Analysis of a Stochastic SIR Model with Vaccination and Nonlinear Incidence Rate
Abstract
We expand an SIR epidemic model with vertical and nonlinear incidence rates from a deterministic frame to a stochastic one. The existence of a positive global analytical solution of the proposed stochastic model is shown, and conditions for the extinction and persistence of the disease are established. The presented results are demonstrated by numerical simulations.
1. Introduction
Mathematical models play an important role in the analysis and control of infectious diseases, and thus effective measures can be taken to reduce its transmission as much as possible. The study of mathematical models in epidemiology has received much attention from many scientists, and some novel results are obtained [1–4]. Recently, stochastic analysis has widely been applied in mathematical modeling in biology [5–9].
- (1)
If α1 = α2 = α3 = 0, g(S, I) becomes a bilinear incidence rate
- (2)
If α1 = α2 = 0 or α1 = α3 = 0, g(S, I) becomes a saturated incidence rate [17]
- (3)
If α3 = 0, g(S, I) becomes a Beddington–DeAngelis functional response [18]
- (4)
If α3 = α1α2, g(S, I) becomes the Crowley–Martin functional response presented in [19]
The organization of this paper is as follows. In Section 2, we show the existence of unique positive global solution to the given SDE system. Extinction and persistence in mean results are explored in Section 3 and Section 4, respectively. In Section 5, the analytical results are illustrated with the support of numerical examples. Finally, we close the paper with conclusion and future directions.
2. Existence and Uniqueness of the Nonnegative Solution
As we are dealing with the population model, the positive solution of the model is of our interest. The coefficients of (3) are locally Lipschitz continuous and do not satisfy the linear growth condition, so the solution of (3) may explode at a finite time. The following theorem shows that the solution is positive and will not explode at a finite time.
Theorem 1. For any given initial value X0 = (S(0), I(0), R(0)) ∈ Δ, there is a unique positive solution X(t) = (S(t), I(t), R(t)) of (3) on t ≥ 0, and the solution will remain in Δ with probability 1, namely, (S(t), I(t), R(t)) ∈ Δ for all t ≥ 0 almost surely (briefly a.s.).
Proof 1. Since the coefficients of system (3) are locally Lipschitz continuous, for any initial value (S(0), I(0), R(0)) ∈ Δ, there is a unique local solution on [0, τe), where τe is the explosion time. To show this solution is global, we need to show that τe = ∞ a.s. For this, we define the stopping time τ by
Using Itô’s formula, we get
Since f(S, I) ≥ 1, and S ≤ 1, I ≤ 1, we get,
Note that some components of X(τ) equal 0; thus, limt⟶τU(X(t)) = +∞.
Letting t⟶τ in (12), we obtain
3. Extinction
Theorem 2. Let (S(t), I(t), R(t)) be the solution of system (3) with initial value (S(0), I(0), R(0)) ∈ Δ. Assume that
- (i)
σ2 > β2/(2(pd + r)) or
- (ii)
Rs < 1 and σ2 < β
Then,
Namely, I(t) tends to zero exponentially a.s., i.e., the disease dies out with probability 1.
Proof 2. Applying Itô’s formula to system (3) leads to
Integrating both sides of (16) from 0 to t, we get
By the large number theorem for martingales [21], we obtain
If condition (i) is satisfied, dividing by t and taking the limit superior of both sides of (17), we get
If the condition (ii) is satisfied, note that
It follows that
Taking the superior limit of both sides of (22), we obtain
Remark 1. From Theorem 1, we can get that the disease will die out if Rs < 1, and the white noise is not large such that σ2 < β, while if the white noise is large enough such that condition (i) is satisfied, then the infectious disease of system (3) goes to extinction almost surely.
4. Persistence
Theorem 3. If then the solution (S(t), I(t), R(t)) of system (3) with initial initial value (S(0), I(0), R(0)) ∈ Δ is persistent in mean. Moreover, we have
Proof 3. We have
Integrating from 0 to t and dividing by t > 0 the third equation of system (3), we get
From (28), one can get
Integrating from 0 to t and dividing by t > 0 on both sides of yields
We can rewrite the inequality (32) as
This completes the proof of theorem.
5. Numerical Simulations
In order to illustrate our theoretical results, we give some numerical simulations. The values of m, σ, and β will be varied over the different examples.
Example 1. We choose the parameters in system (3) as follows:
By calculation, we have R0 = 0.4902; in this case, the disease dies out as shown in Figure 1(a). By choosing m = 0.3, we obtain R0 = 1.635, and we deduce that the disease persists in the population. Figure 1(b) illustrates this result.
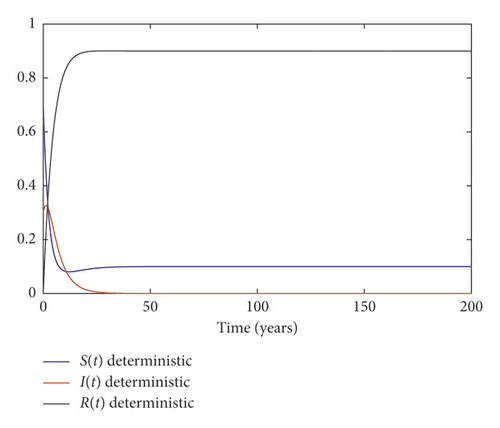
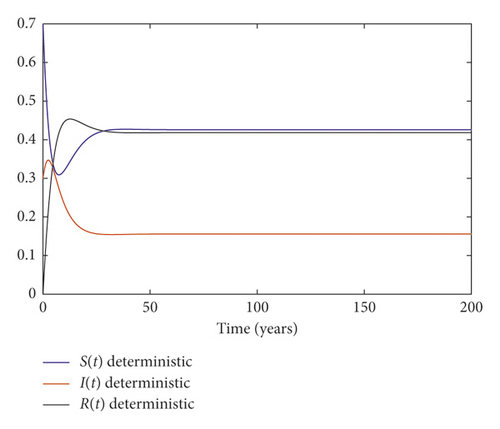
Example 2. We choose the parameters in system (3) as follows:
A simple computation shows that
It follows that the condition of Theorem 2 is satisfied. We conclude that the disease dies out; Figure 2 illustrates this result.
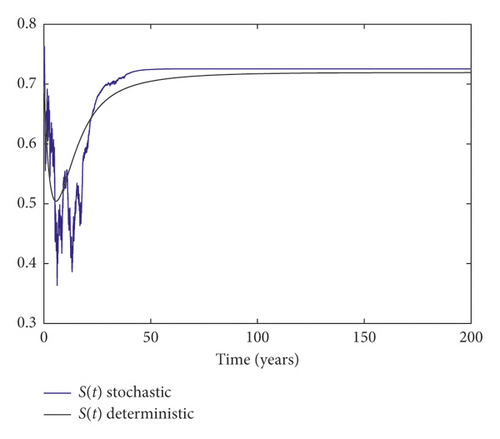
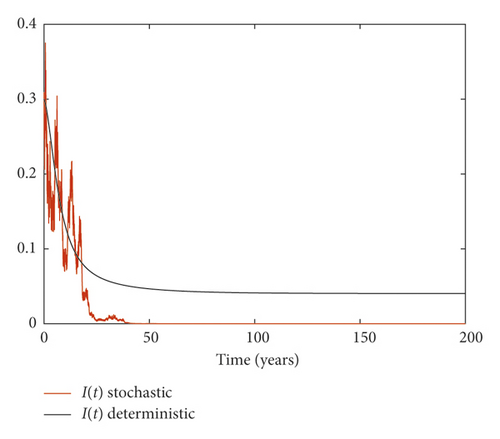
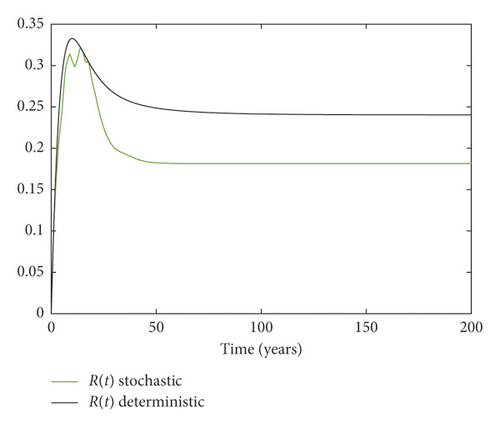
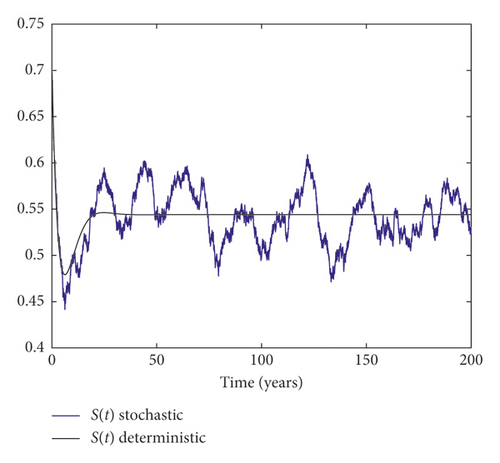
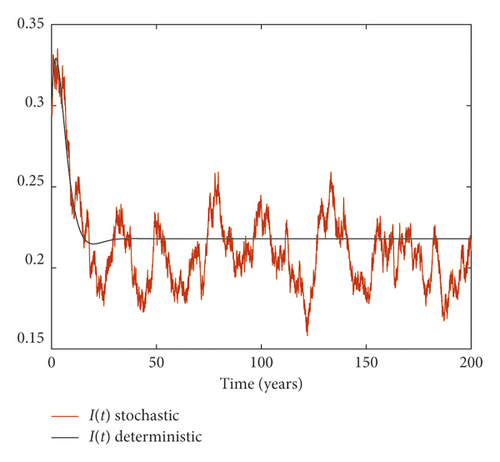
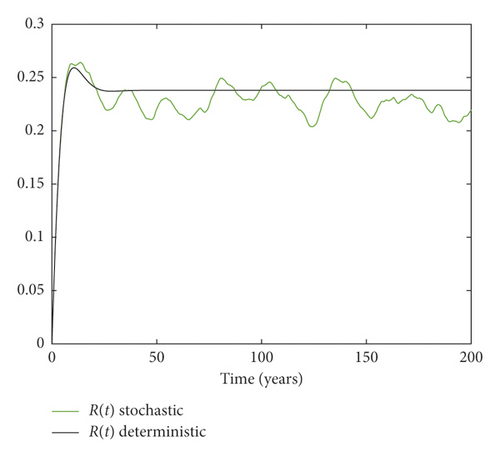
Example 3. On the contrary, we choose m = 0.05 and σ = 0.1 by simple calculation, and it can be found that which implies that the disease persists (Figure 3).
6. Conclusion
This article discusses a stochastic SIR epidemic model with vertical transmission and vaccination and nonlinear incidence rate. We have shown that when the noise is so small such that σ2 < β, the extinction of the disease can be determined by the value of Rs, i.e., if Rs < 1, the disease dies out. Moreover, the disease dies out when the white noise is large enough such that σ2 > (β2/2(pd + r)).
The persistence of the disease is determined by , i.e., if , the disease persists. We presented some numerical simulations to illustrate the obtained analytical results. To go further in this study, we can give a new dimension to the stochastic SIR epidemic model (3) by introducing a different type of noise which is the random telegraph noise (RTN), modeled by the Markov chain. We will investigate this case in our future works.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.