A Thermodynamics Study on the Tetrahydrofuran Effect in Exfoliated Graphite Nanoplatelets and Activated Carbon Mixtures at Temperatures between 293.15 and 308.15 K
Abstract
A thermodynamics study on exfoliated graphite nanoplatelets dispersed in tetrahydrofuran in comparison with activated carbon dispersed in same solvent was realised. The refractive index, speed of sound, and density of diluted mixed binary solutions of exfoliated graphite nanoplatelets and activated carbon in tetrahydrofuran were measured between 0 and 100 kg·m−3 with composition step of 20 kg·m−3 and at temperatures from 293.15 to 308.15 K and at normal pressure. The isentropic compressibility, acoustic impedance, specific refraction, relaxation strength, and space-filling factor have been evaluated for six concentrations, at four different temperatures for each system. The identified possible molecular interactions between the edges and the surface of exfoliated graphite nanoplatelets and tetrahydrofuran molecules, which include modifications in the structure of exfoliated nanostructured materials in tetrahydrofuran solvent and the influence of the temperature, and of the solute concentration have been calculated based on the obtained experimental values.
1. Introduction
Investigations of acoustical and optical parameters of binary solutions of tetrahydrofuran (THF) with exfoliated graphite nanoplatelets (xGnP) and activated carbon (AC) were realised based on their physicochemical behaviour. The physicochemical parameters strongly influence the dispersion of xGnP carbon-based nanomaterials compared with the dispersions of AC in THF, offering new information about the structure and interactions of carbon-based nanostructures in organic solvents [1]. Graphite-based nanomaterials can be classified based on the thickness, the number, and the disposal of the exfoliated layers [2]. Graphite nanoplatelets with dimensions of 3 to 30 nm thick and 10–100 graphene layers, known as exfoliated graphite nanoplatelets, present similar electrochemical behaviour, irrespective of the number of layers [3]. Even though the number of articles about xGnP greatly increased [4, 5], data on their thermodynamic properties, thermophysical behaviour description, and molecular modelling are still missing. The characterization of graphene dispersed in dimethylsulfoxide (DMSO) and dimethylformamide (DMF) polar solvents was published by Shih et al. [6]. It has been noted that DMSO solvent can break the ties in polymerised compounds of oxygenated chemical structures [7]. In the last years, the dispersion of xGnP in DMF and water was also studied [8].
The aim of the present study was to provide new experimental values on thermophysical properties such as refractive indices, speeds of sound, and densities in the THF + xGnP or THF + AC binary systems for which experimental data are not available. The binary mixtures were measured at atmospheric pressure, using various compositions of solutes between 0 and 100 kg·m−3 with increments of 20 kg·m−3 and at temperatures of 293.15, 298.15, 303.15, and 308.15 K. The effect of the variation of these parameters on the concentrations in the studied mixed solutions was evaluated. Based on the understanding of the molecular interactions, several applications could be envisaged, such as sensors with composite nanomaterials [2]. These infield detection systems are used for monitoring the environmental stress. The sorbents based on composite nanomaterials are also used for the removal of contaminants, or to allow the administration of drugs [9] from mixed solutions [10]. Among the applications of nanotechnologies, new types of sorbents based on carbon nanomaterials are studied and further applied for the removal of environmental contaminants based on their interactions at the molecular level [11–13].
2. Experimental Section
2.1. Materials and Methods
Exfoliated graphite nanoplatelets were purchased from XG Sciences (Lansing, MI, USA) and are characterized by mass fraction of >0.95 carbon, thickness of approximately 15 nm, diameter of 25 μm, and surface area of 50–80 m2·g−1. THF was supplied from Merck and activated carbon (mass fraction > 0.99) from Sigma-Aldrich. These chemical compounds have been used without further purification, except drying over P2O5 for 72 h, for those with a mass fraction purity of >0.95. In Table 1, the specifications for the chemical compounds used in the working sample preparation are presented.
| Chemical name | Source | Initial mass fraction purity | Purification method | Final mass fraction purity | Analysis method |
|---|---|---|---|---|---|
| AC | Sigma-Aldrich | >0.99 carbon | None | — | — |
| xGnP | XG Sciences, (Lansing, MI, USA) | >0.95 carbon | None | — | — |
| THF | E. Merck | p.a. | None | — | — |
- AC: activated carbon; xGnP: exfoliated graphite nanoplatelets; THF: tetrahydrofuran.
Working solutions of AC + THF and xGnP + THF with different concentrations have been prepared at 298.15 K using THF of analytical purity (p.a.). The binary mixed solutions have been freshly prepared and kept in airtight bottles by mixing known compositions of stock and pure THF solvent. All precautions have been taken to minimise the errors produced by the evaporation of solutions. Specific concentration was expressed in kg·m−3 as measured unit. The initial compositions of the stock mixtures were prepared with an accuracy of ±0.2 kg·m−3. More details about the refractive indices, speeds of sound, and densities experimental measurements and procedures can be found in previous papers [8, 14, 15].
The refractive index of the samples was measured with an Anton Paar GmbH Abbe automatic refractometer at a controlled temperature within ±0.01 K and a precision of ±0.000001. The calibration of the refractometer has been done by measuring the refractive index of deionised twice distilled pure water. The speeds of sound and densities of mixed solutions have been measured with Anton Paar DSA 5000 digital (Austria) equipment under atmospheric pressure. The precision of the density is of ±0.001 kg·m−3. The speed of sound has been measured at a reduced wavelength and a low frequency of approximately 3 MHz [16, 17], and the precision is ±0.01 m·s−1. The temperature was controlled by several Peltier units with a precision of ±0.001 K for obtaining the speed of sound and density experimental data.
The densitometer instrument was internally calibrated with air and doubly distilled deionised pure water by determining the speed of sound and density at normal pressure, according to the recommendations of the manufacturer. The density, speed of sound, and refractive index values of water at 298.15 K were measured as 0.99706 g·cm−3, 1497.1 m·s−1, and 1.33248, similar to the values described in the literature [18–21], with a reproducibility of ±0.000005 g·cm−3, ±0.04 m·s−1, and ±0.000005 units, respectively. Uncertainties associated with the experimentally measured data for refractive index, speed of sound, and density were presented under each data table, according to the guide for evaluation of measurement data [22].
2.2. Theory and Calculation
The acoustical and optical thermodynamic properties such as isentropic compressibility (kS), impedance (Z), space-filling factor (S), specific refraction (rD), and relaxation strength (r) at various temperatures and atmospheric pressure were estimated from experimental results of density, speed of sound, and refractive index. The calculation relations for the derived thermophysical properties have been described elsewhere [8, 14, 15].
3. Results and Discussion
Experimental data on densities (ρ), speeds of sound (c), and refractive indices (nD) as a function of specific concentration of xGnP or AC in THF solvent at a pressure of 0.1 MPa are reported. The experimental values of these properties at atmospheric pressure for the pure THF solvent in comparison with literature values [23–35] at different temperatures from 293.15 up to 308.15 K are presented in Table 2.
| T (K) | Present work | Reference | |
|---|---|---|---|
| ρ (g·cm−3) | |||
| 293.15 | 0.88762 | 0.88750 | [23] |
| 0.88947 | [25] | ||
| 298.15 | 0.88216 | 0.88205 | [27] |
| 0.88504 | [25] | ||
| 0.88206 | [30] | ||
| 0.88207 | [31] | ||
| 303.15 | 0.87664 | 0.87710 | [27] |
| 0.88076 | [25] | ||
| 0.87114 | 0.87214 | [27] | |
| 308.15 | 0.87663 | [25] | |
| 0.87710 | [35] | ||
| c (m·s−1) | |||
| 293.15 | 1303.65 | 1302.00 | [24] |
| 298.15 | 1279.38 | 1277.60 | [28] |
| 303.15 | 1255.24 | 1258.00 | [32] |
| 1254.00 | [33] | ||
| 308.15 | 1231.10 | 1231.70 | [35] |
| nD | |||
| 293.15 | 1.40731 | 1.4064 | [23] |
| 1.4071 | [26] | ||
| 298.15 | 1.40464 | 1.4049 | [29] |
| 303.15 | 1.40198 | 1.4026 | [29] |
| 1.4030 | [34] | ||
| 308.15 | 1.39932 | 1.4008 | [29] |
The carbon-based nanoplatelets and activated carbon disperse well in the THF solvent with a high dielectric conductivity because it is known that they are miscible in high dielectric liquids, with low viscosity, low melting point, and high solubility for inorganic salts [36].
Tables 3 and 4 present experimental results for the same thermophysical properties of AC and xGnP in THF solvent measured as a function of their specific concentrations at different temperatures.
| T (K) | ρ (kg·m−3) | c (m·s−1) | nD | ρ (kg·m−3) | c (m·s−1) | nD |
|---|---|---|---|---|---|---|
| C (kg·m−3) = 0 | C (kg·m−3) = 20 | |||||
| 293.15 | 887.62 | 1303.65 | 1.40731 | 887.66 | 1303.61 | 1.40731 |
| 298.15 | 882.16 | 1279.38 | 1.40464 | 882.21 | 1279.46 | 1.40465 |
| 303.15 | 876.64 | 1255.24 | 1.40198 | 876.68 | 1255.31 | 1.40200 |
| 308.15 | 871.14 | 1231.10 | 1.39932 | 871.18 | 1231.19 | 1.39935 |
| C (kg·m−3) = 40 | C (kg·m−3) = 60 | |||||
| 293.15 | 887.72 | 1303.59 | 1.40731 | 887.76 | 1303.62 | 1.40732 |
| 298.15 | 882.23 | 1279.52 | 1.40466 | 882.29 | 1279.58 | 1.40467 |
| 303.15 | 876.74 | 1255.37 | 1.40201 | 876.80 | 1255.41 | 1.40202 |
| 308.15 | 871.21 | 1231.26 | 1.39936 | 871.27 | 1231.37 | 1.39938 |
| C (kg·m−3) = 80 | C (kg·m−3) = 100 | |||||
| 293.15 | 887.80 | 1303.72 | 1.40733 | 887.87 | 1303.72 | 1.40733 |
| 298.15 | 882.34 | 1279.64 | 1.40468 | 882.41 | 1279.64 | 1.40468 |
| 303.15 | 876.85 | 1255.49 | 1.40203 | 876.91 | 1255.49 | 1.40203 |
| 308.15 | 871.32 | 1231.46 | 1.39938 | 871.39 | 1231.46 | 1.39938 |
- aC (kg·m−3) is the specific concentration of AC in the THF solvent. Standard uncertainties (u) are u(T) = 0.001 K for ρ and c and u(T) = 0.01 K for nD, and the combined expanded uncertainties (Uc) are Uc(ρ) = 0.01 kg·m−3, Uc(c) = 0.05 m·s−1 (level of confidence = 0.95, k = 2), and Uc(nD) = 0.00001.
| T (K) | ρ (kg·m−3) | c (m·s−1) | nD | ρ (kg·m−3) | c (m·s−1) | nD |
|---|---|---|---|---|---|---|
| C (kg·m−3) = 0 | C (kg·m−3) = 20 | |||||
| 293.15 | 887.62 | 1303.65 | 1.40731 | 887.76 | 1303.60 | 1.40691 |
| 298.15 | 882.16 | 1279.38 | 1.40464 | 882.33 | 1279.43 | 1.40423 |
| 303.15 | 876.64 | 1255.24 | 1.40198 | 876.85 | 1255.30 | 1.40156 |
| 308.15 | 871.14 | 1231.10 | 1.39932 | 871.32 | 1231.21 | 1.39895 |
| C (kg·m−3) = 40 | C (kg·m−3) = 60 | |||||
| 293.15 | 887.88 | 1303.57 | 1.40663 | 888.06 | 1303.56 | 1.40671 |
| 298.15 | 882.44 | 1279.47 | 1.40394 | 882.58 | 1279.49 | 1.40399 |
| 303.15 | 876.95 | 1255.35 | 1.40121 | 877.11 | 1255.38 | 1.40131 |
| 308.15 | 871.44 | 1231.26 | 1.3988 | 871.64 | 1231.31 | 1.39881 |
| C (kg·m−3) = 80 | C (kg·m−3) = 100 | |||||
| 293.15 | 888.22 | 1303.57 | 1.40697 | 888.31 | 1303.59 | 1.40772 |
| 298.15 | 882.78 | 1279.48 | 1.40428 | 882.89 | 1279.46 | 1.40498 |
| 303.15 | 877.34 | 1255.36 | 1.40161 | 877.41 | 1255.32 | 1.40241 |
| 308.15 | 871.78 | 1231.29 | 1.39905 | 871.89 | 1231.27 | 1.39980 |
- aC (kg·m−3) is the specific concentration of xGnP in THF solvent. Standard uncertainties (u) are u(T) = 0.001 K for ρ and c and u(T) = 0.01 K for nD, and the combined expanded uncertainties (Uc) are Uc(ρ) = 0.01 kg·m−3, Uc(c) = 0.1 m·s−1 (level of confidence = 0.95, k = 2), and Uc(nD) = 0.00001.
Figures 1–3 present the comparison of the refractive indices, speeds of sound, and densities experimental and polynomial correlated values as a function of composition of xGnP and AC in the xGnP + THF and AC + THF binary mixed solutions.
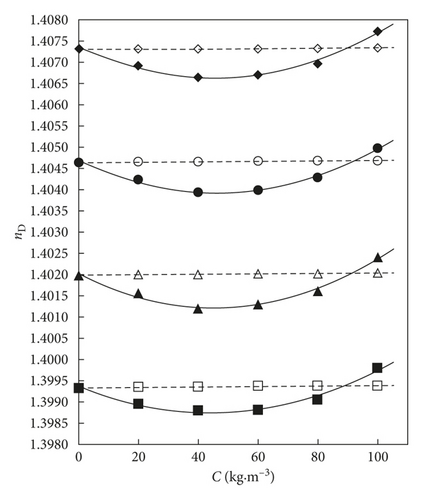
The refractive index variation by concentration of the activated carbon and exfoliated graphite nanoplatelet solutes in THF is presented in Figure 1.
The refractive index behaviour decreased in the xGnP + THF or AC + THF mixtures at the same solute composition by raising the temperature. The refractive index values increase by the temperature in the xGnP + THF system at xGnP concentrations bigger than 90 kg·m−3. The refractive index values rise very slightly by rising the composition of AC solute in the THF organic solvent at all the studied temperatures, too. In the xGnP + THF mixture, a decrease of the refractive index values by increasing up to C = 60 kg·m−3 (xGnP) composition is noticed, followed by an increase up to C = 100 kg·m−3.
The location of oxygen functional groups at the xGnP edges plays an important role in interactions [37], the number of edges, plane surfaces, defects, and the (xGnP) crystallite dimension, inducing significant differences by comparison with the other studied material activated carbon.
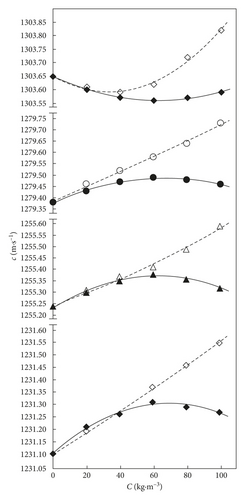
In Figure 2, it is presented the variation of experimental values of speed of sound and its correlation with the solute composition in xGnP + THF and AC + THF studied mixtures.
Figure 2 shows the speeds of sound variation in the AC + THF binary system, which increases by increasing the temperature between 298.15 and 308.15 K. In the same system AC + THF, the speed of sound values decrease up to 60 kg·m−3 and rise at 293.15 K, up to 100 kg·m−3 with a different slope. The speed of sound values in xGnP + THF system varies in a similar way at 293.15 K, increasing up to C = 60 kg·m−3, then decrease slowly by increasing the concentration. The speed of sound variation at the three studied temperatures 298.15, 303.15, and 308.15 K is similar as in AC + THF binary mixture. The values of speed of sound in xGnP + THF mixtures differ than those obtained for the AC + THF system. With regard to the slope, this strongly decreases at 298.15, 303.15, and 308.15 K. The behaviour of speed of sound values by rising xGnP or AC solute concentration shows that the existence of powerful solute-solvent interactions [38] via dipole-dipole ones, which in the domain of studied compositions, can produce displacements of nuclei and electrons. In the xGnP + THF mixture, these interactions increase/intensify because the speed of sound slightly decreasing by temperature is much more in the xGnP + THF system in comparison with that in AC + THF. The thermal energy generated by the increase of the temperature contributes to possible bond breakings and weakens the molecular forces in both studied systems.
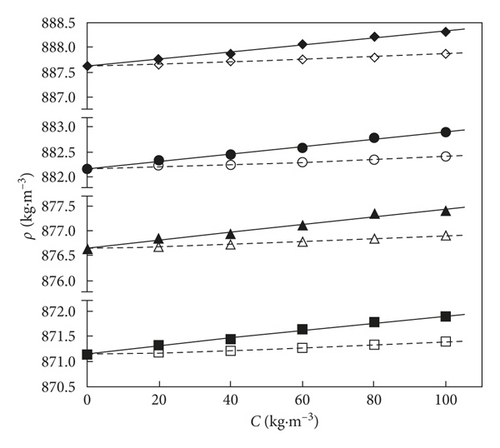
The variation of obtained data for exfoliated graphite nanoplatelets and activated carbon in THF organic solvent as a function of the solute composition is presented in Figure 3.
In the mixed binary solutions of AC or xGnP + THF by rising the temperature at the same solute composition, the density obtained values decrease as it can be observed from Figure 3. The density values in the xGnP + THF and AC + THF mixtures rise by rising xGnP and AC specific compositions, up to C = 100 kg·m−3.
The density of the system xGnP + THF increases in comparison with that of the AC + THF system, probably due to stronger interactions between THF and xGnP compounds than in those between THF and AC compounds. A more pronounced slope of the solute concentration versus density can be mentioned in the xGnP + THF system in comparison with the AC + THF one, based on the interactions occurring between THF and carbon-based nanomaterials, which rise the density by rising the solute specific composition in the mixed solution. This behaviour can be explained by several opposite effects. It can be assumed that dipole-dipole interactions, dispersion forces, and hydrogen bonds are the most important forces that can appear between these unlike molecules [39].
The values of isentropic compressibility empirically evaluated [14], acoustic impedance, space-filling factor, specific refraction, and relaxation strength were computed from experimental data. Table 5 presents measured and derived thermophysical properties as function of AC specific composition fraction in THF solvent at different temperatures.
| T (K) | Z (10−5⋅kg⋅m2⋅s1) | kS(1010⋅m−2⋅N1) | S | rD(103⋅m−3⋅kg1) | r |
|---|---|---|---|---|---|
| C (kg·m−3) = 0 | |||||
| 293.15 | 11.57145 | 6.62904 | 0.24633 | 0.27752 | 0.33613 |
| 298.15 | 11.28617 | 6.92554 | 0.24491 | 0.27762 | 0.36062 |
| 303.15 | 11.00393 | 7.23978 | 0.24348 | 0.27775 | 0.38452 |
| 308.15 | 10.72460 | 7.57400 | 0.24206 | 0.27787 | 0.40797 |
| C (kg·m−3) = 20 | |||||
| 293.15 | 11.57162 | 6.62915 | 0.24633 | 0.27750 | 0.33617 |
| 298.15 | 11.28752 | 6.92428 | 0.24491 | 0.27761 | 0.36054 |
| 303.15 | 11.00505 | 7.23864 | 0.24349 | 0.27775 | 0.38445 |
| 308.15 | 10.72588 | 7.57255 | 0.24208 | 0.27787 | 0.40788 |
| C (kg·m−3) = 40 | |||||
| 293.15 | 11.57223 | 6.62891 | 0.24633 | 0.27749 | 0.33619 |
| 298.15 | 11.28831 | 6.92347 | 0.24492 | 0.27761 | 0.36048 |
| 303.15 | 11.00633 | 7.23745 | 0.24350 | 0.27773 | 0.38439 |
| 308.15 | 10.72686 | 7.57142 | 0.24208 | 0.27787 | 0.40781 |
| C (kg·m−3) = 60 | |||||
| 293.15 | 11.57302 | 6.62830 | 0.24634 | 0.27748 | 0.33616 |
| 298.15 | 11.28961 | 6.92235 | 0.24492 | 0.27760 | 0.36042 |
| 303.15 | 11.00743 | 7.23650 | 0.24351 | 0.27772 | 0.38435 |
| 308.15 | 10.72856 | 7.56955 | 0.24209 | 0.27786 | 0.40771 |
| C (kg·m−3) = 80 | |||||
| 293.15 | 11.57443 | 6.62699 | 0.24634 | 0.27747 | 0.33606 |
| 298.15 | 11.29078 | 6.92131 | 0.24493 | 0.27759 | 0.36036 |
| 303.15 | 11.00876 | 7.23516 | 0.24351 | 0.27771 | 0.38428 |
| 308.15 | 10.72996 | 7.56801 | 0.24209 | 0.27785 | 0.40762 |
| C (kg·m−3) = 100 | |||||
| 293.15 | 11.57623 | 6.62545 | 0.24635 | 0.27746 | 0.33596 |
| 298.15 | 11.29247 | 6.91979 | 0.24493 | 0.27757 | 0.36027 |
| 303.15 | 11.01039 | 7.23351 | 0.24352 | 0.27770 | 0.38418 |
| 308.15 | 10.73160 | 7.56630 | 0.24210 | 0.27783 | 0.40753 |
The computed physicochemical parameters in the xGnP + THF mixed binary solutions are presented in the same conditions in Table 6.
| T (K) | Z(10−5⋅kg⋅m2⋅s1) | kS(109⋅m−2⋅N1) | S | rD(103⋅m−3⋅kg1) | r |
|---|---|---|---|---|---|
| C (kg·m−3) = 0 | |||||
| 293.15 | 11.57146 | 6.62904 | 0.24633 | 0.27752 | 0.33613 |
| 298.15 | 11.28618 | 6.92554 | 0.24491 | 0.27762 | 0.36062 |
| 303.15 | 11.00394 | 7.23978 | 0.24348 | 0.27775 | 0.38452 |
| 308.15 | 10.72460 | 7.57400 | 0.24206 | 0.27787 | 0.40797 |
| C (kg·m−3) = 20 | |||||
| 293.15 | 11.57284 | 6.62851 | 0.24612 | 0.27723 | 0.33618 |
| 298.15 | 11.28879 | 6.92366 | 0.24469 | 0.27732 | 0.36057 |
| 303.15 | 11.00710 | 7.23735 | 0.24326 | 0.27742 | 0.38446 |
| 308.15 | 10.72778 | 7.57108 | 0.24186 | 0.27758 | 0.40786 |
| C (kg·m−3) = 40 | |||||
| 293.15 | 11.57414 | 6.62792 | 0.24597 | 0.27703 | 0.33621 |
| 298.15 | 11.29056 | 6.92237 | 0.24453 | 0.27711 | 0.36053 |
| 303.15 | 11.00879 | 7.23595 | 0.24307 | 0.27718 | 0.38441 |
| 308.15 | 10.72969 | 7.56943 | 0.24178 | 0.27745 | 0.40781 |
| C (kg·m−3) = 60 | |||||
| 293.15 | 11.57639 | 6.62667 | 0.24601 | 0.27702 | 0.33622 |
| 298.15 | 11.29252 | 6.92105 | 0.24456 | 0.27710 | 0.36051 |
| 303.15 | 11.01106 | 7.23428 | 0.24313 | 0.27719 | 0.38438 |
| 308.15 | 10.73259 | 7.56707 | 0.24179 | 0.27739 | 0.40776 |
| C (kg·m−3) = 80 | |||||
| 293.15 | 11.57857 | 6.62538 | 0.24615 | 0.27713 | 0.33621 |
| 298.15 | 11.29499 | 6.91959 | 0.24471 | 0.27721 | 0.36052 |
| 303.15 | 11.01378 | 7.23262 | 0.24329 | 0.27730 | 0.38440 |
| 308.15 | 10.73414 | 7.56611 | 0.24192 | 0.27750 | 0.40778 |
| C (kg·m−3) = 100 | |||||
| 293.15 | 11.57992 | 6.62450 | 0.24655 | 0.27755 | 0.33619 |
| 298.15 | 11.29622 | 6.91895 | 0.24509 | 0.27760 | 0.36054 |
| 303.15 | 11.01430 | 7.23250 | 0.24371 | 0.27777 | 0.38444 |
| 308.15 | 10.73532 | 7.56540 | 0.24232 | 0.27792 | 0.40780 |
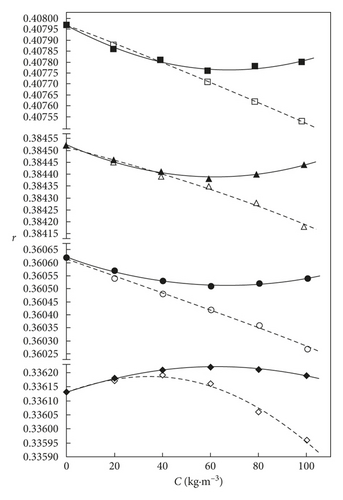
Figures 4 and 5 illustrate the compared variations of xGnP or AC + THF binary mixtures of the estimated and correlated values of isentropic compressibility kS and relaxation strength r of the solute composition.
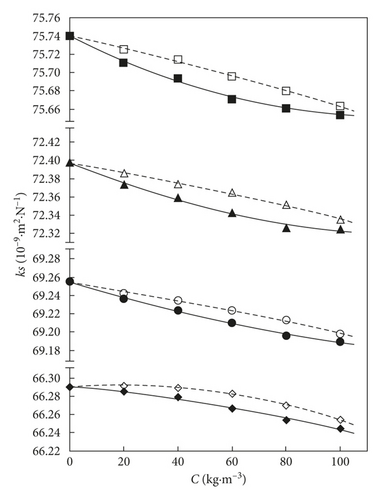
The isentropic compressibility values of the binary AC + THF system (Figure 4) increase by rising the temperature to the same solute composition. In both systems, the isentropic compressibility values decrease with rising the solute compositions up to a C value of 100 kg·m−3. A different trend of variation of the concentration in the AC + THF system at 293.15 K was noticed, till 40 kg·m−3.
The values of the isentropic compressibility for the xGnP + THF mixture are smaller than the AC + THF compressibility values all over the solute concentration range. The two systems present different values of isentropic compressibility: nanoexfoliated graphite modifying the xGnP + THF system strongly than the AC + THF system.
This behaviour of the estimated isentropic compressibility may be the result of several opposite interactions. Dipole-dipole interactions usually improve the dispersion of the structures in a better way than by breaking of molecular clusters. The values of isentropic compressibility in the xGnP + THF system are smaller in comparison with those in the AC + THF system, the repulsive interaction resulting from the compression of the C–H vertical bonds between the nanosheet surfaces [39, 40]. In the mixture of the AC dispersed in THF solvent, the THF molecules are limited at a single-layer structure on the surface of AC particles, possibly losing some of the solvent-solvent interaction energy, due to the smaller number of adjacent solvent molecules. The isentropic compressibility empirically estimated based on the obtained experimental data seems consistent, having the same sign in both studied binary mixtures [14].
In the xGnP + THF and AC + THF binary mixtures, the relaxation strength rises by temperature up to a concentration values of 40 kg·m−3 and 60 kg·m−3, respectively, for 293.15 K, as presented in Figure 5. The relaxation strength decreases at temperatures of 298.15, 303.15, and 308.15 in AC + THF mixture.
Relaxation strength values in the xGnP + THF system decrease up to a solute composition of approximately 60 kg·m−3 at the temperatures from 298.15 up to 308.15 K and rise by raising the solute composition, which reflects a predominance of the molecular interactions [41]. The computed slope and intercept of simple polynomial equation with “statistical functions” from Excel for all properties from the presented figures are shown in Tables 7 and 8.
| T (K) | 293.15 | 298.15 | 303.15 | 308.15 |
|---|---|---|---|---|
| Density | ||||
| Slope | 0.068240877 | 0.002428571 | 0.002742857 | 0.002471429 |
| Intercept | 887.6161905 | 882.1519048 | 876.6328571 | 871.1280952 |
| Speed of sound | ||||
| Slope | 0.00172857 | 0.003357143 | 0.003328571 | 0.004528571 |
| Intercept | 1303.581905 | 1279.38381 | 1255.235238 | 1231.095238 |
| Refractive index | ||||
| Slope | 0.000000314 | 0.000000429 | 0.000000571 | 0.000000657 |
| Intercept | 1.407304286 | 1.404641905 | 1.401984762 | 1.399330476 |
| Isentropic compressibility | ||||
| Slope | −0.000035771 | −0.0000554 | −0.000061057 | −0.000077129 |
| Intercept | 6.629761905 | 6.92556 | 7.239892857 | 7.574161429 |
| Relaxation strength | ||||
| Slope | −0.000001729 | −0.000003357 | −0.000003214 | −0.00000440 |
| Intercept | 0.336198095 | 0.36061619 | 0.384522381 | 0.407973333 |
| T (K) | 293.15 | 298.15 | 303.15 | 308.15 |
|---|---|---|---|---|
| Density | ||||
| Slope | 0.007157143 | 0.007342857 | 0.007828571 | 0.007614286 |
| Intercept | 887.6171429 | 882.1628571 | 876.6585714 | 871.1542857 |
| Speed of sound | ||||
| Slope | −0.00057143 | 0.000814286 | 0.000871429 | 0.001628571 |
| Intercept | 1303.618571 | 1279.410952 | 1255.281429 | 1231.158571 |
| Refractive index | ||||
| Slope | 0.000003300 | 0.000002714 | 0.000003429 | 0.000003871 |
| Intercept | 1.406876667 | 1.404207619 | 1.401508571 | 1.398928095 |
| Isentropic compressibility | ||||
| Slope | −0.000476286 | −0.000664 | −0.000746571 | −0.00086100 |
| Intercept | 66.29384762 | 69.251800 | 72.39146190 | 75.73153333 |
| Relaxation strength | ||||
| Slope | 0.000000571 | −0.000000814 | −0.000000871 | −0.000001629 |
| Intercept | 0.336161429 | 0.360589048 | 0.384478571 | 0.407911429 |
The values for the absolute average percentage deviation (AAD%) obtained from the correlated of equation (1) of ρ, c, nD, kS and r as a function of composition have been computed.
Fitting parameters Ai and AAD% values of all the studied temperatures are summarized in Table 9 for AC + THF binary solutions.
| T (K) | C (kg·m−3) | A1 (kg·m−3) | A2 (kg0·m0) | A3 (kg−1·m3) | R2 | AAD% |
|---|---|---|---|---|---|---|
| ⍴ (kg·m−3) | ||||||
| 293.15 | 0–100 | 887.62 | 0.0021304 | 0.0000031 | 0.99401 | 0.008 |
| 298.15 | 0–100 | 882.17 | 0.0014464 | 0.0000098 | 0.99356 | 0.009 |
| 303.15 | 0–100 | 876.64 | 0.0024750 | 0.0000027 | 0.99821 | 0.009 |
| 308.15 | 0–100 | 871.14 | 0.0014446 | 0.0000103 | 0.99775 | 0.009 |
| T (K) | C (kg·m−3) | A1 (kg0·m−1·s) | A2 (kg·m−4·s) | A3 (kg2·m−7·s) | R2 | AAD% |
| c (m·s−1) | ||||||
| 293.15 | 0–100 | 1303.65 | −0.003539 | 0.000053 | 0.98967 | 0.005 |
| 298.15 | 0–100 | 1279.39 | 0.003134 | 0.000002 | 0.99545 | 0.008 |
| 303.15 | 0–100 | 1255.25 | 0.002302 | 0.000010 | 0.99115 | 0.009 |
| 308.15 | 0–100 | 1231.10 | 0.004171 | 0.000004 | 0.99825 | 0.011 |
| T (K) | C (kg·m−3) | A1 | A2·105 | A3·107 | R2 | AAD% |
| nD | ||||||
| 293.15 | 0–100 | 1.40731 | −0.000000087 | 0.000000004 | 0.98483 | 0.0001 |
| 298.15 | 0–100 | 1.40464 | 0.000000652 | −0.000000002 | 0.98661 | 0.0001 |
| 303.15 | 0–100 | 1.40198 | 0.000000795 | −0.000000002 | 0.99235 | 0.0001 |
| 308.15 | 0–100 | 1.39932 | 0.000001282 | −0.000000006 | 0.97686 | 0.0002 |
| T (K) | C (kg·m−3) | A1 (10−9·kg0·m2·N−1) | A2 (10−9·kg−1·m5·N−1) | A3 (10−9·kg−2·m8·N−1) | R2 | AAD% |
| kS (1010·m2·N−1) | ||||||
| 293.15 | 0–100 | 6.62902 | 0.000020166 | −0.000000559 | 0.99868 | 0.001 |
| 298.15 | 0–100 | 6.92543 | −0.000045489 | −0.000000099 | 0.99601 | 0.001 |
| 303.15 | 0–100 | 7.23971 | −0.000046995 | −0.000000141 | 0.99739 | 0.001 |
| 308.15 | 0–100 | 7.57399 | −0.000064004 | −0.000000131 | 0.99836 | 0.001 |
| T (K) | C (kg·m−3) | A1 (kg0·m0) | A2 (kg−1·m3) | A3 (kg−2·m−6) | R2 | AAD% |
| r | ||||||
| 293.15 | 0–100 | 0.33613 | 0.000003539 | −0.000000053 | 0.98967 | 0.002 |
| 298.15 | 0–100 | 0.36061 | −0.000003134 | −0.000000002 | 0.99545 | 0.002 |
| 303.15 | 0–100 | 0.38451 | −0.000002366 | −0.000000008 | 0.99004 | 0.003 |
| 308.15 | 0–100 | 0.40797 | −0.000004043 | −0.000000004 | 0.99899 | 0.001 |
- aAi and R2 were obtained from (2).
Tables 9 and 10 present the calculated values of the Ai parameters, correlation coefficient R2 obtained for density ⍴, speed of sound c, isentropic compressibility kS, and relaxation strength r [8, 14, 15], together with the AAD% calculated from (2) for AC + THF and xGnP + THF binary mixed solutions.
| T (K) | C (kg·m−3) | A1 (kg·m−3) | A2 (kg0·m0) | A3 (kg−1·m3) | R2 | AAD% |
|---|---|---|---|---|---|---|
| ⍴ (kg·m−3) | ||||||
| 293.15 | 0–100 | 887.61 | 0.0075589 | −0.0000040 | 0.99423 | 0.002 |
| 298.15 | 0–100 | 882.17 | 0.0070750 | 0.0000027 | 0.99438 | 0.002 |
| 303.15 | 0–100 | 876.65 | 0.0086321 | −0.0000080 | 0.98620 | 0.003 |
| 308.15 | 0–100 | 871.14 | 0.0088200 | −0.0000100 | 0.99598 | 0.002 |
| T (K) | C (kg·m−3) | A1 (kg0·m−1·s) | A2 (kg·m−4·s) | A3 (kg2·m−7·s) | R2 | AAD% |
| c (m·s−1) | ||||||
| 293.15 | 0–100 | 1303.65 | −0.002848 | 0.000023 | 0.99669 | 0.001 |
| 298.15 | 0–100 | 1279.38 | 0.003270 | −0.000025 | 0.99509 | 0.001 |
| 303.15 | 0–100 | 1255.24 | 0.004354 | −0.000035 | 0.98499 | 0.002 |
| 308.15 | 0–100 | 1231.10 | 0.005780 | −0.000042 | 0.98843 | 0.001 |
| T (K) | C (kg·m−3) | A1 | A2·105 | A3·107 | R2 | AAD% |
| nD | ||||||
| 293.15 | 0–100 | 1.40735 | −0.000032012 | 0.000000353 | 0.98442 | 0.086 |
| 298.15 | 0–100 | 1.40468 | −0.000032420 | 0.000000351 | 0.98895 | 0.084 |
| 303.15 | 0–100 | 1.40203 | −0.000035411 | 0.000000388 | 0.98336 | 0.119 |
| 308.15 | 0–100 | 1.39935 | −0.000028093 | 0.000000320 | 0.97351 | 0.092 |
| T (K) | C (kg·m−3) | A1 (10−10·kg0·m2·N−1) | A2 (10−9·kg−1·m5·N−1) | A3 (10−9·kg−2·m8·N−1) | R2 | AAD% |
| kS (10−10·m2·N−1) | ||||||
| 293.15 | 0–100 | 6.6291139 | −0.000273 | −0.000002 | 0.991057 | 0.002 |
| 298.15 | 0–100 | 6.9255086 | −0.000910 | 0.000002 | 0.996994 | 0.003 |
| 303.15 | 0–100 | 7.2397718 | −0.001216 | 0.000005 | 0.991389 | 0.003 |
| 308.15 | 0–100 | 7.5739754 | −0.001478 | 0.000006 | 0.995453 | 0.002 |
| T (K) | C (kg·m−3) | A1 (kg0·m0) | A2 (kg−1·m3) | A3 (kg−2·m−6) | R2 | AAD% |
| r | ||||||
| 293.15 | 0–100 | 0.33613 | 0.000002848 | −0.000000023 | 0.99669 | 0.001 |
| 298.15 | 0–100 | 0.36062 | −0.000003270 | 0.000000025 | 0.99508 | 0.001 |
| 303.15 | 0–100 | 0.38453 | −0.000004354 | 0.000000035 | 0.98499 | 0.001 |
| 308.15 | 0–100 | 0.40797 | −0.000005780 | 0.000000042 | 0.98843 | 0.002 |
- aAi and R2 were obtained from (2).
The absolute average percentage deviation for the physicochemical properties: refractive index, speed of sound, density, isentropic compressibility, and relaxation strength are less than 0.0002, 0.011, 0.009, 0.001, and 0.003% for the AC + THF mixture and less than 0.119, 0.002, 0.003, 0.003, and 0.002% for the xGnP + THF mixture, being well correlated for both systems, as it can be seen from Tables 9 and 10.
The isentropic compressibility (kS) rises by the temperature, but it decreases when the concentration rises.
As shown in Tables 9 and 10 and in Figures 1–5, the values of the thermophysical properties experimentally obtained on the basis of polynomial relation (1) have been correlated with good accuracy.
The thermophysical behaviour of studied carbon-based nanomaterial-mixed solutions is well described by using three correlation parameters with polynomial expression (1). The experimental property and computed property values have been then statistically interpreted by analysis of variance “two-factor without replication” by method ANOVA. The obtained values for the both binary mixtures of AC and xGnP in THF solvent are given in Tables 11 and 12, respectively.
| Source of variation | SS | df | MS | F | P value | Fcrit |
|---|---|---|---|---|---|---|
| Density | ||||||
| Concentration | 0.179183 | 5 | 0.035837 | 408.2658 | 1.81E − 15 | 2.901295 |
| Temperature | 906.2948 | 3 | 302.0983 | 3441626 | 5.34E − 44 | 3.287382 |
| Error | 0.001317 | 15 | 8.78E − 05 | |||
| Total | 906.4753 | 23 | 906.4753 | |||
| Speed of sound | ||||||
| Concentration | 0.300683 | 5 | 0.060137 | 22.98217 | 1.54E − 06 | 2.901295 |
| Temperature | 17451.79 | 3 | 5817.263 | 2223158 | 1.42E − 42 | 3.287382 |
| Error | 0.03925 | 15 | 0.002617 | |||
| Total | 17452.13 | 23 | ||||
| Refractive index | ||||||
| Concentration | 6.9E − 09 | 5 | 1.38E − 09 | 23 | 1.53E − 06 | 2.901295 |
| Temperature | 0.000211 | 3 | 7.03E − 05 | 1172184 | 1.72E − 40 | 3.287382 |
| Error | 9E − 10 | 15 | 6E − 11 | |||
| Total | 0.000211 | 23 | ||||
| Isentropic compressibility | ||||||
| Concentration | 9.34E − 05 | 5 | 1.87E − 05 | 39.25078 | 4.28E − 08 | 2.901295 |
| Temperature | 2.96209 | 3 | 0.987363 | 2074115 | 2.38E − 42 | 3.287382 |
| Error | 7.14E − 06 | 15 | 4.76E − 07 | |||
| Total | 2.96219 | 23 | ||||
| Relaxation strength | ||||||
| Concentration | 2.89E − 07 | 5 | 5.79E − 08 | 23.53823 | 1.32E − 06 | 2.901295 |
| Temperature | 0.017114 | 3 | 0.005705 | 2319295 | 1.03E − 42 | 3.287382 |
| Error | 3.69E − 08 | 15 | 2.46E − 09 | |||
| Total | 0.017115 | 23 | ||||
| Source of variation | SS | df | MS | F | P value | Fcrit |
|---|---|---|---|---|---|---|
| Density | ||||||
| Concentration | 1.5783 | 5 | 0.31566 | 816.3621 | 1.03E − 17 | 2.901295 |
| Temperature | 900.9193 | 3 | 300.3064 | 776654.6 | 3.77E − 39 | 3.287382 |
| Error | 0.0058 | 15 | 0.000387 | |||
| Total | 902.5034 | 23 | ||||
| Speed of sound | ||||||
| Concentration | 0.022483 | 5 | 0.004497 | 2.034691 | 0.131603 | 2.901295 |
| Temperature | 17449.86 | 3 | 5816.62 | 2631955 | 3.99E − 43 | 3.287382 |
| Error | 0.03315 | 15 | 0.00221 | |||
| Total | 17449.92 | 23 | ||||
| Refractive index | ||||||
| Concentration | 3.34E − 06 | 5 | 6.68E − 07 | 381.4987 | 2.99E − 15 | 2.901295 |
| Temperature | 0.000209 | 3 | 6.98E − 05 | 39877.28 | 1.77E − 29 | 3.287382 |
| Error | 2.63E − 08 | 15 | 1.75E − 09 | |||
| Total | 0.000213 | 23 | ||||
| Isentropic compressibility | ||||||
| Concentration | 0.01346 | 5 | 0.002692 | 49.93149 | 8.06E − 09 | 2.901295 |
| Temperature | 295.8396 | 3 | 98.61318 | 1829148 | 6.11E − 42 | 3.287382 |
| Error | 0.000809 | 15 | 5.39E − 05 | |||
| Total | 295.8538 | 23 | ||||
| Relaxation strength | ||||||
| Concentration | 2.25E − 08 | 5 | 4.5E − 09 | 2.034691 | 0.131603 | 2.901295 |
| Temperature | 0.01711 | 3 | 0.005703 | 2580691 | 4.62E − 43 | 3.287382 |
| Error | 3.31E − 08 | 15 | 2.21E − 09 | |||
| Total | 0.01711 | 23 | ||||
The ANOVA results suggest that the model coefficients are significant if the F value is higher than Fcrit value with P value < 0.05. In ANOVA statistical analysis, for a 95% confidence level, the “alpha” significance level was used as 5%. In Table 11, the F values are greater than the corresponding Fcrit values of density, speed of sound, refractive index, isentropic compressibility, and relaxation strength in binary AC + THF mixtures at different compositions and temperatures, while the P values are much smaller than the “alpha” value for each property (P < 0.05) [42–44]. In Table 12, the values of F < Fcrit, and P > 0.05 demonstrates an insignificant effect of concentration on the speed of sound and relaxation strength in binary mixture with xGnP. The ANOVA results show that some parameters have a significant influence on the thermophysical properties in the binary mixtures AC and xGnP with THF solvent.
4. Conclusions
The refractive index, speed of sound, density of exfoliated graphite nanoplatelets, and activated carbon dispersed in THF solvent were measured at different temperatures over the 0 to 100 kg·m−3 concentration domain. The derived physicochemical properties from measured experimental data were calculated, and the absolute average percentage deviation (AAD%) values are comparable for both mixtures. In the xGnP + THF mixture, the AAD% values for the physicochemical parameters such as refractive index, speed of sound, density, isentropic compressibility, and relaxation strength are less than 0.119, 0.002, 0.003, 0.003, and 0.002%. Also, in the AC + THF mixture, the AAD% values for the same physicochemical parameters are less than 0.0002, 0.011, 0.009, 0.001, and 0.003% being well correlated by polynomial relation with three correlation parameters. Also, the effects of different parameters such as the temperature and concentration in the AC/xGnP + THF binary mixtures have been statistically investigated by the ANOVA method, and the obtained results, in generally, demonstrate their higher significance influence on of the studied thermophysical properties. The theoretical methodology presented in this study might explain the ability of the organic solvent THF to disperse xGnP and to stabilize the xGnP + THF mixture in comparison with the AC + THF one. THF, which disperses carbon-based nanostructures, is one of the best solvents, and its behaviour based on fundamental principles and practical methods is being emphasized in this work, too.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This research was performed in the frame of ERA-NET SIIN funded by the European Commission within the 7th Framework Program and supported by the Romanian Executive Agency for Higher Education and RDI Funding (Unitatea Executiva pentru Finantarea Invatamantului Superior, Cercetarii, Dezvoltarii si Inovarii (UEFISCDI)). The authors thank the National Research & Development Institute for Chemistry and Petrochemistry ICECHIM of Romania for the financial support. Also, support of the “Ilie Murgulescu” Institute of Physical Chemistry of Romanian Academy by the project EU (ERDF) and Romanian Government Infrastructure POS-CCE O 2.2.1 INFRANANOCHEM (no. 19/200) is gratefully acknowledged.




