Removal of Pb, Cu, Cd, and Zn Present in Aqueous Solution Using Coupled Electrocoagulation-Phytoremediation Treatment
Abstract
This study presents the results of a coupled electrocoagulation-phytoremediation treatment for the reduction of copper, cadmium, lead, and zinc, present in aqueous solution. The electrocoagulation was carried out in a batch reactor using aluminum electrodes in parallel arrangement; the optimal conditions were current density of 8 mA/cm2 and operating time of 180 minutes. For phytoremediation the macrophytes, Typha latifolia L., were used during seven days of treatment. The results indicated that the coupled treatment reduced metal concentrations by 99.2% Cu, 81.3% Cd, and 99.4% Pb, while Zn increased due to the natural concentrations of the plant used.
1. Introduction
The mining industry in Mexico is an important economic activity that provides 4.9% of the National Gross Domestic Product [1], being mostly metallic extraction of Cu, Pb, Ag, Au, Fe, Zn, Mn, Cd, Mo, Bi, Sb, As, Sn, Se, and so forth, through 902 mining complexes [2]. Although mining is considered an important economic sector, it unfortunately causes a high environmental impact with the emission of high concentrations of toxic pollutants (hydrocarbons, mineral salts, heavy metal leaching, particulate material, and acid drainage), which can be dispersed in air, soil, and water with the consequent dispersion towards surface and underground water sources [3, 4].
In recent years, there has been an increasing interest in the development of low cost and high efficiency technologies for the removal of heavy metals in wastewater, which, because of their varied nature, are difficult to eliminate, complicating their treatment and raising their costs [5]. Currently, the elimination is based on unit processes of physicochemical origin such as advanced oxidation processes, adsorption, chemical precipitation, ion exchange, membrane filtration, solvent extraction, and oxidation/reduction methods [6]. These treatments are efficient, but some concentrations remain and can be treated by other systems, including biological treatments, especially phytoremediation.
In this context, electrochemical coupled biologically systems for metal removals of solutions have the potential to achieve high removal efficiencies [7, 8].
The objective of the present study was the evaluation of the removal efficiency of a coupled electrocoagulation process using aluminum electrodes with phytoremediation (Typha latifolia L.), for the treatment of simulated mining water which has the four heavy metals that are commonly present in mining wastewater.
2. Materials and Methods
2.1. Electrocoagulation
2.1.1. Preparation of Simulated Mining Water
A mining water sample of 2 L was prepared by treatment, based on the average concentrations observed during a year in a body of water adjacent to a mining shaft: Pb (16 ppm), Cu (119 ppm), Cd (75 ppm), and Zn (156 ppm).
Sulphate and nitrate metal salts (Fermont) were dissolved in distilled water; the pH was adjusted with H2SO4 to reach a value around 5 ± 0.5; this pH value is characteristic in the mining wastewater zone [25].
The purpose to use a simulated sample was to evaluate the optimal conditions for efficiently eliminating the major pollutants from the mining wastewater in study.
2.1.2. Electrochemical Reactor
A “batch” reactor was constructed, taking into account the optimum operating conditions described by Rodríguez et al. [26] and Mercado et al. [27]. This consists of 5 pairs of aluminum (10∗5∗0.03 cm). The electrodes were connected to a power source that supplied a direct current of 4 A and 8 V [28], during 180 minutes and temperature intervals of 24 ± 4°C [29, 30] and the pH was 5 ± 1.8 [31]. During the treatment period, samples were taken at different times to quantify concentrations of heavy metals by atomic absorption spectrophotometry [29].
2.2. Phytoremediation
2.2.1. Collection and Acclimatization of Plants
The macrophyte, Typha latifolia L., was collected in the municipality of Toluca, State of Mexico, at coordinates 19°16′04.1′′N 99°41′14.7′′W [32]. The macrophyte was collected manually, placed in plastic containers to be kept in water, and transported to the laboratory, where it was grown in dark plastic containers to prevent the formation of algae, with water and Murashige and Skoog nutrient solution “MS” [33] at pH 6.4 ± 0.5. The plants were maintained at room temperature of 20 ± 5°C and light/dark photoperiod. The total period of acclimatization lasted 14 days, after which they were used in phytoremediation systems.
2.2.2. Phytoremediation System
Plastic containers with a capacity of 2 liters of electrocoagulated water were distributed in seven treatments systems: control (electrocoagulated water and Typha latifolia + nutritional solution “MS” in water). The remaining treatments had the following conditions: a dilution of 12.5% electrocoagulated water, three plants with a biomass of 90 ± 10 g, pH 6.4 ± 1, temperature ranges of 23 ± 3°C, and nutrient solution MS. The phytoremediation system lasted 7 days. During the treatment of phytoremediation, the levels of concentration of heavy metals in solution were determined [30] and the translocation factor, bioaccumulation factor [34–36], and removal kinetics were calculated.
2.2.3. Tolerance to Exposure
2.2.4. Determination of Metals Concentrations in Water and Biomass
The metals concentration was analyzed by atomic absorption spectrophotometry using Avanta GBC 3000 equipment. The liquid samples were filtered using Whatman filter paper with 0.45 μm of pore size and it acidified with concentrated HNO3. The biomass (roots and aerial part) was weighed, dried, and ground to a particle size of 20 mesh and then were digested with H2SO4-HClO4 (4 : 1).
Analyze both the electrocoagulation and phytoremediation treatment (water and biomass) by atomic absorption spectrophotometry using Avanta GBC 3000 equipment. The liquid samples previously were filtered with filter paper with 0.45 μm pore size and acidified with concentrated HNO3. Regarding the biomass, the root and aerial part were weighed, dried, and ground to a particle size of 20 mesh and then digested with H2SO4-HClO4 (4 : 1).
3. Results
3.1. Electrocoagulation
3.1.1. Reactions of Electrocoagulation System with Aluminum Electrodes
During the electrocoagulation process, with aluminum electrodes, we have the in situ formation of the coagulant by the corrosion of the sacrificial anode and the parallel evolution of the hydrogen in the cathode that allows the elimination of contaminants by flotation or sedimentation. All this removal process is carried out in three main reactions [31, 40, 41].
3.1.2. Effects of pH during Electrocoagulation
The pH influence is one of the most important parameters in electrocoagulation processes, since this determines the ionic species that will act as a coagulant [42–44], furthermore influencing the current efficiency in the metal solubility process to form hydroxides [45, 46]. To analyze this variable in the simulated mining sample, the pH value is 5.27, which is characteristic of mining wastewater [25]; pH was monitored throughout the electrochemical treatment.
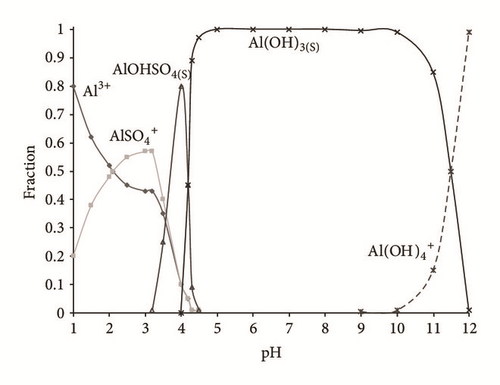
During the first treatment hour, a decrease in the pH value caused by buffering processes between OH- production and consumption was observed, in addition to the formation of monomeric species. Through the Hydra-Medusa software [47] the species present in the solution were calculated by means of speciation diagrams from equilibrium constants, pH (2–12), 5–60 minutes, []: 1.29 mM, []: 3.14 mM, and [Al3+]: 2 mM. Figure 1(a) shows a chemical predominant speciation diagram at the first 5 minutes of treatment. It is interesting to note that there are 5 species; there are between them Al3+, , AlOHSO4, Al(OH)3(s), and [46–48]. Figure 1(b) shows the mechanisms in the chemical speciation diagram, when applying 60 minutes of treatment. It can be observed that the predominant species are Al3+ and Al(OH)3,(s); according to the diagram the formation of the coagulant agent exhibited a minimum solubility at pH a from 3.5 to 10 [49, 50]. In both figures we can see the formation of another polymorphic compound with values of pH = 10, , which may cause passivation of the cathode [51].

3.1.3. Kinetics of Removal
| Heavy metals | CD (mA/cm2) | First-order model k (h−1) |
|---|---|---|
| Pb | 8 | 1.080 |
| Cu | 8 | 1.372 |
| Cd | 8 | 0.483 |
| Zn | 8 | 1.658 |
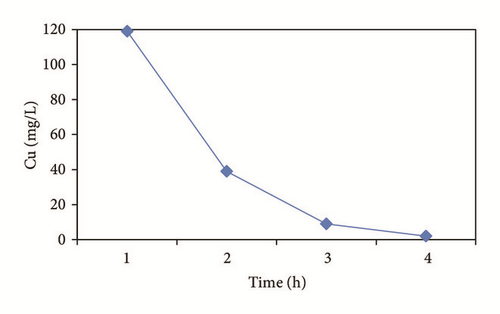
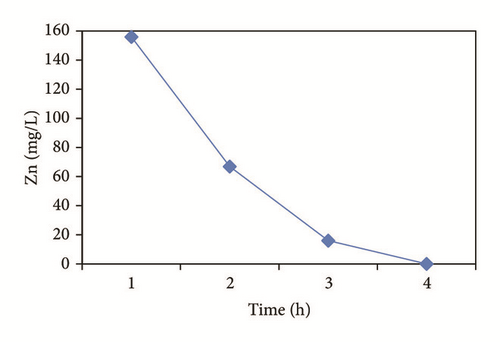
In the heavy metals removal kinetic (Figure 2), it can be noted that Cu and Zn follow a similar behavior with metal hydroxide precipitation around the cathode [47, 49]. In the case of copper (Figure 2(a)), its removal started from a pH of 5.65 in the form of Cu(OH)2(c) [29]. While the removal of Zn was in 3 h of treatment, the species formed are as follows (Figure 2(b)): Zn2+, ZnSO4, ZnOH+, and Zn(OH)2(c). Works by Adhoum et al. [40] and Al-Shannag et al. [53] report percentages of removal similar to this study [52].
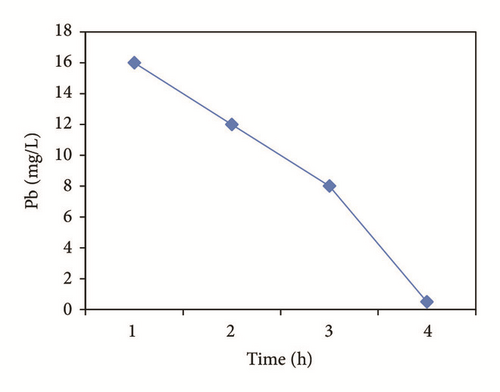
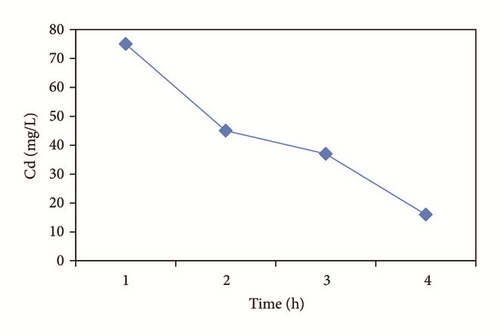
Lead precipitated prior Heidmann and Calmano [47] reported that some metals may exhibit this phenomenon and that the concentration decrease will continue as pH increases during electrochemical treatment. In Figure 2(c) it is observed that the precipitation was present due to the presence of the SO4 anion with which it becomes complexed. The removal process involved the species formed of Pb2+, PbSO4(c), PbOH+, Pb(OH)2, , and .
In the case of cadmium the maximum removal is around 80% (Figure 2(d)) in 3 hours of electrochemical treatment. This can be explained using a Medusa-Hydra chemical program (Figure 3) where it can be observed that Cd(OH)2(c) require to increase the pH until 9.5.
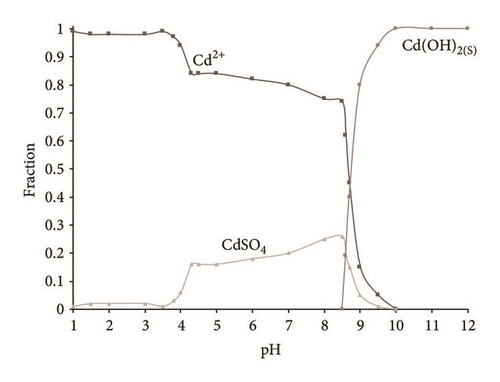
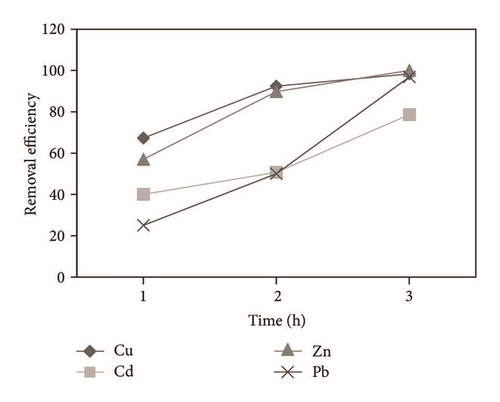
Figure 4 shows the percentage of removal of the metal ions (Cu2+, Cd2+, Pb2+, and Zn2+) which was increased with the time of electrocoagulation. Removal efficiencies were obtained up to 98.3% for Cu, 78.7% for Cd, and 100% for Zn in 3 hrs. In the case of Pb, a 96.9% removal was obtained by precipitation prior to the electrocoagulation process and the reduction made by the electrochemical treatment. The concentration of the aluminum generated in the reactor was calculated at 2680 mg/l, which decreases during the treatment; Mouedhen et al. [30] indicate that during the early stages of electrocoagulation the concentration of aluminum increases and subsequently reduces due to its participation in the formation of polymorphic species that give rise to Al(OH)3(s).
3.2. Phytoremediation
3.2.1. Determination of Exposure Tolerance for Typha latifolia L.
For the tolerance analysis, the plants were exposed to 100% and dilutions to 75, 50, 25, and 12.5% of electrocoagulated water for 7 and 14 days, making daily observations. Significant differences (p < 0.05, U of Mann–Whitney) were obtained for chlorophyll production at 14 days in all treatments. At dilutions of >50% after 7 days, phytotoxic effects on macrophytes were observed (Figures 5(a), 5(b), and 5(c)).


The concentration of the electrocoagulated water metal mixture to which the Typha was tolerant was at a dilution at 12.5% (mixture of 1.25 mg/l of Cu, 9 mg/l of Cd, 0.2625 mg/l of Pb, and Zn < the limit of quantification), after 7 days of exposure. The concentrations at which no significant differences were found between the values obtained of chlorophyll, carotene, and their relation to control plants, ANOVA (p < 0.05), are shown in Figures 6(a) and 6(b) [52].
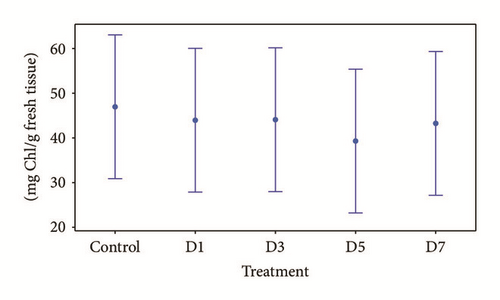
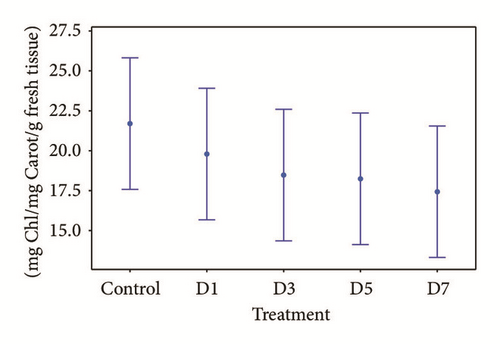
Ruiz et al. [25] reported that Typha latifolia is tolerant to concentrations of the mixture of 0.309 mg/l of Cd and 23.60 mg/l of Zn present in the wastewater of a mining company. Anning et al. [55] worked with a mixture of 0.31 mg/l of Cu and 0.19 mg/l of Pb present in municipal wastewater and tested the tolerance of T. latifolia to these concentrations. Both works indicate the potential of the macrophytes in the removal of these four metals, all without showing any signs of phytotoxicity (chlorosis or wilt).
3.2.2. Kinetics of Heavy Metal Removal
To the metal tolerant mixture for the plant, the kinetics of removal were performed. Figure 7(a) shows that Cu2+ had a reduction of 20% at day 1 and subsequently remained constant; similar results were reported by Anning et al., 2013, with the removal of 33.84% Cu present in wastewater by Typha latifolia. The reduction of Pb concentration was gradual, obtaining a total removal at 7 days of 61.9%, close to that observed by Oquendo [56] when removing 62% lead solution at 2 ppm using the same macrophytes.
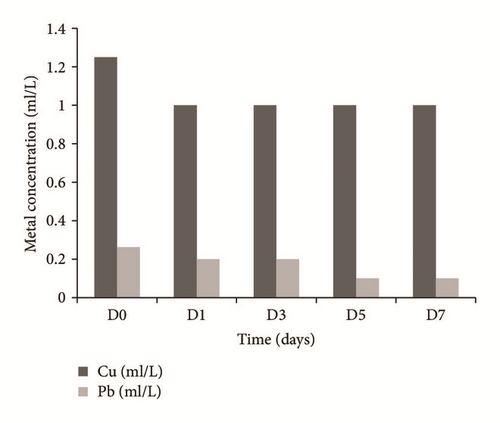
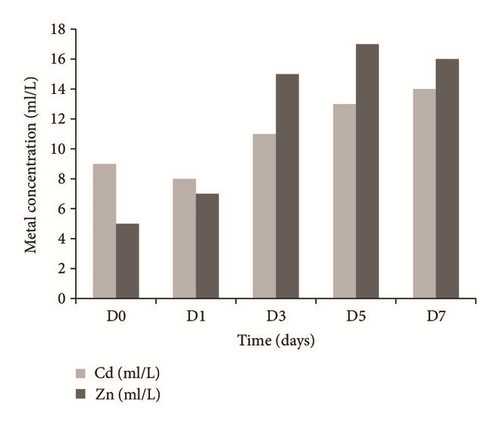
In Figure 7(b) increases in concentrations of both Zn and Cd (11 mg/l and 5 mg/l), respectively, are observed; this may be due to desorption processes of both metals by the plant; the days of greatest desorption for both metals were day 5 for zinc and day 7 for cadmium. Probably the increase of the concentration of both metals is because the collect site of the Typha was contaminated with these heavy metals; Kabata-Pendias [57] mentions that such metals are associated in the soil with concentration range which can reach up to 4832 mg/kg for Zn and 222 mg/kg for Cd. In the phytoremediation system when exposed to the mixture with other metals the root could be saturated, which would lead to the desorption and increase of Zn-Cd in the water.
3.2.3. Translocation Factor (FT)
To evaluate the ability of plants to translocate heavy metals from the root to aerial parts, the FT was calculated by aerial concentration/root concentration [58]; we recall that the criterion for establishing that a plant has the translocation capacity of the metal in the vegetative structure is that FT > 1 metals [39]. During the treatment with the electrocoagulated mineral water the plants showed high values of FT (Table 2) showing the following trend of Pb > Zn > Cu > Cd translocation.
| Cu | Cd | Zn | Pb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BCFA | BCFR | FT | BCFA | BCFR | FT | BCFA | BCFR | FT | BCFA | BCFR | FT | |
| D1 | 18 | 22 | 0.82 | 4.25 | 7 | 0.61 | 5.86 | 6 | 0.98 | 1485 | 1510 | 0.98 |
| D3 | 10 | 29 | 0.34 | 3.09 | 6.27 | 0.49 | 2.73 | 2.80 | 0.98 | 1445 | 1365 | 1.06 |
| D5 | 20 | 18 | 1.11 | 2.85 | 4.85 | 0.59 | 1.88 | 2.12 | 0.89 | 2820 | 2810 | 1.00 |
| D7 | 13 | 22 | 0.59 | 2.43 | 5.07 | 0.48 | 1.44 | 2.38 | 0.61 | 2690 | 2570 | 1.05 |
| Average | 15.25 | 22.75 | 0.72 | 3.15 | 5.80 | 0.54 | 2.98 | 3.32 | 0.86 | 2110 | 2063 | 1.02 |
- BCFA: bioaccumulation factor aerial/medium, BCFR: bioaccumulation factor root: root/medium, and FT: translocation factor: aerial/root.
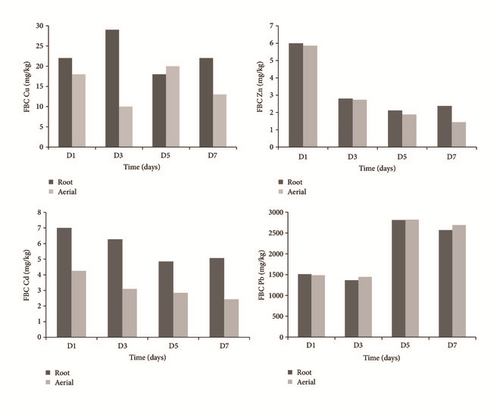
The higher values of lead in the treatment indicate an efficient transport capacity of the root metal to the aerial part of the plant and its probable accumulation in the plant [34, 59], while Zn, Cu, and Cd presented a TF < 1, indicating that the mechanism of accumulation of these metals is the adsorption in the root [60].
3.2.4. Bioaccumulation Factor in Leaves and Roots (BCFA and BCFR)
In this study, higher values of BCF than FT were observed, indicating that the plant possesses phytoremediation and phytostabilization potential [61, 62]. The concentrations of BCF > 1 in both leaves and root (Table 2), same behavior as Ye et al. [16] and Sasmaz et al. [34]. The Pb is the one with the greatest accumulation in the vegetative structure.
The bioaccumulation factor in leaves (BCFA) of Typha latifolia L. presented high accumulation of Pb despite being cataloged of low mobility and accumulation in root [56, 63]. The tendency of observed bioaccumulation was Pb > Cu > Cd > Zn, very similar to that reported by Lyubenova et al. [64].
The bioaccumulation of the metals in the roots was greater than those observed in the aerial parts, except for Pb (Figure 8), as follows: Pb > Cu > Cd > Zn, behavior probably due to a passive adsorption process and metal accumulation in the rhizosphere, as reported by Chandra and Yadav [65] and Hazra et al. [66].
3.3. Electrocoagulation-Phytoremediation Coupled System
The electrocoagulation process used in the treatment of simulated mineral water showed a good effectiveness in the removal of the metal mixture. Residual concentrations were treated by phytoremediation with Typha latifolia L., achieving on average an additional 2% removal (Table 3).
| Metal | Initial concentration (mg/l) | % removal with Electrocoagulation | % removal with Phytoremediation | % removal of coupled system |
|---|---|---|---|---|
| Cu | 119 | 98.3 | 20 | 99.2 |
| Cd | 75 | 78.7 | 11 | 81.3 |
| Zn | 156 | 100 | — | Desorption |
| Pb | 16 | 96.9 | 43 | 99.4 |
4. Conclusions
The mechanisms present in the removal of the Cu, Cd, Pb, and Zn mixture from the simulated mining solution are as follows: (1) In the cathode a reaction of reduction forming metal hydroxides Cu(OH)2(c), Cd(OH)2(c), Pb(OH)3, and Zn(OH)2(c) is performed, which were formed by electrolysis, and (2) coprecipitated with aluminum hydroxide (flocs). The removal rates during the 180 minutes of electrochemical treatment achieved a 100% efficiency in Zn, 98.3% in Cu, 96.4% in Pb, and 78.7% in Cd at pH 5.27. The slower removal of the Cd in comparison to the rest of the metals present in the solution is attributed to a difference in the pH value (9.5) required for this metal as evidenced in the speciation diagram. The electrocoagulation process had a calculated energy consumption of 26 kWh/m3.
In the present study it was shown that Typha latifolia L. is tolerant to residual concentrations of the metals mixture present in the electrocoagulated water, as evidenced by the content of pigments (chlorophyll and carotene) in the plant. The translocation factor values showed a tendency of the pass of the metals from the root to the aerial part of the plant in the following order: Pb > Zn > Cu > Cd. Likewise the bioconcentration factor indicates that the root of this macrophyte has the ability to perform the phytostabilization and phytoaccumulation processes of the metals studied.
The coupled process electrochemical-phytoremediation had a high removal efficiency of Cu (99.2%), Cd (82.7 to 89.3%), and Pb (99.4%) to the concentrations present in mining wastewater. In this context such combination of treatments is viable both environmentally and economically.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The present paper is a product of the Master thesis by Francisco Ferniza García, student of the M.S. of Environmental Science of the Chemistry Faculty of the Autonomous University of the Mexico State. The authors are also grateful to CONACyT, Pharmacy Laboratory of the Chemistry Faculty of the Autonomous University of the Mexico State, and Center of Research in Sustainable Chemistry, UAEM-UNAM, for the materials and instrumentation used during the development of this study.




