Global Dynamics of a Periodic SEIRS Model with General Incidence Rate
Abstract
We consider a family of periodic SEIRS epidemic models with a fairly general incidence rate of the form Sf(I), and it is shown that the basic reproduction number determines the global dynamics of the models and it is a threshold parameter for persistence of disease. Numerical simulations are performed using a nonlinear incidence rate to estimate the basic reproduction number and illustrate our analytical findings.
1. Introduction
Epidemiological models in mathematics have been recognized as valuable tools in analyzing the dynamics of an infectious disease nowadays. They are used to describe the spread of disease and also to make control measures known to avoid its persistence, for example, via vaccination terms or treatment terms. These models consider the total population divided into compartments, given by the biological assumptions on the model and represented by functions depending on time t. The most common categories used are susceptible (S), infected (I), recovered (R), exposed (E), quarantined (Q), and vaccinated (V), and the dynamics of model is given by transmission rates from a compartment to another. We have then indicated that the models could be of type SIR, SIRS, SEIR, SEIRS, SEIVR, SEIQV, and so forth.
To ensure that the model can give a justified qualitative description of the disease, the choice of the incidence rate plays an important role. An incidence rate is defined as the number of new health related events or cases of a disease in a population exposed to the risk in a given time period. Some examples are the bilinear incidence rate, the saturated incidence rate, or a general incidence rate. The bilinear incidence rate has been repeatedly used by several authors. It is given by βSI, where β is the transmission rate and the product SI represents the contact between infected and susceptible individuals (based on the law of mass action). It was introduced by Kermack and McKendrick [1] in 1927, and even when it is mathematically simple to use, it faces multiple problems and challenges when it is used to describe disease propagation among gregarious animals or persons [2], because it goes to infinity when I becomes larger. In order to improve the modelling process to study the dynamics of infection among a large population, Capasso and Serio [3] in 1978 introduced a saturated incidence rate by studying the Cholera epidemic spread in Bari, given by βSI/(1 + kI), where β is the transmission rate and k the saturation constant. Unlike the bilinear incidence, saturated incidence does not grow up without a limit, but it goes to a saturation limit as I goes to infinity. Multiple types of saturated incidence have been used in the literature; see, for example, [2] for a list of them. To avoid the use of a single incidence function, the use of a general incidence rate that includes a family of particular functions with similar properties has become a topic of interest by several authors (see, e.g., [4–8]).
The basic reproduction (represented by ) is defined as “the average number of secondary cases produced by a single infected case when it is introduced in a susceptible population” and it has an important role in the study of disease transmission. In biological terms, usually when this number is less than one, the disease is eradicated from population, but when it is greater than one, the infection persists. Mathematically, it is of interest to compute a threshold parameter with the properties of the basic reproduction number. A method to compute this number for certain compartmental disease models is via the next-generation matrix method developed in [9]; however, it is not useful when the model presents time periodic seasonal terms. Authors like [10, 11] have defined its basic reproduction number for periodic models as an average, to give some results about extinction or persistence of infection. However Bacaër and Guernaouni in [12] introduced the definition of basic reproduction number for periodic environments, and, later, Wang and Zhao [13] made a formal definition of it, via the monodromy matrix.
In the present work, we focus on a family of SEIRS epidemic models with a time periodic seasonal term, improving the model of Moneim and Greenhalgh in [14], by introducing an incidence rate with a general function taken from [4] and the references therein.
- (A1)
is continuously differentiable.
- (A2)
f(0) = 0, f′(0) > 0 and f(I) > 0 for all I > 0.
- (A3)
f(I) − If′(I) ≥ 0.
Under these assumptions, function f(I) includes various types of incidence rate; in particular, when f(I) = I, we are on the bilinear case considered in [14].
- (A4)
f′′(0) ≤ 0.
- (A5)
There exists ϵ∗ > 0 such that when 0 < I < ϵ∗, f(I) ≥ f(0) + If′(0) + (1/2)I2f′′(0).
This set of assumptions on the function f allows for more general incidence functions than the bilinear one, like saturated incidence functions and functions of the form βSI/(1 + kIq); in particular, in the case when q > 1, they represent psychological or media effects depending on the infected population. In this last case the incidence function is nonmonotone on I. (A3) regulates the value of f(I) comparing it with the value at I of a line containing the origin of slope f′(I) (note that this line varies as I increases), (A4) requires a concave f(I) at the origin, and (A5) imposes the geometrical condition that in a small neighborhood of the origin f(I) must lie between the tangent line of f at I and a concave parabola tangent to f at I.
We consider a family of SEIRS epidemic models with periodic coefficients and general incidence rate in epidemiology. Then we show that the global dynamics of solutions is determined by the basic reproduction number , generalizing the results in [5]. The layout of this paper is as follows: In Section 2, we prove the existence of a disease-free periodic solution and we introduce the basic reproduction number via the theory developed in [12, 13]. In Section 3, we adapt the arguments given in [5] to prove that the disease-free periodic solution of system (1) is globally asymptotically stable if and it is persistent when . Finally, in Section 4, we give some numerical simulations of our results, making a comparison between our basic reproduction number and the average reproduction number used by several authors (see, e.g., [10, 11]).
2. The Basic Reproduction Number
First of all, we prove nonnegativity of the solutions under nonnegative initial conditions.
Theorem 1. Let S0, E0, I0, R0 ≥ 0. The solution (S(t), E(t), I(t), R(t)) of (1) with
Proof. Let N(t) = S(t) + E(t) + I(t) + R(t); then, adding all equations of system (1), we can see that dN/dt = 0, so the value of N is constant. Now, set x(t) = (S(t), E(t), I(t), R(t)) as the solution of system (1) under initial conditions x0 = (S(0), E(0), I(0), R(0)) = (S0, E0, I0, R0) ≥ 0. By the continuity of solutions, for all of S(t), E(t), I(t) and R(t) that have a positive initial value at t = 0, we have the existence of an interval (0, t0) such that S(t), E(t), I(t), R(t) ≥ 0 for 0 < t < t0. We will prove that t0 = ∞.
If S(t1) = 0 for a t1 ≥ 0 and other components of x(t) remain nonnegative at t = t1, then
When I(t1) = 0 for a t1 ≥ 0 and other components remain nonnegative,
Finally, when R(t1) = 0 for a t1 ≥ 0 and other components remain nonnegative,
Therefore, whenever x(t) touches any of the axes S = 0, E = 0, I = 0, or R = 0, it never crosses them.
Therefore, is a disease-free periodic solution of (7); moreover, from [5] we have that ; therefore, lives in X.
- (i)
: the rate of new infection in compartment i,
- (ii)
: the rate of individuals into compartment i by other means,
- (iii)
: the rate of individuals transfer out of compartment i.
is the distribution of accumulative new infections at time t produced by all those infected individuals ϕ(s) introduced before t, with kernel K(t, a) = Y(t, t − a)F(t − a). The coefficient Ki,j(t, a) in row i and column j represents the expected number of individuals in compartment Ii that one individual in compartment Ij generates at the beginning of an epidemic per unit time at time t if it has been in compartment Ij for a units of time, with I1 = E, I2 = I [16].
3. The Threshold Dynamics of R0
3.1. Disease Extinction
Theorem 2. Let be defined as (20); then the disease-free periodic solution is asymptotically stable if and unstable if .
Proof. We use Theorem 2.2 of [13] and check conditions (A1)–(A7). Conditions (A1)–(A5) are clearly satisfied from the definitions of and given in Section 2. We prove only conditions (A6) and (A7). Define
- (A6)
ρ(ΦM(LT)) < 1. Let ΨM be a fundamental matrix for system dz/dt = M(t)z, with M defined as before and LT periodic; the monodromy matrix ΦM(LT) is given by . The general solution of (22) is
(23) -
so and . Note that , so ΦM(LT) = ΨM(LT) and
(24) -
Due to the fact that ΦM(LT) is a constant, its eigenvalue is itself and ρ(ΦM(LT)) < 1 for μ, δ, r(s) > 0.
- (A7)
ρ(Φ−V(LT)) < 1. Solving the system dz/dt = −V(t)z, we arrive at the general solution
(25) -
so
(26) -
Computing , we have
(27) -
Clearly, ρ(Φ−V(LT)) = max{e−(μ+σ)LT, e−(μ+γ)LT} < 1 for μ, γ, σ > 0.
Note 1. Due to the fact that ΨA is a fundamental solution of a periodic system, we can always choose it such that Ψ(0) = I, so the monodromy matrix satisfies ΦA(LT) = ΨA(LT). This property is used in further analysis.
In order to prove the global stability of the disease-free periodic solution, we enunciate some useful definitions and some lemmas.
Let A(t) be continuous, cooperative, irreducible, and ω-periodic k × k matrix function, and ΨA(t) the fundamental matrix of system x′(t) = A(t)x(t). Denote by ρ(ΨA(ω)) the spectral radius of ΨA(ω).
Lemma 3. Let p = (1/ω)lnρ(ΨA(ω)). Then there exists a positive, ω-periodic function v(t) such that eptv(t) is a solution of x′(t) = A(t)x(t) (see proof in Lemma 2.1 of [18]).
Lemma 4. Function f(I) of model (1) satisfies f(I) ≤ f′(0)I, ∀I ≥ 0.
Proof. Using assumptions on function f, we have
Lemma 5. Let (S(t), E(t), I(t)) be a solution of system (7) with initial conditions (S0, E0, I0) ≥ 0, and the disease-free periodic solution of (7); then
Proof. Proof is similar to Lemma 4.1 of [14]. S(t) satisfies the first equation of system (7); then
Now, we are able to enunciate our theorem for global stability of disease-free periodic solution.
Theorem 6. The disease-free periodic solution of system (7) is globally asymptotically stable if .
Proof. From Theorem 2 we have that is unstable for and asymptotically stable for , so it is sufficient to prove that any solution (S(t), E(t), I(t)) with nonnegative initial conditions (S0, E0, I0) approaches as t tends to infinity.
Let ϵ > 0; from Lemma 5 we have
Then, we have proved that for all ϵ > 0 we can find a t1 > 0 such that for all t > t1.
Now, using Lemma 4, for ϵ > 0 we can find a t1 > 0 such that for t > t1
Matrix (F − V + ϵH)(t) is LT-periodic, cooperative, irreducible, and continuous. Using Lemma 3, if q = (1/LT)lnρ(ΨF−V+ϵH(LT)), then there exists a positive and LT-periodic function v(t) = (v1(t), v2(t)) T such that eqtv(t) is solution of system (38). Note that for all k > 0, function is also a solution of system (38) with initial condition kv(0) at t = ti.
Choose a and α1 > 0 such that ; then from (37) we have that
From Theorem 2.2 of [13], iff ρ(ΦF−V(LT)) < 1. By the continuity of the spectrum for matrices (see [20], Section II.5.8), we can choose ϵ > 0 small enough so that ρ(ΦF−V+ϵH(LT)) < 1 and then q < 0 (see Note 1). Thus, using positivity of solutions and comparison,
We need only to prove that S(t) approaches . At disease-free periodic solution , where satisfies
Let ϵ1 > 0 be arbitrary and rmax = maxu∈[0,LT]r(u). Due to (43) we can find a t2 > 0 such that I(t) < ϵ1 for t > t2; moreover, we can find a t3 > 0 such that for t > t3. Then, let t4 = max{t2, t3}; we have for t > t4
So, , where ϵ1(rmax + γ)/(μ + δ) is arbitrarily small. Then , and using similar arguments for S and ϵ2 > 0, we can find a t5 > 0 with for t > t5. Also, from (43), we can find t6 > 0 with E(t) + I(t) < ϵ2/2 for t > t6, so, for t > max{t5, t6}, we have
Or, equivalently, , with ϵ2 being arbitrarily small, and this implies that . We conclude by comparison and using Lemma 5 that , completing the proof.
Theorem 6 shows that disease will completely disappear as long as . Thus, reducing and keeping below the unity would be sufficient to eradicate infection, even in a periodic environment and a general incidence rate.
3.2. Disease Persistence
Uniform persistence is an important concept in population dynamics, since it characterizes the long-term survival of some or all interacting species in an ecosystem [21].
In this section we consider the dynamics of the periodic model when . We will show that actually is a threshold parameter for the extinction and the uniform persistence of the disease. Our results are inspired by [5, 15, 18, 22].
Note that ∂X0 is not the boundary of X0, but it is a standard notation of persistence theory.
Lemma 7. Set X0 is positively invariant under system (7).
Proof. Let x0 = (S0, E0, I0) ∈ X0, that is, E0 > 0, I0 > 0, and let
Let x0 = (S0, 0,0) ∈ X and (S(t), E(t), I(t)) be the solution that passes through that initial condition. We have that ϕ(t, x0) = (S1(t), 0,0), with S1(t) being a solution of (9) and S1(0) = S0 being a solution that satisfies the initial condition. By uniqueness of solutions we have E(t) = 0 = I(t) ∀ t ≥ 0, so x0 lives on M∂.
By continuity of E(t) and sign of derivative of I, we have that, for small 0 < t ≪ 1, E(t) > 0, I(t) > 0, so, for 0 < t ≪ 1, ϕ(t, x0) ∈ X0. Using invariance of X0 (Lemma 7) we have ϕ(t, x0) ∈ X0 for all t > 1. Finally, for a m > 0 such that mLT > 1, we have Pm(x0) = ϕ(mLT, x0) ∈ X0 and this implies (56). By the existence of a disease-free periodic solution (proved in Section 2), it is clear that there is one fixed point of P in M∂ given by ([23]).
Now, we are in a position to introduce the following result of uniform persistence of the disease.
Theorem 8. Let ; then there exists an ϵ > 0 such that any solution (S(t), E(t)I(t)) of (7) with initial values (S(0), E(0), I(0)) ∈ X0 satisfies
Proof. We first prove that P is uniformly persistent (see Definition 1.3.2 from [21]) with respect to (X0, ∂X0), because this implies that the solution of (7) is uniformly persistent with respect to (X0, ∂X0) (see [21], Theorem 3.1.1). Clearly, X0 is relatively open in X, so ∂X0 is relatively closed.
Define
By Theorem 2.2 of [13], if and only if r(ΨF−V(LT)) > 1. Choose an η > 0 small enough with the property (see Appendix A). For α > 0, let us consider the following perturbed equation:
Since is continuous in α, for all ϵ > 0 there is a δ > 0 such that for |α | < δ we have . Moreover, by continuity of solutions with respect to initial values we can find for all an such that if , then
Therefore, for η established before, we can find α small enough such that , ∀t > 0.
Again, by continuity of solutions with respect to initial values, for this small α > 0, there exists a δ > 0 such that for all (S0, E0, I0) ∈ X0 with ‖(S0, E0, I0) − M0‖ ≤ δ we have ‖ϕ(t, (S0, E0, I0)) − ϕ(t, M0)‖ < α, ∀t ∈ [0, LT].
We now claim that
Without loss of generality, we can assume that ‖Pm(S0, E0, I0) − M0‖ < δ for all m ≥ 0 (see Appendix C). From the discussion above, ‖ϕ(t, Pm(S0, E0, I0)) − ϕ(t, M0)‖ < α, ∀m ≥ 0 and t ∈ [0, LT].
For any t ≥ 0, let t = mLT + t1, where t1 ∈ [0, LT) and m = [t/LT] is the greatest integer less than or equal to t/LT. Then, we get
If we set ϕ(t, (S0, E0, I0)) = (S(t), E(t), I(t)), then we have E(t) ≤ α, I(t) ≤ α, ∀t ≥ 0, and from the first equation of (7) and Lemma 4 we arrive at
We have E(t), I(t) ≤ α, and α is fixed small, so we can take α < ϵ∗ and use assumption (A5) in Introduction (see Appendix D) to obtain
By Theorem 2.2 of [13], we have iff ρ(ΦF−V(LT)) > 1. By continuity of spectrum (see [20] Section II), we can find α, ϵ such that
The claim above shows that P is weakly uniformly persistent with respect to (X0, ∂X0). Note that P has a global attractor (see Lemma 5). It follows that M0 is an isolated invariant set in X, Ws(M0)∩X0 = ∅. Every orbit in M∂ converges to M0 and M0 is acyclic. By the acyclicity theorem on uniform persistence for maps ([21] Theorem 1.3.1 and Remark 1.3.1), it follows that P is uniformly persistent with respect to (X0, ∂X0); that is, there exists ϵ > 0 such that any solution of (7) satisfies limt→∞E(t) ≥ ϵ, limt→∞I(t) ≥ ϵ.
4. Numerical Simulations
In this section we provide some numerical simulations to illustrate the results obtained in our theorems and compare them with previous results.
Set an initial population N = 2,200,000 and take time t in years. Suppose μ = 0.02 per year, corresponding to an average human life time of 50 years. Following [5] take the parameters as follows: σ = 38.5 per year, γ = 100 per year, p = 0.85, δ = 0, and a = 1. Choose the periodic transmission as β(t) = β0 + 0.0002cos(2πt), with β0 being the transmission parameter, and the periodic vaccination rate r(t) = 0.1 + 0.004cos(2πt). Both functions have period LT = 1.
Example 1. To illustrate our results, fix β0 = 0.0018. Computing , we have , which is a first approximation of R0. To solve system (80) numerically, we substitute the terms of expression of in (10):
The previous integral cannot be computed analytically, so we approach using Taylor expansion around 0 (remember that we want so solve ρ(W(LT, 0, λ)) = 1, where LT = 1), so even when we cannot find an explicit expression for , the Taylor expansion is a good way to estimate it in (0,1). It could be of interest to also use an approach of around t = 1 and compare the results with those obtained in the present work (see Section 5 for a discussion about this topic).
Setting an initial value λ0 = 0.98 and letting λi = λ0 + i(0.0001), we solve system (80) numerically for each λi (using initial conditions w(0) = (1,0) and w(0) = (0,1), to satisfy W(0,0) = I2×2), and compute ρ1 = ρ(W(LT, 0, λi)) until ρ1 ~ 1. With previous process we arrive at ρ1 = 1.00120166209265 for λ = 0.9872 and ρ1 = 0.997826338969630 for λ = 0.9873; therefore . Using a finer step size 0.0000001 to have more accuracy, we arrive at .
Set initial values as S(0) = 1,500,000, E(0) = 400,000, I(0) = 40,000, and R(0) = N − (S(0) + E(0) + I(0)).
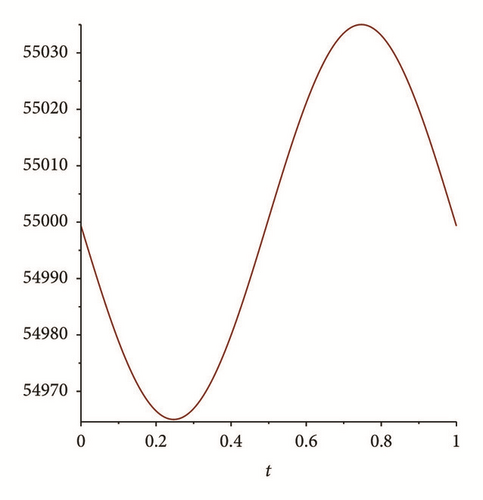
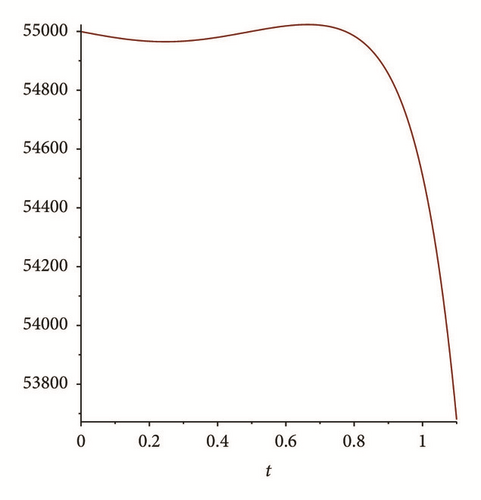
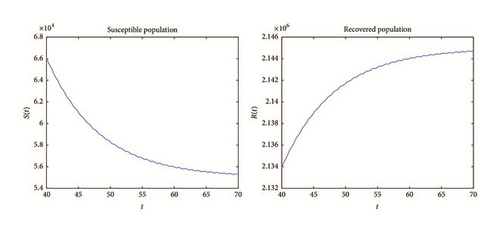
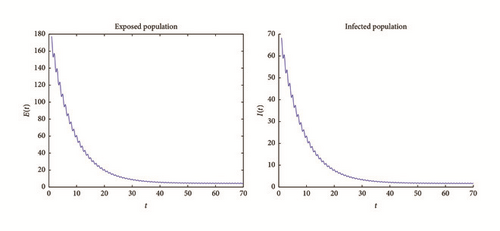
There exist multiple numerical methods to compute and plot the solutions of nonautonomous differential equations; see, for example, the Adomian method, the homotopy analysis method, or the modified homotopy methods (see, e.g., [24, 25]). For this work we use Matlab algorithms (ODE 45) to graph the solution of system (76) with these initial conditions. Figures 2 and 3 shows the results. We can see that I(t), E(t) goes to zero, while S(t), R(t) tend to stabilize; also S(t) is tending to with values between 54,000 and 56,000 (see Figure 1); this shows the results obtained in Theorem 6.
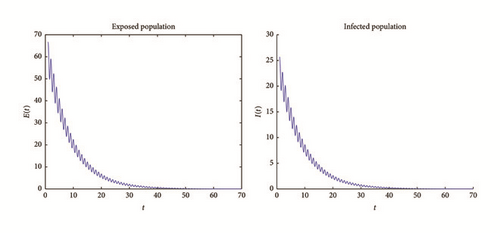
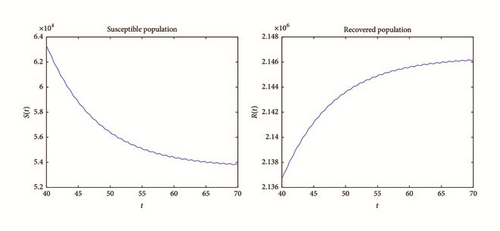
Example 2. Now, choose β0 = 0.005. As we can see in Figures 4 and 5, the solutions of system (1) remain persistent when t tends to infinity; this fact suggests that from Theorem 8. In fact, if we compute the basic reproduction number and its average (using the process described in example 1), and ; therefore it is bigger than one. In fact, this shows the results of persistence obtained in Theorem 8.
5. Conclusion
In this paper we presented a model with seasonal fluctuation with a general incidence function Sf(I) that includes the bilinear case βSI (studied by [5]) and a family of saturated incidence rate of the form βSI/(1 + kIq). We proved the existence of a disease-free periodic solution and defined the basic reproduction number , proving that it is a threshold parameter for disease, in the sense that when , the disease-free periodic solution is globally asymptotically stable, and when , the disease is persistent. A next step of this work is to consider a family of incidence rates more generally, changing Sf(I) by f(S, I) and trying to obtain results of persistence and stability similar to the ones obtained in this work. Another interesting topic is to ask what the behavior of system at is, in order to complete the analysis that we have made.
Several authors (e.g., [10, 11]) define as an average, which we denoted as to distinguish between it and the basic reproduction number defined by [13], via the monodromy matrix (which is a real threshold parameter for extinction and persistence of disease). We compute , approximate (with the help of Taylor theorem), and compare these values, obtaining that is not equal to ; moreover in both examples (similar comparisons can be observed also in the works made by [13, 17]). This fact suggests that the use of for persistence overestimates the threshold. To emphasize this conclusion, it would be helpful to find an example where but and then compute the solutions to observe the behavior (we affirm that the disease will go extinct due to Theorem 6).
To obtain the estimation of we used a code in Maple, which is based on numerical computing of ρ1 = ρ(W(LT, 0, λi)) until ρ1 ~ 1, where λi = λ0 + Δλi, Δλ is the step size, and the initial estimation λ0 is taken as . For this approximation we have used a Taylor expansion of the periodic solution ; another interesting possibility could be varying the approximation used for , for example, changing the Taylor approach of around t = 1 instead of t = 0. The graphs of the solutions were obtained with ODE 45 from Matlab, but other methods can be used to improve them, for example, Adomian methods or homotopy methods [24, 25]. The Maple code used to estimate is available for anyone who wants to use it.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This article was supported in part by Mexican SNI under Grants nos. 15284 and 33365.
Appendix
A. Assumption on η Used in Theorem 8
Note that has a positive minimum value (it is periodic, positive, and continuous, so it is bounded for t ∈ [0, LT] and then for all t > 0) and we can choose a η > 0 with the property , sufficiently small such that .
B. Periodic Solution of (61)
C. Assumption on Pm Used in Theorem 8
So, we can assume without loss of generality that ‖Pm(S0, E0, I0) − Mi‖ < δ for all m ≥ 0.