Evaluation of Two Different Solvents for Azolla pinnata Extracts on Chemical Compositions and Larvicidal Activity against Aedes albopictus (Diptera: Culicidae)
Abstract
Limited success for Aedes control program has impelled the necessities for new insecticide search. Hence, alternative plant compounds may be competent to overcome the pesticide resistance problem and to lead a chemical-free environment. Following go-green conceptions, larvicidal effects of the Azolla pinnata extracts using methanol and acetone solvent against Aedes albopictus late 3rd instar larvae were evaluated. The A. pinnata fresh plant from Kuala Krai, Kelantan, Malaysia (5° 31′ N 102° 12′ E) was used for crude extraction with Soxhlet apparatus using methanol and acetone solvents. Next, larvicidal test following WHO guidelines was tested against late 3rd instar to early 4th instar larvae of Ae. albopictus mosquitoes. Meanwhile, the chemical composition of extracts and their structures have been identified using GCMS-QP2010 Ultra (Shimadzu) fitted with Rtx-5MS capillary column (30 m × 0.25 mm inner diameter, ×0.25 μm film thickness; maximum temperature, 370°C), coupled to QP2010 Ultra (Shimadzu) MS. Results of methanol solvent showed the highest larvicidal activity against late 3rd instar to early 4th instar Ae. albopictus larvae with LC50 and LC95 values of 867 ppm and 1293 ppm at 24 hours, respectively, and 647 ppm and 972 ppm at 48 hours, respectively. Meanwhile, acetone solvent compounds were recorded with LC50 and LC95 values of 1072 ppm and 1302 ppm at 24 hours, respectively, and 904 ppm and 1126 ppm at 48 hours, respectively. Finally, the chemical composition of A. pinnata plant extracts has been characterized for 35 active compounds from methanol solvent and 37 active compounds with acetone solvent. In conclusion, A. pinnata plant bioactive molecules are efficient and could be developed as an eco-friendly, “go-green” approach for mosquitoes′ larvicidal control programs. Thus, our study suggests that future research can be conducted on A. pinnata bioactive ingredients against Ae. albopictus larvae in small-scale field trials as botanical insecticide for environmentally friendly approach.
1. Introduction
Mosquitoes cause great health problems in the world because of their predominant role in causing malaria, dengue fever, yellow fever, Zika, and several other disease transmissions [1]. A total of 124 countries were affected by dengue epidemics with approximately 3.61 billion humans at high risk of being infected and yearly 500 million people in denture infection effectively [2]. Meanwhile, Malaysia has recorded 55,744 dengue cases with 131 deaths between January and July 2017 [3].
Vector control programs are requisite part of global strategy for managing mosquito-borne diseases, and commonly used insecticide applications are the most essential components for this sector [4]. In addition to that, the mosquitoes in the juvenile stages can be killed before it emerges into haematophagous adults, by the larvicidal applications [4]. Since larvae are only bound to their habitats, the control operations will be much easier with larvicides. Although current synthetic chemical control agents are common and effective, their constant repetitive applications have resulted in resistant mosquitoes and environmental pollutions. Hence, the limited success of biocontrol programs on Aedes has encouraged the necessity for new insecticide search [5, 6].
Plant products produced positive outcomes as an alternative for synthetic chemical agents for insect biocontrol programs. In this context, the phytochemicals ranging from various classes such as alkaloids, terpenes, steroids, and phenolic constituents were investigated earlier for biocontrol potency, and it has positive results [7–10]. Moreover, the ability to control mosquito larvae and their efficacies of application varies with age, species, part extracted, collection site of plants, and the solvent used [11, 12]. Following this conception, the solvent factor could be compared and examined for its larvicidal efficacies. As an example, Markouk et al. [13] evaluated the differences between ethanolic and aqueous extracts of Calotropis procera flowers and leaves at 1,000 ppm, which did not exhibit any activity and the aqueous phase pose activity (with LC50 = 28 ppm) against Anopheles labranchiae. Rahuman et al. [14] have stated that the highest Culex quinquefasciatus larval mortality was found in stembark for acetone and methanol extracts of Cedrus deodara (LC50 = 141.60 and 95.19 ppm; LC95 = 624.19 and 639.99 ppm). Referring to all these studies, we can conclude that different solvents pose different larvicidal efficacies. Moreover, it contributes to the search of new alternative resources for biocontrol applications. The new search would minimize the environmental pollutions.
Azolla pinnata is commonly known as a mosquito weed that has been used for paddy growth nourishments [15]. It provides nitrogen sources for paddy plants and forms a thick mat layer on the water surface, which may prevent the breeding of mosquitoes [15]. Meanwhile, A. pinnata field study has reported that the breeding of malaria-transmitting mosquitoes was completely suppressed in pools, wells, and ponds [16]. In paddy fields of Tanzania, Africa, cultivation of Anabaena azollae plant has reduced the larvae productivity and larvae densities of An. gambiae, An. funestus, and Cx. quinquefasciatus [17]. Further, suggesting the mosquito productivity is low when the Azolla sp. coverage is high (>80%) in paddy fields [17].
Considering the potentials of A. pinnata applications, the phytochemical properties have been characterized as alkaloids, flavonoids, phenols, saponins, quinones, tannins, carboxylic acids, proteins, xanthoproteins, coumarins, steroids, and carbohydrates [18]. Till to date, no other studies have been done on the specific chemical compounds and their structures for A. pinnata plant. Additionally, no other explicit study has stated the efficacies of either methanol or acetone solvent for A. pinnata plant against Ae. albopictus larvae. Hence, the purpose of this study is to identify the chemical compounds and their structures from acetone and methanol solvent extraction for A. pinnata plant and to test its efficacies compared with both solvents against the late 3rd instar to early 4th instar larvae of Ae. albopictus.
2. Materials and Methods
All experimental procedures were approved by animal ethics: USM/IACUC/2018/111/909 from Vector Control Research Unit, School of Biological Sciences, Universiti Sains Malaysia, Minden, Penang, Malaysia. According to WHO [21] guidelines, the test is only specific to the Aedes late 3rd instar to early 4th instar larvae.
2.1. Plant Materials
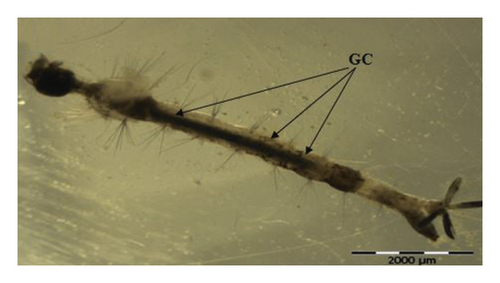
A total of 50 kg fresh A. pinnata (Figure 1) was sampled from Kuala Krai, Kelantan, Malaysia (5°31′N 102°12′E), and its species was identified based on a morphological view of phyllotaxis. Next, A. pinnata fresh samples were prepared using a sun-dried (30°C ± 4°C room temperature) for 2 days. Then, the dried samples were powdered electrically using grinding machine, Faber FBG-460 K, and were sieved as fine powder. The fine powders would increase the surface area and, thus, increase the rate of extractions [19]. Finally, methanol and acetone extractions as a solvent were used in this study using Soxhlet extraction procedures.

2.2. Soxhlet Extraction
Following Zuharah et al. [20], Soxhlet extraction apparatus (Favorit, Malaysia) with a total of 40 g of dried plant powder was placed into the paper thimble. Next, some cotton wool was placed on the top part of extraction flask to prevent the sample from overflow into other apparatus parts. One litre of methanol or acetone solvent was placed in a round-bottom flask with the heating mantle underneath. The solvent was repetitively refluxed and heated along with the fine grinded plant materials. It was done in order to extract the desired plant compound into the round bottom flask. The extraction in the Soxhlet apparatus was performed at boiling point 70°C for about 3 hours until the solvent in the siphon arm becomes clear, which indicates the sample has been extracted entirely. Finally, the extracts were evaporated to dryness in the vacuum evaporator.
2.3. Larvae Rearing
Aedes albopictus eggs were obtained from the Vector Control Research Unit (VCRU) at University Sains Malaysia (USM), Penang, Malaysia. We followed the method used by Zuharah et al. [20] in larvae rearing. Then, the eggs were hatched in seasoned water for 24 hours. The larval food with ratio 2 : 1 : 1 : 1 of cat biscuit, beef liver, yeast, and milk powder was used to trigger the hatching process with 0.2 g. The eggs were maintained at 25°C to 30°C (room temperature), a pH of 6.95 to 7.03, relative humidity of 80 ± 10%, and dissolved oxygen from 5.5 to 6.1 mg/L in the laboratory. After 5 to 6 days, the late 3rd instar larvae were used for the bioassay test.
2.4. Larvicidal Bioassay
Larvicidal bioassays were performed in accordance with the standard WHO [21] larval susceptibility test methods. The bioassay was conducted with 25 of late 3rd instar larvae (homogeneous population consisting of 5 days old 4 to 5 mm in length), in total, four replicates per set for each concentration. Initially, the mosquito larvae were exposed to a wide range of test concentrations and a control to find out the activity range of the extract solution [22]. After determining the mortality of larvae in this wide range of concentrations, a narrow range (seven concentrations ranging between 10 and 1500 ppm, yielding between 0 and 100% mortality in 24 hours of exposure) was selected as test concentrations for larvicidal bioassays [22]. The control solutions were prepared with distilled water of 1 ml of 10% of the respective solvent for each of the experiment [22]. Solvent was added into the control containers to ensure it was identical to the test solutions [22]. Experiments were conducted at room temperature of 28 ± 2°C. Mortality observations of Ae. albopictus larvae were recorded at 24 hours and 48 hours, respectively. Immobilization and total absence from the larvae, even after touch, were the end points of the bioassay [21]. The data were analyzed using a probit analysis, IBM SPSS Statistics 24 [22].
2.5. Photomicrograph View
Aedes aegypti late 3rd instar to early 4th instar larvae were observed under an optical microscope (Leica USA), with a magnification of 40–400x [23].
2.6. GC-MS Analysis
The GC-MS analysis of the crude extracts from Azolla pinnata was performed on a GCMS-QP2010 Ultra (Shimadzu). We followed the method used by previously published research findings of plant extracts [24]. The GCMS-QP2010 Ultra (Shimadzu) system, fitted with Rtx-5MS capillary column (30 m × 0.25 mm inner diameter, ×0.25 μm film thickness; maximum temperature, 370°C), coupled to a QP2010 Ultra (Shimadzu) MS. Ultra-high-purity helium (99.99%) was used as carrier gas at a constant flow rate of 1.0 mL/min. The injection, transfer line, and ion source temperatures were all 280°C. The oven temperature was programmed from 80°C (hold for 2 min) to 280°C at a rate of 3°C/min. The crude samples were diluted with appropriate solvent (1/100, v/v) and filtered. The particle-free diluted crude extracts (1 μL) were taken in a syringe and injected into an injector with a split ratio of 10 :1. All data were obtained by collecting the full-scan mass spectra within the scan range of 40–550 amu. The percentage composition of the crude extract constituents was expressed as the percentage by peak area. The identification and characterization of chemical compounds in various crude extracts were based on the GC retention time. The mass spectra were computer matched (>70%) with those of the standards available in the NIST 08 mass spectrum libraries.
3. Results and Discussion
3.1. Larvicidal Bioassay
The bioassay testing from the acetone solvent of A. pinnata was tested at 500 ppm, 700 ppm, 1000 ppm, 1100 ppm, 1300 ppm, 1500 ppm, and 1600 ppm; meanwhile, methanol solvent was tested at 300 ppm, 500 ppm, 700 ppm, 1000 ppm, 1300 ppm, 1500 ppm, and 1700 ppm. The entire larvae bioassay test with A. pinnata extracts showed a significant increase in the mortality rate with the increase of concentration. Among the plant extracts tested, highest larvicidal activity was observed in the methanol solvent compounds against the late 3rd instar Ae. albopictus larvae with LC50 and LC95 values of 867 and 1293 ppm at 24 hours, 647 and 972 ppm at 48 hours, respectively (Table 1). Meanwhile, the acetone solvent compounds were recorded with LC50 and LC95 values of 1072 and 1302 ppm at 24 hours, 904 ppm and 1126 ppm at 48 hours, respectively (Table 1). No significant mortality for control assays.
| Extraction solvent | Na | LC50 (ppm) (95% LCL–UCL) | LC95 (ppm) (95% LCL–UCL) | Time (h) |
|---|---|---|---|---|
| Acetone | 700 |
|
|
24 |
| Acetone | 700 |
|
|
48 |
| Methanol | 700 |
|
|
24 |
| Methanol | 700 |
|
|
48 |
- aTotal number of larvae used in this study; n = 25 with 4 replicates per concentration; LC50: lethal concentration with 50% mortality; LC95: lethal concentration with 95% mortality; LCL: lower confidence limits; UCL: upper confidence limits.
The biological activity of plant-based insecticides against mosquito larvae extensively varies according to the solvent used for its extraction [25]. The findings of current study are inconformity with the past findings whereby methanol solvent for plant extract resulted in higher larvicidal activity against Ae. albopictus larvae compared with acetone extract. The reason for choosing methanol and acetone in this study was due to the similar polarity index of 5.1, but the viscosity value varies for acetone and methanol between 0.32 and 0.6, respectively. According to Khayyat and Roselin [24], lower viscosity level on solvents will provide higher coefficient diffusion and yield with active compounds from plants. Additionally, the efficacy of the extracted plant compounds increases with decreasing polarities [26]. According to Ghosh et al. [27], the application of moderate polarity of solvents for plant extraction would produce excellent results on larvicidal bioassays. Hence, following all these conceptions, our current study has selected acetone and methanol solvent as the best test solvent for larvicidal bioassays. Till to date, this would be the first study with A. pinnata plant for chemical compound identifications, characterizations, and larvicidal bioassays.
3.2. Photomicrograph View
Photomicrograph view of Ae. albopictus larvae shown in Figure 2(b) indicates the presence of A. pinnata plant extracts in the midgut content (dark colour) in comparison with the control test (Figure 2(a)). The presence of the midgut content (dark colour) of extracts was indicative of the ingestion mechanisms by larvae towards A. pinnata plant extracts. Similarly, Procopio et al. [23] have shown photomicrography for the application of Moringa oleifera lectin on the gut content of Ae. aegypti larvae.

3.3. GC-MS Analysis and Identification of Compounds
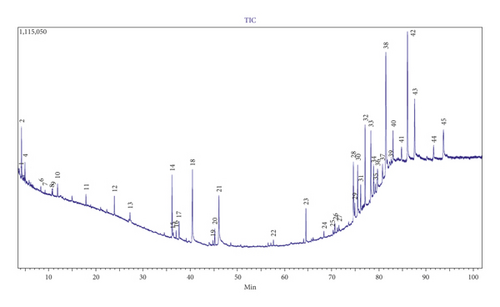
The GC-MS analysis of acetone solvent extracts of Azolla pinnata showed 45 peaks, which indicated the presence of 45 phytochemical compounds (Figure 3). In comparison (more than 70% similarity) of the mass spectra on the constituents with NIST 08 library, only 37 compounds were characterized and identified (Table 2). The identified chemical compounds in A. pinnata acetone extracts were cis-11,12-epoxytetradecen-1-ol (13.359%), glycerin (8.633%), stigmastane-3,6-dione, (5.alpha.) (7.585%), n-hexadecanoic acid (6.461%), stigmast-4-en-3-one(5.610%), 2,6,10,14,18,22-tetracosahexaene,2,6,10,15,19,23-h (5.378%), 1,37-octatriacontadiene (4.640%), 3,7,11,15-tetramethyl-2-hexadecen-1-ol (4.145%), oleic acid (3.998%), phenol, 2,4-bis(1,1-dimethylethyl) (3.925%), tetracontane-1,40-diol (3.607%), 9,19-cyclolanost-24-en-3-ol (3.beta.) (3.654%), benzofuran, 2,3-dihydro (3.268%), diethyl phthalate (2.831%), 1-decene, 2,4-dimethyl (2.01%), heptacosyl heptafluorobutyrate (1.928%), gamma-sitosterol (1.619%), phytol (1.571%), tetracontane-1,40-diol (1.514%), 1,2-O-isopropylidene-beta-l-idofuranurono-6,3 (1.399%), vitamin E (1.171%), octatriacontyl trifluoroacetate (1.155%), 1,2-benzenedicarboxylic acid, diisooctyl ester (1.133%), oxirane, hexadecyl (1.042%), 9,19-cyclo-27-norlanostan-25-one,3-(acetyloxy)-24(0.939%), 3,7,11,15-tetramethyl-2-hexadecen-1-ol (0.9%), tetracosyl pentafluoropropionate (0.701%), 4-heptanone, 2,3:5,6-diepoxy-2,6-dimethyl-(0.613%), 2H-pyran-2-one, tetrahydro-6-tridecyl (0.691%), 3-hexen-2-one (0.519%), 2,2,6,6-tetramethyl-4-piperidone (0.439%), butanamide 3-(2-methylpropinonylhydrazono)-N (0.414%), 2-hexadecene 3,7,11,15-tetramethyl (0.374%), 1-hentetracontanol (0.289%), 1,1′-bicyclooctyl (0.231%), 9-octadecenoic acid, methyl ester (E) (0.221%), cyclopentane, and 1,2-dimethyl-3-(1-methylethyl) (0.2%). The attached supplementary pdf file contains the NIST 08 library search for chemical compound structures and details.
| Peak no. | Retention time | Area | Area % | Compound name | Activity |
|---|---|---|---|---|---|
| 2 | 4.235 | 246212 | 8.633 | Glycerin | Pesticides and herbicidal |
| 3 | 4.581 | 6587 | 0.231 | 1,1′-Bicyclooctyl | Herbicidal and insecticidal |
| 4 | 4.970 | 57338 | 2.010 | 1-Decene, 2,4-dimethyl | Pesticides |
| 5 | 5.097 | 5753 | 0.200 | Cyclopentane, 1,2-dimethyl-3-(1-methylethyl) | Pesticides |
| 6 | 8.326 | 12614 | 0.439 | 2,2,6,6-Tetramethyl-4-piperidone | Antimicrobial |
| 7 | 9.235 | 14795 | 0.519 | 3-Hexen-2-one | Natural pesticides and insecticidal |
| 8 | 10.713 | 11812 | 0.414 | Butanamide, 3-(2-methylpropinonylhydrazono)-N | Insecticidal and herbicidal |
| 9 | 10.831 | 17477 | 0.613 | 4-Heptanone, 2,3:5,6-diepoxy-2,6-dimethyl | Pesticides |
| 10 | 11.903 | 93194 | 3.268 | Benzofuran, 2,3-dihydro | Insecticidal |
| 11 | 17.921 | 39904 | 1.399 | 1,2-O-Isopropylidene-beta-l-idofuranurono-6,3 | Unknown |
| 12 | 23.947 | 111946 | 3.925 | Phenol, 2,4-bis(1,1-dimethylethyl) | Pesticides |
| 13 | 27.262 | 80732 | 2.831 | Diethyl phthalate | Insecticidal |
| 14 | 36.194 | 118203 | 4.145 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | Antimicrobial |
| 15 | 36.442 | 10670 | 0.374 | 2-Hexadecene, 3,7,11,15-tetramethyl- | Insecticidal, nematicide, and pesticide |
| 18 | 40.526 | 184262 | 6.461 | n-Hexadecanoic acid | Insecticidal, nematicide, and pesticide |
| 19 | 44.871 | 6310 | 0.221 | 9-Octadecenoic acid, methyl ester, (E) | Antimicrobial |
| 20 | 45.258 | 44798 | 1.571 | Phytol | Insecticidal, fungicide, and miticide |
| 21 | 46.138 | 114014 | 3.998 | Oleic acid | Pesticides and insecticidal |
| 22 | 57.704 | 32308 | 1.133 | 1,2-Benzenedicarboxylic acid, diisooctyl ester | Antimicrobial |
| 23 | 64.602 | 153370 | 5.378 | 2,6,10,14,18,22-Tetracosahexaene, 2,6,10,15,19,23-h | Antimicrobial |
| 24 | 68.430 | 19721 | 0.691 | 2H-Pyran-2-one, tetrahydro-6-tridecyl | Antimicrobial |
| 25 | 70.395 | 8230 | 0.289 | 1-Hentetracontanol | Antimicrobial |
| 26 | 70.777 | 20002 | 0.701 | Tetracosyl pentafluoropropionate | Insecticidal |
| 27 | 71.602 | 33387 | 1.171 | Vitamin E | Antimicrobial and antioxidant |
| 28 | 74.685 | 132335 | 4.640 | 1,37-Octatriacontadiene | Antimicrobial |
| 29 | 74.986 | 32935 | 1.155 | Octatriacontyl trifluoroacetate | Insecticidal |
| 30 | 75.599 | 46184 | 1.619 | Gamma-sitosterol | Antibacterial and antioxidant |
| 31 | 76.218 | 26772 | 0.939 | 9,19-Cyclo-27-norlanostan-25-one, 3-(acetyloxy)-24 | Pesticides |
| 32 | 77.157 | 104216 | 3.654 | 9,19-Cyclolanost-24-en-3-ol, (3.beta.) | Pesticides |
| 33 | 78.383 | 159996 | 5.610 | Stigmast-4-en-3-one | Antimicrobial and antibacterial |
| 34 | 78.956 | 54988 | 1.928 | Heptacosyl heptafluorobutyrate | Pesticides |
| 37 | 80.873 | 29709 | 1.042 | Oxirane, hexadecyl | Pesticides |
| 38 | 81.578 | 216330 | 7.585 | Stigmastane-3,6-dione, (5.alpha.) | Antimicrobial and anti-inflammatory |
| 40 | 83.088 | 43183 | 1.514 | Tetracontane-1,40-diol | Antibacterial |
| 41 | 84.906 | 25657 | 0.900 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | Insecticidal |
| 42 | 86.157 | 380998 | 13.359 | cis-11,12-Epoxytetradecen-1-ol | Biopesticides and insecticidal |
| 43 | 87.677 | 102881 | 3.607 | 43-Tetracontane-1,40-diol | Antimicrobial and antibacterial |
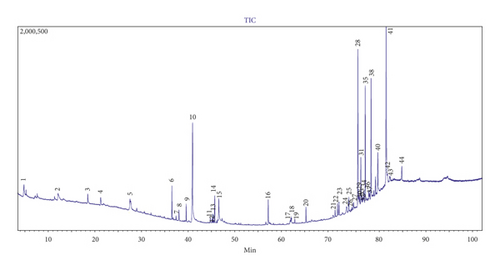
The GC-MS analysis of methanol solvent extracts using maceration extraction of A. pinnata showed 44 peaks, which indicated the presence of 44 phytochemical compounds (Figure 4). In the comparison (more than 70% similarity) of the mass spectra on the constituents with the NIST 08 library, only 35 compounds were characterized and identified (Table 3). The identified chemical compounds in methanol solvent extracts were stigmastane-3,6-dione, (5.alpha.) (11.933%), n-hexadecanoic acid (11.909%), stigmast-4-en-3-one (10.892%), glycerin (9.375%), DL-proline, 5-oxo-, methyl ester (5.992%), 9,19-cyclolanost-24-en-3-ol (3.beta.) (5.479%), gamma-sitosterol (5.342%), benzoic acid 2-4-(4-hydroxy-4-methylpentyl) (3.950%), benzaldehyde, 2-hydroxy-4-methyl (3.130%), phytol (3.222%), alpha-tocopherol-beta-D-mannoside (2.904%), hexadecanoic acid, methyl ester (2.762%), 2,6,10,14,18,22-tetracosahexaene, 2,6,10,15,19,23-h (2.680%), neophytadiene (2.015%), 9,19-cyclo-27-norlanostan-25-one, oleic acid (1.895%), 3-(acetyloxy)-24 (1.868%), hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)eth (1.445%), 3,7,11,15-tetramethyl-2-hexadecen-1-ol (0.877%), vitamin E (0.835%), cholest-4-en-3-one (0.801%), Lup-20(29)-en-3-ol, acetate (3.beta.) (0.732%), ergost-5-en-3-ol (3.beta.) (0.642%), 17-pentatriacontene (0.560%), tetracosanoate methyl (0.519%), 7-tetradecenal, (Z)- (0.500%), oxirane, 2-decyl-3-(5-methylhexyl), cis (0.496%), 9-octadecenoic acid (Z)-methyl ester (0.487%), cholestan-3-one (0.472%), stigmastanol (0.428%), cholesterol (0.337%), ergosterol (0.328%), n-nonadecanol-1 (0.284%), 9-octadecenoic acid, 1,2,3-propanetriyl ester (0.261%), 9,12-octadecadienoic acid (Z,Z)-, methyl ester (0.230%), and stigmasterol (0.198%). The attached supplementary pdf file contains the NIST 08 library search for chemical compound structures and details.
| Peak no. | Retention time | Area | Area % | Compound name | Activity |
|---|---|---|---|---|---|
| 1 | 4.827 | 504294 | 9.375 | Glycerin | Pesticides and herbicides |
| 3 | 18.362 | 322274 | 5.992 | DL-Proline, 5-oxo-, methyl ester | Antibacterial and antifungal |
| 4 | 21.099 | 168354 | 3.130 | Benzaldehyde, 2-hydroxy-4-methyl- | Pesticides |
| 5 | 27.310 | 212453 | 3.950 | Benzoic acid, 2-4-(4-hydroxy-4-methylpentyl) | Pesticides |
| 6 | 36.206 | 108402 | 2.015 | Neophytadiene | Larvicidal, insecticidal, and antimicrobial |
| 8 | 37.695 | 47152 | 0.877 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | Insecticidal |
| 9 | 39.234 | 148583 | 2.762 | Hexadecanoic acid, methyl ester | Insecticidal, nematicide, and pesticide |
| 10 | 40.590 | 640591 | 11.909 | Hexadecanoic acid <n-> | Insecticidal, nematicide, and pesticide |
| 11 | 44.331 | 15262 | 0.284 | n-Nonadecanol-1 | Pesticides |
| 12 | 44.703 | 12360 | 0.230 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | Antifeedant and insecticidal |
| 13 | 44.903 | 26198 | 0.487 | 9-Octadecenoic acid (Z)-, methyl ester | Antifeedant and insecticidal |
| 14 | 45.280 | 173301 | 3.222 | Phytol | Insecticidal, fungicide, and miticide |
| 15 | 46.132 | 101905 | 1.895 | Oleic acid | Pesticides and insecticidal |
| 16 | 56.629 | 77698 | 1.445 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)eth | Antimicrobial |
| 17 | 61.312 | 14017 | 0.261 | 9-Octadecenoic acid, 1,2,3-propanetriyl ester | Antimicrobial |
| 18 | 61.490 | 26880 | 0.500 | 7-Tetradecenal, (Z) | Pesticides |
| 19 | 62.247 | 27895 | 0.519 | Tetracosanoate methyl | Pesticides |
| 20 | 64.625 | 144155 | 2.680 | 2,6,10,14,18,22-Tetracosahexaene, 2,6,10,15,19,23-h | Antimicrobial |
| 21 | 70.800 | 30142 | 0.560 | 17-Pentatriacontene | Pesticides |
| 22 | 71.325 | 18144 | 0.337 | Cholesterol | Unknown |
| 23 | 71.624 | 156179 | 2.904 | Alpha-tocopherol, beta-D-mannoside | Antimicrobial |
| 24 | 73.259 | 17620 | 0.328 | Ergosterol | Unknown |
| 25 | 73.704 | 34527 | 0.642 | Ergost-5-en-3-ol, (3.beta.) | Antimicrobial |
| 26 | 74.399 | 10666 | 0.198 | Stigmasterol | Antimicrobial |
| 27 | 74.707 | 26693 | 0.496 | Oxirane, 2-decyl-3-(5-methylhexyl)-, cis-(.+/-.) | Pesticides |
| 28 | 75.627 | 287315 | 5.342 | Gamma-sitosterol | Antibacterial and antioxidant |
| 29 | 75.820 | 22998 | 0.428 | Stigmastanol | Antimicrobial |
| 31 | 76.249 | 100498 | 1.868 | 9,19-Cyclo-27-norlanostan-25-one, 3-(acetyloxy)-24 | Pesticides |
| 32 | 76.560 | 43084 | 0.801 | Cholest-4-en-3-one | Insecticidal |
| 33 | 76.725 | 25375 | 0.472 | Cholestan-3-one | Insecticidal |
| 35 | 77.196 | 294724 | 5.479 | 9,19-Cyclolanost-24-en-3-ol, (3.beta.) | Pesticides |
| 36 | 77.942 | 44894 | 0.835 | Vitamin E | Antimicrobial and antioxidant |
| 38 | 78.423 | 107567 | 10.892 | Stigmast-4-en-3-one | Antimicrobial and anti-inflammatory |
| 39 | 79.365 | 7507 | 0.732 | Lup-20(29)-en-3-ol, acetate, (3.beta.) | Insecticidal |
| 41 | 81.635 | 641850 | 11.933 | Stigmastane-3,6-dione, (5.alpha.) | Antimicrobial and anti-inflammatory |
Furthermore, as discussed previously, the conception of lower viscosity values gave more yields of active compounds using acetone solvent compared to methanol, which was evidentially proven from this study. Azolla pinnata plant yields 37 chemical compounds from acetone solvent extract compared to 35 compounds from methanol solvent. As stated in Table 1, acetone solvent extracts produced highest chemical composition of cis-11,12-epoxytetradecen-1-ol (13.359%), glycerin (8.633%), stigmastane-3,6-dione (5.alpha.) (7.585%), n-hexadecanoic acid (6.461%), stigmast-4-en-3-one (5.610%), 2,6,10,14,18,22 tetracosahexaene, 2,6,10,15,19,23-h (5.378%), which were extensively used for insecticidal, pesticidal, and antimicrobial properties [28–30]. Besides that, methanol solvent (Table 2) extracts have yielded highest chemical compositions of stigmastane-3,6-dione (5.alpha.) (11.933%), hexadecanoic acid (11.909%), stigmast-4-en-3-one (10.892%), glycerin (9.375%), DL-proline, 5-oxo-, methyl ester (5.992%), and 9,19-cyclolanost-24-en-3-ol (3.beta.) (5.479%), which were used for antimicrobial, anti-inflammatory, insecticidal, nematicidal, and pesticidal applications [31–36].
According to the chemical composition results in our study, it showed that less number of active compounds have been extracted for methanol solvent on A. pinnata plant compared to acetone solvent. However, the efficacy of extracts for larvicidal was superior for methanol solvent compared to acetone solvent. These can be further discussed as the total composition (34.734%) of compounds from stigmastane-3,6-dione, (5.alpha.), hexadecanoic acid, and stigmast-4-en-3-one has the ability of antimicrobial properties when compared to major components of acetone solvent extracts. According to Minard et al. [37], there was an interaction from all the midgut bacterial diversity for Ae. albopictus mosquitoes in their life cycle. Meanwhile, a recent study has stated that Ae. aegypti larvae require live gut bacteria for its development and that they rely on multiple bacterial diversity [38]. According to previous microbial findings, our recent results on methanol-extracted chemical compounds may be more active in its antimicrobial properties on its gut microbial interference within Ae. albopictus larvae compared to acetone extracts.
4. Conclusion
In conclusion, the findings of this study have shown the effectiveness of A. pinnata extracts by acetone and methanol solvents against one major mosquito species in the late 3rd instar to early 4th instar larvae stages. Moreover, our findings showed that the A. pinnata bioactive molecules can be effective as larvicides for Ae. albopictus mosquito vector control programs. Finally, this study suggests that future research work can be conducted on the field evaluation of its larvicidal effectiveness against Ae. albopictus species for environmentally safer botanical insecticide inventions.
Conflicts of Interest
The authors have declared that no conflicts of interest exist.
Acknowledgments
This work was supported by Ministry of Higher Education, Malaysia, through the Fundamental Research Grant Scheme (R/FRGS/A08.00/00425A/002/2017/000440).
Open Research
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.




