Prognostic Significance of Pretreatment Apolipoprotein A-I as a Noninvasive Biomarker in Cancer Survivors: A Meta-Analysis
Abstract
Background. Numerous studies have reported the prognostic significance of serum apolipoprotein A-I (ApoA-I) in various cancers, but the results have been inconsistent. The current meta-analysis was performed to investigate the association between ApoA-I level and prognosis in human malignancies. Methods. A literature search was performed using the electronic platforms of the PubMed, Cochrane Library, Web of Science, Embase, Wanfang, and China National Knowledge Infrastructure (CNKI) databases to obtain eligible articles published up to May 20, 2018. Pooled hazard ratios (HRs) with 95% confidence intervals (95% CIs) were calculated to assess the prognostic values of the ApoA-I level in cancers using STATA 12.0 software. Results. A total of 14 studies involving 9295 patients were included. The results indicated that low ApoA-I level was significantly associated with poor overall survival (OS) (HR = 0.52, 95% CI: 0.44–0.61). Significant relationships between the ApoA-I level and OS were specifically detected in nasopharyngeal carcinoma (NPC, HR = 0.63, 95% CI: 0.54–0.73), colorectal cancer (CRC, HR = 0.48, 95% CI: 0.19–0.76), and hepatocellular carcinoma (HCC, HR = 0.46, 95% CI: 0.27–0.65). The subgroup analyses for OS also further confirmed the prognostic significance of the ApoA-I level in cancers. Moreover, lower Apo A-I was associated with unfavorable cancer-specific survival (CSS, HR: 0.47, 95% CI: 0.19–0.76) in cancers, and low ApoA-I level was clearly associated with inferior total time to recurrence (TTR, HR: 0.43, 95% CI: 0.29–0.58) in HCC, poorer locoregional recurrence-free survival (LRFS) and distant metastasis-free survival (DMFS) (HR: 0.58, 95% CI: 0.42–0.74 for LRFS; HR: 0.65, 95% CI: 0.41–0.89 for DMFS) in NPC, and shorter disease-free survival (DFS, HR: 0.64, 95% CI: 0.43–0.84) in cancers. Conclusions. Low ApoA-I level might be an unfavorable prognostic factor in multiple malignancies, and serum ApoA-I could serve as a noninvasive marker to predict cancer prognosis.
1. Introduction
Cancer is a major public health problem and causes huge burdens on developed and developing countries. It is the third leading cause of death in China and ranks second in the USA [1, 2]. Although great progress has been achieved in the diagnosis and treatment of cancer, effective treatment for many cancer patients is still lacking. In view of the current situation, there has been great interest in prognostic biomarkers because of their usefulness in predicting clinical outcomes and guiding therapy [3–8].
Previous studies have investigated the correlations between serum lipid levels and human cancers [9, 10]. Serum cholesterol, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were shown to be associated with the prognosis in multiple human cancers, such as colorectal cancer, liver cancer, and breast cancer [11–13]. As the major protein component of plasma HDL, apolipoprotein A-I (ApoA-I) synthesized in the liver and small intestine has been reported to be associated with clinical survival in multiple human cancers, including gastric cancer, nasopharyngeal carcinoma, and breast cancer [14–17]. However, the results on the clinical value of serum ApoA-I as a useful indicator have been debatable and inconsistent, and given the limited sample size and varied methodologies of individual studies, we therefore conducted this meta-analysis to provide a systematic evaluation of the significance of serum ApoA-I as a promising prognostic marker based on all related published data.
2. Materials and Methods
2.1. Search Strategy and Study Selection
PubMed, Cochrane Library, Web of Science, Embase, Wanfang and China National Knowledge Infrastructure (CNKI) were comprehensively searched by the end of May 20, 2018. The search terms used were “apolipoprotein A-I,” “ApoA-I,” “apolipoprotein” combining with “tumor,” “neoplasms,” “malignancy,” “carcinoma” OR “cancer.” The search language was limited to English and Chinese. The references of the retrieved articles were also checked to obtain relevant studies.
Published studies that met the following criteria were included: (1) the study investigated the survival outcomes of human malignancies with low ApoA-I level versus high ApoA-I levels, (2) a cutoff value to identify pretreatment low/high ApoA-I level was given, (3) complete information for assessment of hazard ratios (HRs) and the corresponding 95% CIs for cancer prognosis were included, and (4) all patients were divided into two groups based on serum ApoA-I level.
A study was excluded if it was a nonoriginal study (review, comments, editorial, or abstract) or no useful data was available.
2.2. Data Extraction and Quality Assessment
The data from all studies were independently extracted by two reviewers according to an extraction template. The general information included first author, country, year of publication, included time, total sample size, survival type, follow-up period, cutoff value, cutoff selection, treatment methods, and disease stage. The HRs along with 95% CI were directly obtained from published articles, and the multivariate analysis mode was preferred. In this meta-analysis, an HR < 1 indicated a worse prognosis for subjects with low ApoA-I. If a study considered cases with low ApoA-I as a reference, then the data were converted to HR estimations that considered cases with high ApoA-I as a reference group to reflect the impact of low ApoA-I levels on cancer patients. The Newcastle–Ottawa scale (NOS) was utilized to assess the quality of the included studies, and a study with a NOS score ≥ 6 was considered to be of high quality.
2.3. Statistical Analysis
All pooled analyses were conducted using STATA 12.0 software (Stata, College Station, TX, USA). For the prognostic index, e.g., the overall survival (OS), HR and corresponding 95% CI were used as the summary measure. The chi-square test and I2 statistic were used to evaluate the heterogeneity. I2 > 50% or p < 0.1 determined significant heterogeneity, and then the random-effect model was applied. Visual funnel diagrams and Begg’s test and Egger’s test were utilized to assess the potential publication bias. Sensibility analysis was performed to evaluate the robustness of the combined results. All p values < 0.05 were regarded as statistically significant.
3. Results
3.1. Characteristics of the Studies
The detailed steps involved in the literature search are shown in Figure 1. After reading the full text and further examination according to the selection criteria, ultimately, 13 publications (including 14 studies) [14–26] containing 9295 patients were included in this systematic review and meta-analysis.
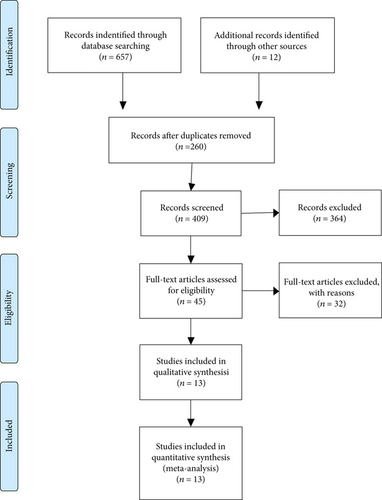
All were retrospective studies, and a total of 9295 cancer patients were included from 13 studies that were carried out in China and one study from Finland. The sample size varied from 144 to 1927. With respect to prognostic outcomes, 13 studies reported OS, 1 covered disease-specific survival (DSS), 1 covered cancer-specific survival (CSS), 3 covered disease-free survival (DFS), 1 reported progression-free survival (PFS), and 2 reported total time to recurrence (TTR), locoregional recurrence-free survival (LRFS), and distant metastasis-free survival (DMFS). For calculating the prognostic values of serum ApoA-I, CSS was integrated into the meta-analysis of DSS, and PFS was integrated into the meta-analysis of DFS. Furthermore, as for cancer type, 10 different types of cancers were investigated, including non-small cell lung cancer (NSCLC); bladder cancer; gastric cancer (GC); nasopharyngeal carcinoma (NPC); breast cancer (BC); colorectal cancer (CRC); hepatocellular carcinoma (HCC); extranodal natural killer (NK)/T-cell lymphoma, nasal type (ENKTL); esophageal squamous cell carcinoma (ESCC); and renal cell carcinoma (RCC). The NOS evaluating the quality of included studies varied from 6 to 8. The main characteristics of the included studies are presented in Table 1.
| Study | Year | Cancer type | Country | Included time | Total sample | Follow-up | Cutoff value | Cutoff selection | Treatment methods | Stage | Survival type | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen S | 2018 | NSCLC | China | 2008–2010 | 141 | ≥5 years | 1.17 g/l | X-tile software | Mixed | Mixed | OS | 6 |
| Shang Z | 2018 | Bladder cancer | China | 2004–2011 | 470 | ≥5 years | 1.19 g/l | ROC analysis | With surgery | No metastasis | OS, CSS | 7 |
| Ma M | 2018 | GC | China | 2005–2010 | 1201 | ≥5 years | 1.4 g/l | X-tile software | With surgery | Mixed | OS | 8 |
| Chang H | 2018 | NPC | China | 2010–2011 | 1927 | ≥5 years | 1.125 g/l | ROC analysis | No surgery | No metastasis | OS, DFS, LRFS, DMFS | 8 |
| Li X | 2017 | BC | China | 2008–2011 | 1044 | ≥5 years | 1.56 g/l | ROC analysis | With surgery | No metastasis | OS, DFS | 7 |
| Quan Q | 2017 | CRC | China | 2005–2013 | 721 | ≥5 years | 1.105 g/l | ROC analysis | No surgery | Metastasis | OS | 7 |
| Sirnio P | 2017 | CRC | Finland | 2006–2010 | 144 | ≥5 years | 1.235 g/l | ROC analysis | With surgery | Mixed | OS | 6 |
| Ma XL-1 | 2016 | HCC | China | 2012–2015 | 224 | <5 years | 1.04 g/l | X-tile software | With surgery | Mixed | OS, TTR | 7 |
| Ma XL-2 | 2016 | HCC | China | 2012-2013 | 219 | <5 years | 1.04 g/l | X-tile software | With surgery | Mixed | OS, TTR | 6 |
| Quan Q | 2016 | ENKTL | China | 2002–2014 | 236 | ≥5 years | 0.95 g/l | ROC analysis | No surgery | Mixed | OS, PFS | 7 |
| Wang XP | 2016 | ESCC | China | 2007–2009 | 210 | ≥5 years | 1.21 g/l | ROC analysis | With surgery | Mixed | OS | 6 |
| Guo S | 2016 | RCC | China | 2000–2012 | 755 | ≥5 years | 1.04 g/l | ROC analysis | With surgery | Mixed | OS, DFS | 7 |
| Luo XL | 2015 | NPC | China | 2004–2007 | 1196 | ≥5 years | 1.025 g/l | ROC analysis | No surgery | No metastasis | DSS, LRFS, DMFS | 8 |
| Jiang R | 2014 | NPC | China | 2003–2009 | 807 | ≥5 years | 1.065 g/l | ROC analysis | No surgery | Metastasis | OS | 7 |
- NSCLC: non-small cell lung cancer; GC: gastric cancer; NPC: nasopharyngeal carcinoma; BC: breast cancer; CRC: colorectal cancer; HCC: hepatocellular carcinoma; ENKTL: extranodal natural killer (NK)/T-cell lymphoma, nasal type; ESCC: esophageal squamous cell carcinoma; RCC: renal cell cancer; OS: overall survival; CSS: cancer-specific survival; TTR: time to recurrence; DFS: disease-free survival; PFS: progression-free survival; DSS: disease-specific survival; DMFS: distant metastasis-free survival; LRFS: locoregional recurrence-free survival; ROC: the receiver operating characteristic curve.
3.2. Apolipoprotein A-I Level and OS
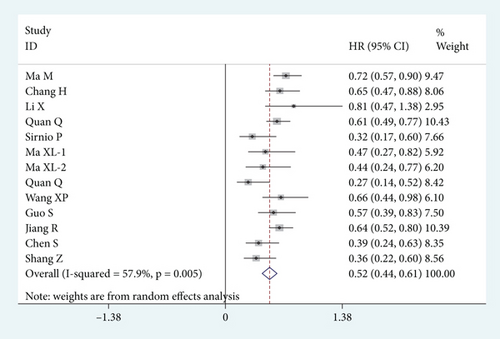
A total of 13 studies with 8099 subjects was involved in the meta-analysis of OS. Because of the significant heterogeneity among studies (I2 = 57.9%, p = 0.005), the random-effect model was applied. As shown in Figure 2, the results indicated that the low ApoA-I group was significantly associated with shortened OS time (HR = 0.52, 95% CI: 0.44–0.61, p < 0.001).
3.3. Subgroup Analysis
The subgroup analysis for OS was conducted to further explore the correlation between ApoA-I level and various cancers. The results showed that the ApoA-I level could serve as a prognostic biomarker especially in NPC (HR = 0.63, 95% CI: 0.54–0.73, p < 0.001), CRC (HR = 0.48, 95% CI: 0.19–0.76, p < 0.001), and HCC (HR = 0.46, 95% CI: 0.27–0.65, p < 0.001). As shown in Table 2, the subgroup analyses were implemented based on cancer type, cutoff value selection, stages, follow-up time, analysis models, and treatments. The calculated pooled HR values were significantly less than 1.0 in those subgroup analyses, which all suggested that low ApoA-I level was a significant unfavorable prognostic factor for OS.
| Stratified analysis | No. of studies | Pooled HR (95% CI) | p value | Heterogeneity | |
|---|---|---|---|---|---|
| I2 (%) | Ph | ||||
| (1) Cancer type | |||||
| Gastrointestinal cancer | 6 | 0.55 (0.43–0.68) | <0.001 | 52.1 | 0.064 |
| Non-gastrointestinal cancer | 7 | 0.50 (0.37–0.63) | <0.001 | 63.9 | 0.011 |
| (2) Cutoff value selection | |||||
| ROC analysis | 9 | 0.52 (0.41–0.63) | <0.001 | 62.1 | 0.007 |
| X-tile software | 4 | 0.52 (0.35–0.69) | <0.001 | 59.4 | 0.061 |
| (3) Stage | |||||
| No metastasis | 3 | 0.56 (0.31–0.81) | <0.001 | 64.7 | 0.059 |
| Metastasis | 2 | 0.63 (0.53–0.72) | <0.001 | 0.0 | 0.773 |
| Mixed | 8 | 0.48 (0.36–0.60) | <0.001 | 60.9 | 0.012 |
| (4) Follow-up time | |||||
| <5 years | 2 | 0.46 (0.27–0.65) | <0.001 | 0.0 | 0.878 |
| ≥5 years | 11 | 0.53 (0.43–0.63) | <0.001 | 64.1 | 0.002 |
| (5) Analysis modes | |||||
| Univariate analysis | 2 | 0.73 (0.57–0.88) | <0.001 | 0.0 | 0.707 |
| Multivariate analysis | 11 | 0.49 (0.41–0.58) | <0.001 | 53.6 | 0.018 |
| (6) Treatments | |||||
| Mixed | 1 | 0.39 (0.24–0.63) | <0.001 | — | — |
| No surgery | 4 | 0.55 (0.39–0.71) | <0.001 | 73.1 | 0.011 |
| With surgery | 8 | 0.53 (0.40–0.65) | <0.001 | 52.5 | 0.040 |
3.4. Apolipoprotein A-I Level and Secondary Outcomes
We also investigated the relationships between and serum ApoA-I level and secondary outcomes in cancer patients. The detailed results are provided in Table 3, and we found that lower Apo A-I was associated with poor CSS (HR: 0.47, 95% CI: 0.19–0.76, p < 0.01) in cancers, and low ApoA-I level was a prognostic factor for inferior TTR (HR: 0.43, 95% CI: 0.29–0.58, p < 0.01) in HCC, LRFS (HR: 0.58, 95% CI: 0.42–0.74, p < 0.01) and DMFS (HR: 0.65, 95% CI: 0.41–0.89, p < 0.01) in NPC, and DFS (HR: 0.64, 95% CI: 0.43–0.84, p < 0.01) in various cancers.
| Secondary outcomes | No. of studies | No. of cases | Pooled HR (95% CI) | p value | Heterogeneity | |
|---|---|---|---|---|---|---|
| I2 (%) | Model | |||||
| CSS | 2 | 1666 | 0.47 (0.19–0.76) | <0.001 | 77.5 | Random |
| TTR | 2 | 443 | 0.43 (0.29–0.58) | <0.001 | 0.0 | Fixed |
| LRFS | 2 | 3123 | 0.58 (0.42–0.74) | <0.001 | 0.0 | Fixed |
| DMFS | 2 | 3123 | 0.65 (0.41–0.89) | <0.001 | 73.0 | Random |
| DFS | 4 | 3962 | 0.64 (0.43–0.84) | <0.001 | 65.9 | Random |
3.5. Publication Bias
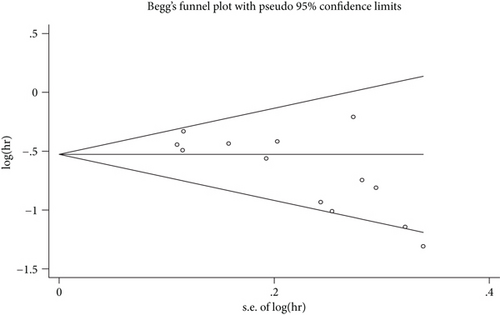
Begg’s plot is shown in Figure 3, and Begg’s test and Egger’s test showed that there was potential publication bias in OS (PrBegg’s test > |z| = 0.006 (continuity corrected); PrEgger’s test > |z| = 0.005). Then, the “trim and fill method” was also adopted, and after correction, the adjusted pooled HR was 0.555 (95% CI: 0.480–0.642, p < 0.001), which indicated that no significant publication bias existed.
3.6. Sensitivity Analysis
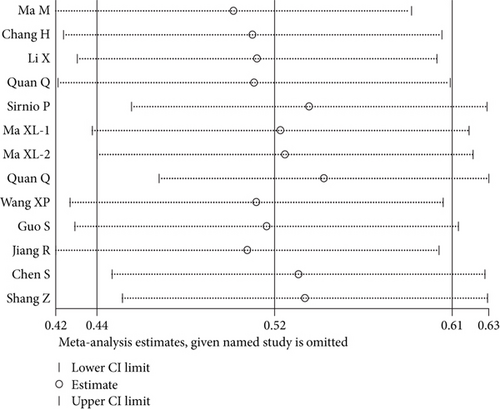
The results indicated that any individual study had little effect on the overall results (Figure 4), which suggested that our results were relatively stable and credible.
4. Discussion
ApoA-I belongs to the apolipoprotein A1/A4/E family, and it plays pivotal roles in both lipid metabolism (such as liver excretion of cholesterol, intracellular reuse of fatty acids, and as an important carrier and cofactor) and diverse human diseases [27–31]. It is well known that systemic inflammatory responses are actively involved in oncogenesis, progression, and survival prediction in cancer patients [32–34]. ApoA-I, as an indispensable component of HDL, inhibits monocyte chemotaxis and recruitment and was shown to be involved in inflammatory reactions, and ApoA-I/HDL can be activated and participate in antitumor activities in mature immune systems [31, 35]. ApoA-I can also inhibit tumor progression and protect against tumor development in vivo and in vitro. It was reported that ApoA-I established antitumor properties by interacting with C1QBP in colon cancer, and ApoA-I could also inhibit colitis-propelled carcinogenesis and modulate tumorigenicity and immunogenicity [36–39]. Studies also demonstrated the antitumorigenic effects of ApoA-I in vivo and that ApoA-I could potently suppress tumor growth and metastasis in vivo and improve survival in mouse tumor models [40, 41]. Additionally, ApoA-I exerted multiple functional effects in the tumor microenvironment [31, 41].
As far as we know, this is the first meta-analysis that has provided an updated understanding of the prognostic value of the serum ApoA-I level in various malignancies. After a synthesized literature search, a total of 13 published articles was collected involving a total of 9295 patients. From the pooled results, we found that a low level of serum ApoA-I was significantly associated with poor OS (HR = 0.52, 95% CI: 0.44–0.61) in human cancers. Furthermore, we also investigated the prognostic values of ApoA-I level in certain types of cancers and found that the pretreatment of ApoA-I level could act as a prognostic indicator in NPC (HR = 0.63, 95% CI: 0.54–0.73), CRC (HR = 0.48, 95% CI: 0.19–0.76), and HCC (HR = 0.46, 95% CI: 0.27–0.65). The subgroup analyses further revealed the prognostic significance of ApoA-I for OS in cancer patients. In addition, the relationships between serum ApoA-I level and secondary outcomes were also investigated, and the ApoA-I level was found to be associated with CSS (HR: 0.47, 95% CI: 0.19–0.76) in cancers, and the ApoA-I level might be a prognostic indicator for TTR (HR: 0.43, 95% CI: 0.29–0.58) in HCC, LRFS (HR: 0.58, 95% CI: 0.42–0.74) and DMFS (HR: 0.65, 95% CI: 0.41–0.89) in NPC, and DFS (HR: 0.64, 95% CI: 0.43–0.84) in cancers. Thus, the serum ApoA-I level might be a candidate biomarker with clinical utility in human malignant tumors.
Several limitations should be acknowledged in our meta-analysis. First, the total sample size was insufficient, and the number of studies included was also relatively small. Second, most of the participants were Chinese, and only one study was conducted with a European cohort. Third, only eligible articles published in English or Chinese were included in this meta-analysis. Fourth, potential bias might exist for OS in studies, although the “trim and fill method” was adopted and no significant bias was found. The sensitivity analysis also indicated the robustness of the pooled HR for OS. In addition, there was obvious heterogeneity for OS, and the heterogeneity could not be totally eliminated by subgroup analysis. Finally, the cutoff values for low ApoA-I level varied in different studies.
In summary, our meta-analysis showed that pretreatment low ApoA-I level was related to worse survival in patients with various tumors. Serum ApoA-I level might be a powerful and noninvasive biomarker to predict cancer prognosis. However, before ApoA-I levels are routinely applied in clinical management, large-scale and well-designed studies with unified cutoff values are necessary to validate our results.
Abbreviations
-
- ApoA-I:
-
- Apolipoprotein A-I
-
- HDL-C:
-
- High-density lipoprotein cholesterol
-
- LDL-C:
-
- Low-density lipoprotein cholesterol
-
- CNKI:
-
- China National Knowledge Infrastructure
-
- HRs:
-
- Hazard ratios
-
- 95% CIs:
-
- 95% confidence intervals
-
- NSCLC:
-
- Non-small cell lung cancer
-
- GC:
-
- Gastric cancer
-
- NPC:
-
- Nasopharyngeal carcinoma
-
- BC:
-
- Breast cancer
-
- CRC:
-
- Colorectal cancer
-
- HCC:
-
- Hepatocellular carcinoma
-
- ENKTL:
-
- Extranodal natural killer (NK)/T-cell lymphoma, nasal type
-
- ESCC:
-
- Esophageal squamous cell carcinoma
-
- RCC:
-
- Renal cell cancer
-
- OS:
-
- Overall survival
-
- CSS:
-
- Cancer-specific survival
-
- TTR:
-
- Time to recurrence
-
- DFS:
-
- Disease-free survival
-
- PFS:
-
- Progression-free survival
-
- DSS:
-
- Disease-specific survival
-
- DMFS:
-
- Distant metastasis-free survival
-
- LRFS:
-
- Locoregional recurrence-free survival
-
- ROC:
-
- Receiver operating characteristic curve.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
Our deepest gratitude goes to the editor and anonymous reviewer for their expertise and careful work and thoughtful suggestions that have helped improve this paper substantially.