Total cost/infections averted | ||||||
|---|---|---|---|---|---|---|---|
| E | I1 | I2 | V | Cost (C) | |||
| 1 | 2611.359 | 993.9245 | 3089.437 | 6694.72 | 1428001 | 24083.05 | 506.7692 |
| 2 | 2733.639 | 1047.799 | 3235.659 | 7017.097 | 1289513 | 23169.77 | 409.1593 |
| 3 | 1764.43 | 629.6668 | 2014.547 | 4408.643 | 2062500 | 27112.9 | 1711.077 |
| 4 | −5760.75 | −2641.78 | −7402.5 | −15805 | −6571334 | −88600 | 4028.777 |
| 5 | 2570.829 | 978.5093 | 3047.142 | 6596.481 | 1714556 | 26802.12 | 512.8923 |
| 6 | 2666.764 | 1018.451 | 3143.797 | 6829.012 | 1206425 | 22069.25 | 412.3039 |
| 7 | 936.6537 | 340.6382 | 1059.084 | 2336.376 | 1727553 | 20718.88 | 1006.99 |
| 8 | 1386.963 | 531.5784 | 1605.459 | 3524 | 1283545 | 18012.2 | 222.5826 |
| 9 | 2548.109 | 987.5465 | 3006.603 | 6542.258 | 3394931 | 43533.45 | 315.2923 |
| 10 | 2546.287 | 985.938 | 2985.105 | 6517.33 | 3155681 | 41113.4 | 266.7208 |
| 11 | 2733.361 | 1070.782 | 3275.858 | 7080.001 | 2332750 | 33676.25 | 820.8051 |
| 12 | 2595.299 | 1010.626 | 3084.501 | 6690.426 | 1580560 | 25596.63 | 475.1345 |
| 13 | 2616.866 | 1016.852 | 3088.199 | 6721.918 | 3412642 | 43973.63 | 299.1677 |
| 14 | 2685.096 | 1044.349 | 3147.14 | 6876.585 | 3216295 | 42246.81 | 240.0045 |
| 15 | 2962.699 | 1192.866 | 3585.314 | 7740.879 | 2692650 | 38226.51 | 285.9898 |
For each of the outputs E, I1, I2, V, , and , the control strategies are ranked (see Table 5) from the most cost-effective to the least effective in minimizing the output, using incremental cost-effectiveness ratio (ICER). The ICER is used to compare the cost and benefits of two competing strategies and is defined as follows.
| Output used for ranking | |||||
|---|---|---|---|---|---|
| E | I1 | I2 | V | ||
| 8 | 8 | 8 | 8 | 10 | 10 |
| 7 | 7 | 7 | 7 | 14 | 14 |
| 12 | 15 | 15 | 15 | 13 | 13 |
| 15 | 12 | 12 | 12 | 9 | 9 |
| 4 | 4 | 4 | 4 | 15 | 15 |
| 2 | 10 | 2 | 2 | 7 | 7 |
| 10 | 2 | 6 | 6 | 8 | 8 |
| 6 | 6 | 1 | 10 | 12 | 12 |
| 1 | 3 | 10 | 11 | 11 | 11 |
| 5 | 1 | 5 | 5 | 3 | 3 |
| 3 | 14 | 3 | 3 | 4 | 4 |
| 14 | 13 | 14 | 14 | 6 | 6 |
| 13 | 9 | 13 | 13 | 2 | 2 |
| 9 | 5 | 9 | 9 | 1 | 1 |
| 11 | 11 | 11 | 11 | 5 | 5 |
It is observed that the most cost-effective strategy in minimizing infections only (without environmental contamination) is strategy 8 which consists of implementing only control u1, and if minimizing environmental contamination is also included, the most cost-effective strategy is 10, which involves implementing only controls u2 and u3. We note, however, that person-to-person infection continues to be the main source of infections and, hence, strategy 8 is recommended. That is, all efforts (physical distancing, wearing of nose masks among others) aimed at reducing/preventing person-to-person transmission should be adhered to strictly.
5. Conclusions
In this paper, a deterministic mathematical model has been proposed to study the dynamics of the COVID-19 in a variable-sized population. It is shown that unless the immigration of infected persons is restricted, the disease cannot be eradicated, and that if border closure is strictly adhered to, the disease can be eradicated if a threshold parameter ℛ0 is kept below unity. The model is modified into an optimal control problem by seeking to minimize an objective function that measures total infected persons and surface viral concentration and the total cost associated with implementing the various controls. It is shown that the best strategy in controlling the spread of COVID-19 is social distancing and use of nose masks.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Appendix
Coefficients of λ∗ in Equation (10)
Open Research
Data Availability
All data used to support the findings of this study are included within the article.