A Turn-On Fluorescent Sensor for Hg2+ Based on Graphene Oxide
Abstract
A graphene oxide- (GO-) boradiazaindacenes (BODIPY) charge-transfer complex (BGO) has been easily synthesized, and the structure of BGO was confirmed by FT-IR and atomic force microscopy (AFM). Moreover, the BGO was found that could be used as a turn-on fluorescent sensor for Hg2+. Upon addition of Hg2+, the fluorescence of BGO would be enhanced since the energy transfer between BODIPY and GO was inhibited. The selectivity and the competition performance of BGO towards Hg2+ were good among other heavy metal ions.
1. Introduction
Most heavy metal ions are very toxic and pose risks to human health and environment. Among the metal ions, Hg2+ is one of the most dangerous toxic metal ions, which leads to many health problems including digestive, cardiac, kidney, and especially neurological diseases [1, 2]. Thus, development of specific and facile method for detecting Hg2+ in environment is highly desirable [3]. Among the well-known analytical methods, fluorescent sensors have received intense interests owing to their potential promises to on-site and real-time detection of toxic metal ions with low detection limits [1, 3]. Therefore, a lot of fluorescent sensors based on small molecules for monitoring Hg2+ have been reported [4]. Moreover, the chemists prefer to use organic-inorganic materials instead of organic dye as report part for fluorescent sensor [5]. The receptor-immobilized inorganic materials have some important advantages [6], such as being free from the pollution of organic molecules, easily recyclable, applicable in heterogeneous solid-liquid phase.
GO nanosheet, a two-dimensional oxidized derivative of graphene, also contains isolated polyaromatic clusters and can be easily exfoliated from graphite with a high yield under simple oxidizing conditions. It has been widely studied in regard to electrical conductivity, drug delivery, self-assembly, surface functionalization [7], and the application in optical sensor [8]. Usually, GO could act as a fluorescence quencher due to the energy transfer in the sensing system. However, it needs multiple steps to build up GO-based sensors [8].
BODIPY are very attractive functional groups for construction of molecular sensors because of their advantageous characteristics, such as sharp absorption and fluorescence bands, high extinction coefficients, high fluorescence quantum yields, and high stability against light and chemical reactions [9, 10]. Stankovich et al. reported synthesis of isocyanate-treated GO which is stable in polar aprotic solvents such as DMF and DMSO [11]. Herein, a chemically modified GO derivative BGO via treatment of GO with 4,4-difluoro-8-(4-isothiocyanatophenyl)-1,3,5,7-tetramethyl-4-bora-3a,4a-diaza- s-indacene (BODIPY 1) was synthesized in one step, and its application for selective sensing Hg2+ was also studied (Scheme 1).
2. Materials and Methods
The compound BODIPY 1 was synthesized similarly as the reported reference [12]. In a round-bottomed flask, a solution of thiophosgene (0.04 mL, 0.56 mmol) in distilled CH2Cl2 (5 mL) was added dropwise to a solution of 1 [13] (0.1 g, 0.3 mmol) in anhydrous CH2Cl2/NEt3 (20 mL/0.2 mL) under argon at 0°C. As soon as total consumption of the starting material was observed by TLC (one spot, about 5 min), the reaction was quenched with water (10 mL). The organic layer was then washed with water (3 × 20 mL), dried over anhydrous MgSO4, and filtered, and the solvent was evaporated. The residue was then passed through a very short pad of silica gel and the resulting BODIPY isothiocyanate was used without further purification for the following reaction (60 mg, 52%). 1H NMR (CDCl3, 300 MHz): δ 7.36 (d, 2H, 8.4 Hz, phenyl), 7.29 (d, 2H, 8.4 Hz, phenyl), 6.00 (s, 2H, pyrrolyl), 2.55 (s, 6H, -CH3), 1.40 (s, 6H, -CH3); 13C NMR (CDCl3, 75 MHz): 156.0, 142.8, 134.0, 132.3, 129.6, 126.5, 121.5, 14.7, 14.6 ppm.
The graphite oxide was prepared by oxidation of natural graphite powder (325 mesh) according to the method developed by Hummers Jr. and Offemann [14].
Preparation of BGO. Graphite oxide (20 mg) was dispersed in 10 mL DMF and sonicated for 3 hours. 40 mg BODIPY 1 was added to the dispersion and the mixture was allowed to stir under nitrogen at 60°C for 24 h. Then the reaction mixture was poured into methylene chloride (30 mL) to coagulate the product. The product was filtered, washed with additional methylene chloride (100 mL), and dried under vacuum to afford BGO.
The 1H and 13C NMR spectra were collected on Jeol JNM-AL300 spectrometer at 300 MHz and 75 MHz, respectively. FT-IR experiments were conducted on a JASCO FT/IR-4200 spectrometer (0.5 cm−1, 64). HRMS spectra were recorded on Jeol JMS-700 spectrometer. UV-vis absorption spectra were obtained on a Shimadzu UV-3100 spectrophotometer. Fluorescence spectra were recorded with a FL-4500 spectrofluorophotometer (EX 480 nm, Slit 5 nm). Atomic force microscopy (AFM) was taken on Bruker dimension icon.
3. Results and Discussion
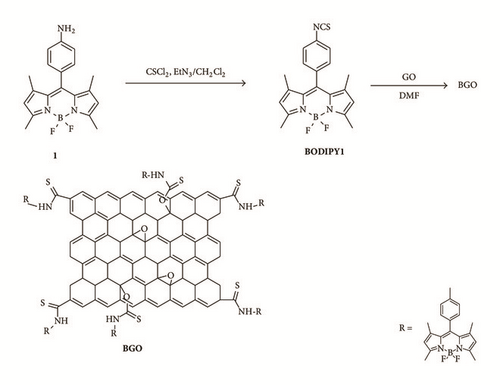
Figure 1 illustrates the changes occurring in the FT-IR spectra of BODIPY 1, GO, and BGO. The most characteristic features in the FT-IR spectrum of GO are the absorption bands corresponding to the C=O stretching at 1727 cm−1, the O-H deformation vibration at 1414 cm−1, the C-OH stretching at 1229 cm−1, and the C-O stretching at 1071 cm−1; besides the O-H stretches at 3400 cm−1 (not shown), the vibration of the absorbed water or skeletal vibrations of unoxidized graphitic domains at 1621 cm−1 [11]. Upon treatment with BODIPY 1, the C=O stretching vibration at 1727 cm−1 in GO becomes obscured by the appearance of a weaker absorption at 1721 cm−1 that may be attributed to the consumption of carbonyl group. The new stretch at 1642 cm−1 may be assigned to the thioamide stretching. And the adsorption bands corresponding to the NCS at 2183 cm−1 and 2125 cm−1 disappeared in BGO which indicated that the BODIPY 1 was consumed during the reaction [11] and no free BODIPY 1 was absorbed by GO.
AFM was performed to check the topology of GO and BGO. The GO and BGO samples (1 mg/mL in DMF, respectively) were prepared by spin-coating on a fresh mica surface. Figure 2 is the tapping mode AFM images of GO (a) and BGO (b). As shown in the AFM analysis, the average thickness for GO sheets was about 1.1 nm, and the average thickness for BGO sheets was about 1.3 nm. This suggested that the BGO was single layer and the GO may be functionalized by BODIPY 1.
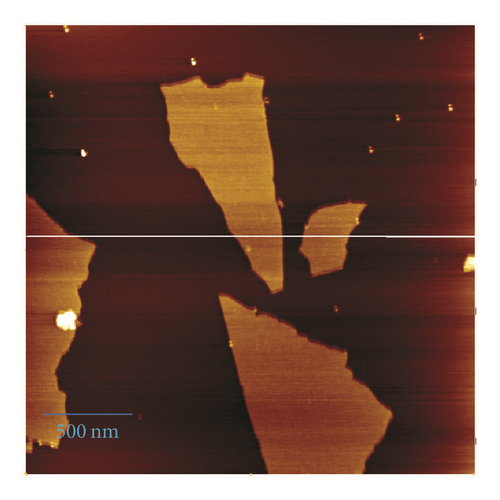

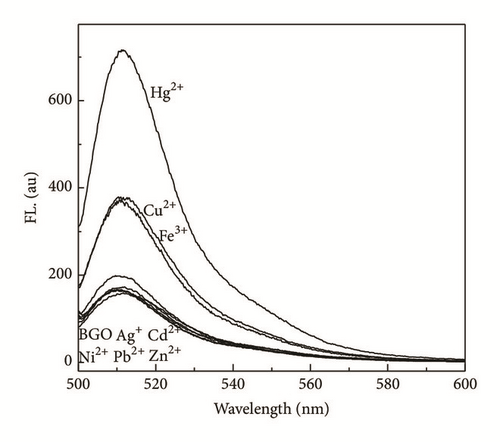
The effects of metal ion addition on the absorption and fluorescence properties of BGO in DMF were investigated to evaluate the metal ion binding properties of BGO. There is no change in absorption spectra upon addition of metal ions (Figure 3(a)). The fluorescence spectra of BGO (0.02 mg/mL) were recorded in following 480 nm excitation. Figure 3(b) showed the fluorescence spectra of compound 1 upon the addition of various kinds of metal ions, Ag+, Cd2+, Cu2+, Fe3+, Ni2+, Pb2+, Zn2+, Hg2+. After addition of Hg2+, the emission at 510 nm was increased about 4-fold and almost no changes of BGO were observed in the presence of Ag+, Cd2+, Ni2+, Pb2+, and Zn2+ (400 μM), respectively. The free BGO solution exhibited fluorescence (excited at 480 nm) in DMF, but the fluorescence of BGO was quenched dramatically compared to BODIPY 1 (Figure S1 in the Supplementary Material available online at https://doi.org/10.1155/2017/9431605); the reason can be attributed to two possible competitive processes: photo-induced electron transfer (PET) and energy transfer (ET) [15]. The PET of BODIPY 1 could not be inhibited after addition of Hg2+ because of desulfurization; the fluorescence of BODIPY 1 would be quenched about 50% through the lone pair electrons on nitrogen (Figure S1b). However, the PET or ET would be suppressed by Hg2+ due to its strong thiophilic affinity of Hg2+; therefore the fluorescence of BGO was increased. The result could be supported by FT-IR spectra. From the FT-IR spectra of BGO in presence of Hg2+, the peaks correspond to C=O and thioamide would be red shift. That indicates the carbonyl group and thioamide participate in the binding with Hg2+ (Figure S2). Furthermore, the fluorescence of BGO in presence of Hg2+ would be reversed by addition of KI, which means that no desulfuration occurred (Figure S3).
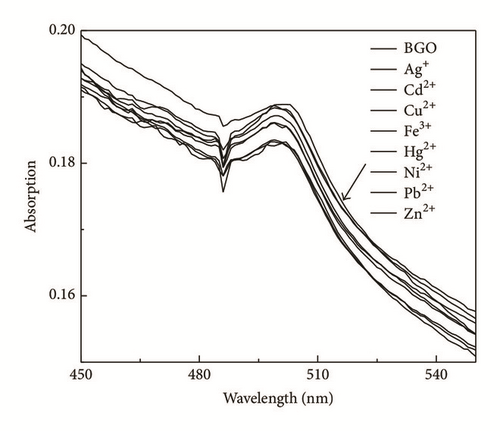
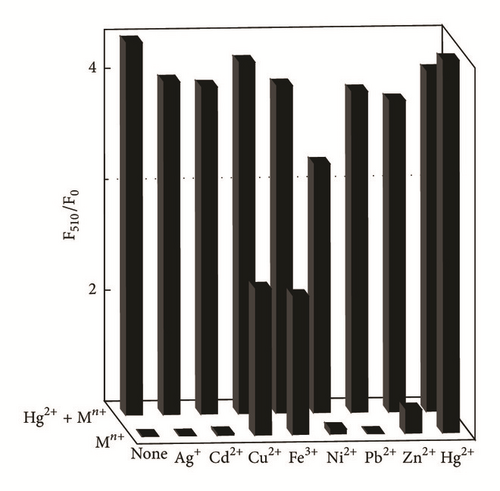
To study the recognition of hybrid material for metal ions, the selectivity of BGO towards Ag+, Cd2+, Cu2+, Fe3+, Ni2+, Pb2+, Zn2+, and Hg2+ was also examined at the concentration of 400 μM in DMF. As shown in Figure 4, the competition experiments revealed that the Hg2+-induced luminescence enhancement was almost unaffected in the presence of Ag+, Cd2+, Cu2+ Ni2+, Fe3+, and Zn2+; only Ni2+ showed a little interference, confirming the remarkable high selectivity of BGO for Hg2+.
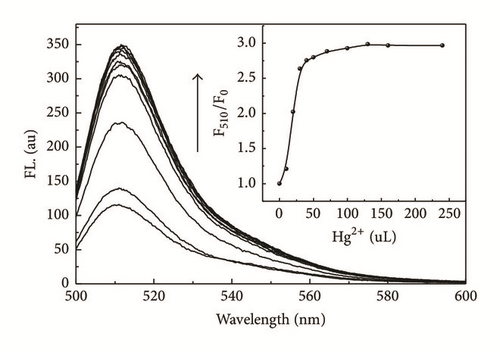
Titration experiments were performed with BGO (0.01 mg/mL) with different concentrations of Hg2+ ranging from 0 to 125 μM in 2 ml DMF. Figure 5 illustrated the fluorescence titration spectra of BGO upon addition of various amounts of Hg2+ (0, 5, 10, 15, 20, 25, 35, 50, 60, 80, and 125 μM). As shown in Figure 5, the fluorescence intensity increased continuously with increasing Hg2+ concentration. The inset showed the fluorescence titration profile of BGO at 510 nm upon addition of Hg2+.
4. Conclusions
In conclusion, an organic-inorganic hybrid complex BGO has been synthesized. And the BGO can be used as a selective fluorescent sensor for Hg2+. Further studies on the accurate structure of the BGO-Hg2+ complex and other application of BGO are in progress in our laboratory. In addition, the present work inspires the development of multifunctional GO-based hybrid materials through a simple charge-transfer process. Taking advantage of the high surface area of GO, the organic-inorganic hybrid complex based on GO could be potentially used for treating wastewater and sustained drug release.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This work was supported by the Research Foundation of Department of Education of Hebei Province, China (Grant no. QN2016173), the Research Foundation of Administration of Science and Technology of Handan, China (Project no. 1629209045-4), and Foundations of Hebei University of Engineering.




