Optimization of Physiochemical Parameters during Bioremediation of Synthetic Dye by Marasmius cladophyllus UMAS MS8 Using Statistical Approach
Abstract
In many industrial areas such as in food, pharmaceutical, cosmetic, printing, and textile, the use of synthetic dyes has been integral with products such as azo dye, anthrax, and dyestuffs. As such, these industries produce a lot of waste by-products that could contaminate the environment. Bioremediation, therefore, has become an important emerging technology due to its cost-sustainable, effective, natural approach to cleaning up contaminated groundwater and soil via the use of microorganisms. The use of microorganisms in bioremediation requires the optimisation of parameters used in cultivating the organism. Thus the aim of the work was to assess the degradation of Remazol Brilliant Blue R (RBBR) dye on soil using Plackett-Burman design by the basidiomycete, M. cladophyllus UMAS MS8. Biodegradation analyses were carried out on a soil spiked with RBBR and supplemented with rice husk as the fungus growth enhancer. A two-level Plackett-Burman design was used to screen the medium components for the effects on the decolourization of RBBR. For the analysis, eleven variables were selected and from these four parameters, dye concentration, yeast extract concentration, inoculum size, and incubation time, were found to be most effective to degrade RBBR with up to 91% RBBR removal in soil after 15 days.
1. Introduction
Synthetic dyes are chemicals that are important alternatives in many industries. Since 1856, synthetic dyes have been used as they are economical to produce and create brighter, more colour-fast, and easy applications. However, most industrial dye processes involve the dye solution to be released to the surrounding water and soils. It has been reported that about 10–15% of all dyestuff is directly lost to wastewater [1] because of inadequate treatment of wastewater and poor waste management. Thus, this will lead to dye contamination in soil and water bodies [2]. Azo, anthraquinone, and phthalocyanine are the most common groups of synthetic dyes used [3]. Remazol Brilliant Blue R (RBBR) is a compound that is normally used to analyse azo dye degradation in laboratory condition [4]. RBBR, or Reactive 19, is an anthraquinone-based dye [5] which is an important dye in textile industries. It also represents a class of toxic and recalcitrant organopollutants that has identical structure to some polycyclic aromatic hydrocarbons (PAH) [6]. RBBR, an anthraquinone-based dye, is not easily degraded even by bacteria due to the presence of the aromatic structure [7]. The chemical structure and specification of the RBBR dye are shown in Table 1 [5].
| Synonym | Reactive Blue 19 |
| Molecular formula | C22H16N2Na2O11S3 |
| Molecular weight | 626.54 |
| CAS number | 2580-78-1 |
| Colour index number | 61200 |
| MDL number | MFCD00001215 |
| Chemical structure | 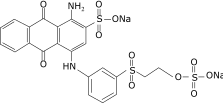 |
Among industrial effluents, wastewater from textile and dyestuff industries is one of the most difficult to be treated. This is because industrial textile dyes have been purposely designed to be highly resistant to washing, chemical agents, solvents, and environmental factors such as action by sunlight. The synthetic and complex aromatic molecular structures of synthetic dyes also make them more stable and difficult to be degraded by microbial attack [8].
Conventional wastewater treatment plants using activated sludge treatment are unable to sufficiently treat dye containing wastewater with up to 90% of reactive textile dyes still remaining after the treatment [9]. In another study done by Shaul et al. (1991) [10], 11 out of 18 azo dyes studied were found to pass through activated sludge process substantially untreated while another 4 azo dyes were just adsorbed onto the waste activated sludge. Only 3 dyes (Acid Orange 7, Acid Orange 8, and Acid Red 88) were biodegraded by the activated sludge process.
These environmental pollutants are the contaminants that enter the environment and cause adverse changes. The pollutants can be cleaned up but at a high cost to the people. Furthermore, there are several limitations in the use of physicochemical methods such as adsorption, coagulation, precipitation, filtration, and oxidation [11]. This is because those methods are not environmental friendly and cost competitive compared to bioremediation [12]. Bioremediation on the other hand, by using fungi or bacteria, is an alternative way of cleaning up pollutants [11].
White rot fungi are important and have been used to discover organisms that can degrade synthetic compounds. For example, lignin biodegradation of white rot fungi involves the degradation of aromatic xenobiotics, heterocyclic aromatic hydrocarbons, synthetic high polymers, chlorinated aromatic compounds, and various dyes which are all environmentally persistent pollutants [13]. In the paper and pulp industry, degradation of lignin is needed to eliminate lignin from the wastewater [14]. For the degradation of lignin, white rot fungi produce extracellular enzymes, namely, lignin peroxidase (LiP), manganese peroxidase (MnP), and laccase (Lac), which are involved to effectively depolymerize lignin producing carbon dioxide and water [14, 15]. Apart from that, white rot fungi have a high lignin degradation ability due to their strong oxidative activity and low substrate specificity of their ligninolytic enzymes [14]. Previous studies showed RBBR biodegradation by various fungi such as Phanerochaete chrysosporium and Irpex lacteus, [4, 16]. Irpex lacteus is one of white rot fungi that have been reported to be one of the first bioremediation agents of soil [16].
Here we report on the bioremediation of RBBR by M. cladophyllus UMAS MS8, in soil and under aerobic conditions. Eleven parameters in the bioremediation process were tested with the aim to determine the optimal parameters. Statistical approaches utilizing Plackett-Burman design were applied to optimize the concentration of dye, concentration of yeast, and incubation time as variables that affected the bioremediation rate.
2. Materials and Methods
2.1. Microorganism Preparation and Culture Maintenance
Marasmius cladophyllus UMAS MS8 was used in this study and obtained from Molecular Biology Laboratory, Universiti Malaysia Sarawak (UNIMAS). The fungus was grown on malt extract agar (MEA) for a period of 7 to 10 days at 29°C. Malt extract agar (MEA) was prepared by dissolving 33.6 gL−1 of malt extract in distilled water, with pH 5.6, and autoclaved for 1 h at 121°C. Stock fungus culture was maintained in 20% (v/v) glycerol stored at −20°C, in sterile distilled water and on plates of malt extract agar (MEA) medium added with 0.05% (w/v) chloramphenicol.
2.2. Soil and Substrate Preparation
Top soil sample was used and oven-dried for 4 days at 70°C before being used in the experiment. The soil was sieved through 2.0 mm mesh sieve and the physicochemical properties were partially characterized. Results showed that the soil used was clay textured with pH of 4.73 (1 : 5 H2O) and contained organic carbon of 70.133 g kg−1 (w/w) glucose equivalent, a total nitrogen of 80.06 mg L−1, and bioavailable P of 1.96 mg kg−1. These indicated a ratio of C : N : P of 35.8 : 40.8 : 1 which was lower than the commonly used C : N : P ratio of 100 : 10 : 1. The sterile soil was prepared by autoclaving three times continuously at 24-hour intervals for 55 min at 121°C.
2.3. Screening of RBBR Dye Degradation on Agar Medium and Bioremediation Experiments on Soil
Marasmius cladophyllus UMAS M8 was screened for RBBR decolourization capability on malt extract agar (MEA) supplemented with 0.02%, 0.06%, and 0.1% (w/v) RBBR dye. The agar plates were inoculated with 1.0 cm2 (in diameter) of mycelium plug from a 7-day-old fungal mycelium. The fungal isolate together with the control plate was prepared in triplicate and incubated for 3 to 15 days in the dark at room temperature [17]. Bioremediation experiments were performed in 50 mL of Erlenmeyer flasks with 6 g of dry soil that was homogenously spiked with 0.1% (w/v) RBBR solution. A 30% (w/w) rice husk was used and added onto the top of the soil. The spiked soil was individually treated with 1.0 cm2 of fungal mycelial plug with 70% (w/v) water moisture content. Noninoculated flasks with the respective dye concentrations were used as controls. Each culture condition was prepared in triplicate and incubated in the dark at room temperature under static condition. Other parameters were standardized using the experimental design layout.
2.4. Screening of Important Nutrient Components Using Plackett-Burman Design
Plackett-Burman design was used for screening the important medium components with respect to their main effects but not their interaction effects on RBBR decolourization. Plackett-Burman design provides an efficient and rapid method to screen and select ingredients with maximum number of variables [17, 18]. A 12-run Plackett-Burman design was applied to this study to evaluate eleven variables at two levels: maximum (+1) and minimum (−1). The design and levels of each variable are shown in Table 2 and all trials were performed in triplicate. The medium was formulated as per the design and the response was calculated as the rate of dye decolourization and expressed in percentage. Table 3 represented the list of ingredients and concentrations chosen of 12 screening experiments.
| Independent variable (Xi) | Range and levels | |
|---|---|---|
| −α | +α | |
| Lactose (%) | 8 | 12 |
| Glucose (%) | 8 | 12 |
| Dye concentration (ppm) | 200 | 1000 |
| Ammonium sulphate (%) | 9 | 13 |
| Ammonium nitrate (%) | 9 | 13 |
| Tryptone (%) | 9 | 13 |
| Yeast (%) | 9 | 13 |
| Ammonium chloride (%) | 9 | 13 |
| Inoculum size (cm) | 0.2 | 1.0 |
| Moisture content (%) | 40 | 80 |
| Incubation time (days) | 3 | 15 |
| Run | A | B | C | D | E | F | G | H | I | J | K |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8 | 12 | 1000 | 9 | 13 | 13 | 13 | 9 | 3 | 40 | 1 |
| 2 | 12 | 12 | 1000 | 9 | 9 | 9 | 13 | 9 | 15 | 80 | 0.2 |
| 3 | 12 | 8 | 1000 | 13 | 9 | 13 | 13 | 13 | 3 | 40 | 0.2 |
| 4 | 8 | 12 | 1000 | 13 | 9 | 9 | 9 | 13 | 3 | 80 | 1 |
| 5 | 8 | 8 | 200 | 13 | 9 | 13 | 13 | 9 | 15 | 80 | 1 |
| 6 | 8 | 12 | 200 | 13 | 13 | 9 | 13 | 13 | 15 | 40 | 0.2 |
| 7 | 12 | 8 | 200 | 9 | 13 | 9 | 13 | 13 | 3 | 80 | 1 |
| 8 | 8 | 8 | 1000 | 9 | 13 | 13 | 9 | 13 | 15 | 80 | 0.2 |
| 9 | 8 | 8 | 200 | 9 | 9 | 9 | 9 | 9 | 3 | 40 | 0.2 |
| 10 | 12 | 8 | 1000 | 13 | 13 | 9 | 9 | 9 | 15 | 40 | 1 |
| 11 | 12 | 12 | 200 | 13 | 13 | 13 | 9 | 9 | 3 | 80 | 0.2 |
| 12 | 12 | 12 | 200 | 9 | 9 | 13 | 9 | 13 | 15 | 40 | 1 |
- A: lactose (%), B: glucose (%), C: dye concentration (rpm), D: ammonium sulphate (%), E: ammonium nitrate (%), F: tryptone (%), G: yeast (%), H: ammonium chloride (%), I: incubation time (days), J: moisture content (%), and K: inoculum size (cm).
2.5. Residual RBBR Extraction and Quantification
3. Results and Discussion
3.1. Screening of Degradation Assay in Agar
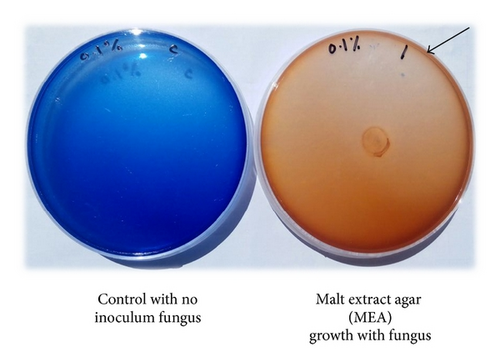
Marasmius cladophyllus UMAS MS8 was used to decolourize RBBR and evaluate the dye decolourization characteristics. This work was carried out on solid agar medium and observation showed that the fungus grew on MEA supplemented with RBBR dye. MEA supplemented with 0.1% (w/v) of RBBR showed complete decolourization with formation of halo on agar plate after 9 days of incubation with 1.0 cm2 of mycelial plug (Figure 1). Other results were also recorded and shown in Table 4. The fungal isolate together with the control plate was prepared in triplicate and incubated for a period of 3 to 15 days at 28°C.
| Incubation time (days) | Concentration RBBR (%) | ||
|---|---|---|---|
| 0.02 | 0.06 | 0.1 | |
| 3 | + | + | + |
| 6 | ++ | + | + |
| 9 | ++ | ++ | + |
| 12 | ++ | ++ | ++ |
| 15 | ++ | ++ | ++ |
- (+): partial or weak dye decolourisation; (++): complete dye decolourisation.
3.2. Screening Using Plackett-Burman Design
The effects of eleven variables, lactose, glucose, dye concentration, (NH4)2SO4, NH4NO3, tryptone, yeast, NH4Cl, inoculum size, moisture content, and incubation time, supplemented with rice husk on RBBR dye degradation on soil by M. cladophyllus UMAS MS8 were analysed and results were listed in Table 5. The results indicated a wide variation in RBBR dye degradation, from 1.99% to 91.01%, in the 12 trials. Runs 11 and 1 resulted in the highest RBBR dye degradation of 91.01% and 63%, respectively, at initial RBBR concentrations of 0.02 ppm. The Plackett-Burman design showed results that were generated by Design Expert 10.0.0 (Stat-Ease, Inc., Minneapolis, USA). The two values of each variable {maximum (+) and minimum (−)} were chosen such that the difference between the two values (+ and −) is large enough, as shown in Table 2.
| Run | A | B | C | D | E | F | G | H | I | J | K | Biodegradation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 | 8 | 0.02 | 9 | 13 | 9 | 13 | 13 | 0.2 | 80 | 15 | 63.0 |
| 2 | 12 | 12 | 0.02 | 13 | 13 | 13 | 9 | 9 | 0.2 | 80 | 3 | 13.8 |
| 3 | 12 | 12 | 0.02 | 9 | 9 | 13 | 9 | 13 | 1.0 | 40 | 15 | 53.3 |
| 4 | 12 | 12 | 0.1 | 9 | 9 | 9 | 13 | 9 | 1.0 | 80 | 3 | 14.23 |
| 5 | 8 | 12 | 0.1 | 9 | 13 | 13 | 13 | 9 | 0.2 | 40 | 15 | 10.71 |
| 6 | 12 | 8 | 0.1 | 13 | 13 | 9 | 9 | 9 | 1.0 | 40 | 15 | 12.54 |
| 7 | 8 | 12 | 0.02 | 13 | 13 | 9 | 13 | 13 | 1.0 | 40 | 3 | 51.31 |
| 8 | 8 | 8 | 0.1 | 9 | 13 | 13 | 9 | 13 | 1.0 | 80 | 3 | 6.03 |
| 9 | 8 | 8 | 0.02 | 9 | 9 | 9 | 9 | 9 | 0.2 | 40 | 3 | 20.28 |
| 10 | 8 | 12 | 0.1 | 13 | 9 | 9 | 9 | 13 | 0.2 | 80 | 15 | 15.53 |
| 11 | 8 | 8 | 0.02 | 13 | 9 | 13 | 13 | 9 | 1.0 | 80 | 15 | 91.01 |
| 12 | 12 | 8 | 0.1 | 13 | 9 | 13 | 13 | 13 | 0.2 | 40 | 3 | 1.99 |
- A: lactose (%), B: glucose (%), C: dye concentration (ppm), D: ammonium sulphate (%), E: ammonium nitrate (%), F: tryptone (%), G: yeast (%), H: ammonium chloride (%), I: inoculum size (cm), J: moisture content (%), and K: incubation time (days).
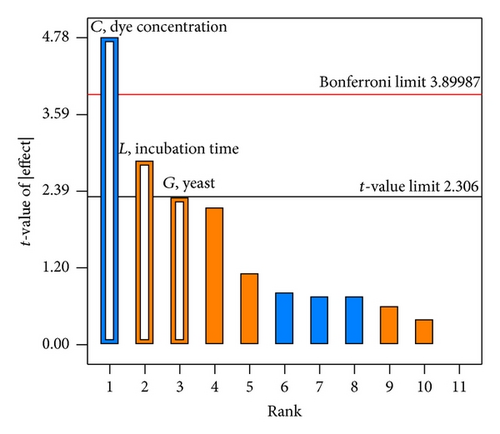
The maximum RBBR dye degradation (Run 11) was obtained by having 8% lactose, 8% glucose, 0.02 ppm dye concentration, 13% (NH4)2SO4, 9% NH4NO3, 13% tryptone, 13% yeast, 9% NH4Cl, inoculum size of 1.0 cm, 80% moisture content, and incubation time of 15 days. Regression coefficients analysis and t-values of 5 factors (Table 6) were calculated and showed that D, G, H, K, and L had positive effects on RBBR dye degradation, whereas A, B, D, E, and F had negative effects. This positive effect indicated that these parameters will increase the RBBR dye degradation by increasing their concentration from low to high level. The variable with confidence level of above 95% is considered as significant parameters. Values of greater than 0.1000 indicated that the model is not significant. However, in this case, the model F-value of 22.44 implies that the model was significant since its value is largely greater than 0.1000. There is only a 0.08% chance that an F-value this large could occur due to noise. It was clear that variables C, G, J, and L were the significant factors, while K, with confidence levels below 95%, was considered as insignificant and thus was not selected.
| Source | Sum of squares | DF ∗ | Mean square | F-value | Prob > F | Remarks |
|---|---|---|---|---|---|---|
| Model | 8216.67 | 5 | 1643.33 | 22.44 | 0.0008 | Significant |
| C, dye concentration | 4472.58 | 1 | 4472.58 | 61.08 | 0.0002 | |
| G, yeast | 1022.50 | 1 | 1022.50 | 13.96 | 0.0097 | |
| J, inoculum size | 885.97 | 1 | 885.97 | 12.10 | 0.0132 | |
| K, moisture content | 238.25 | 1 | 238.25 | 3.25 | 0.1213 | |
| L, incubation time | 1597.37 | 1 | 1597.37 | 21.81 | 0.0034 | |
| Residual | 439.34 | 6 | 73.22 | |||
| Cor total | 8656.02 | 11 |
- ∗DF = degree of freedom.
The significant effects (factors and interactions) having p value less than 0.05 were selected as it is statistically different from zero at the 95% confidence level. On the other hand, the insignificant effects (factors and interactions) having p value higher than 0.05 were then excluded. Since the values of Prob > F less than 0.0500 indicated that the model terms are significant, thus in this case C, G, J, and L are the significant model terms. However, for this present study, only three variables (dye concentration, yeast concentration, and incubation time) were chosen since they were having the least number of p values. Moreover, for a better response surface method, it is advisable to have only three parameters.
Results obtained indicated that the ratio of C : N : P (35.8 : 40.8 : 1) was lower than the commonly used C : N : P ratio of 100 : 10 : 1 for soil bioremediation. Hence, addition of carbon and nitrogen source can accelerate decolourization of dye in soil. Yeast concentration was utilized as nitrogen source and it has been reported to influence the synthesis of ligninolytic enzymes, laccase, and peroxidase [21]. Yeast extract (organic compound) has been suggested to accelerate dye decolourization and important nitrogen source in decolourization of mix dye [22]. However, high concentration of nitrogen source can inhibit the production of fungal ligninolytic enzyme [23]. Since yeast extract appeared to be significant in this design, M. cladophyllus UMAS MS8 was expected to produce more laccase and peroxidase. Since there was a need for the addition of carbon, RBBR dye was thus utilized as the sole carbon source. Summary of statistics of the model including standard deviation, predicted residual sum of squares (PRESS), and coefficients of determination is presented in Table 7.
| Parameters | Value |
|---|---|
| Standard deviation | 8.56 |
| Mean | 29.48 |
| Coefficient of variation (CV) (%) | 29.03 |
| R2 | 0.9492440752809 |
| Adjusted R2 | 0.90694747134832 |
| Predicted R2 | 0.7969763011236 |
| Predicted residual sum of squares (PRESS) | 1757.377 |
| Adequate precission | 14.508 |
The significance of the model examined by the determination coefficient (R2) showed that more than 0.9492% of variance was attributable to the variables 29.03% of the total variance that cannot be explained by the model. The adjusted coefficient (R2 adjusted) and predicted coefficient (R2 predicted) were calculated to ensure the quality of fit of the polynomial model for RBBR bioremediation [24]. The R2 Pred of 0.7969 was in agreement with the R2 Adj of 0.90694. This indicated that the overall mean was a better predictor of the response than the current model. Adequate precision, which measures the signal to noise ratio, showed a value of 14.508 (>4 value is desirable) indicated an adequate signal. Thus, this model can be used to navigate the design space. The Pareto chart (Figure 2) offers a convenient way to view the results obtained by Plackett-Burman design and the order of significance of the variable affecting RBBR degradation. The vertical line in the chart defines the 95% confidence level.
| Run order | Actual value | Predicted value | Error (%) |
|---|---|---|---|
| 1 | 63.00 | 65.42 | −3.841 |
| 2 | 13.80 | 23.88 | −73.04 |
| 3 | 53.30 | 55.23 | −3.62 |
| 4 | 14.23 | 20.91 | −41.88 |
| 5 | 10.71 | 17.89 | −67.0 |
| 6 | 12.54 | 16.61 | −32.46 |
| 7 | 51.31 | 50.61 | 1.36 |
| 8 | 6.03 | 2.45 | 59.37 |
| 9 | 20.28 | 14.97 | 26.18 |
| 10 | 15.53 | 8.34 | 46.3 |
| 11 | 91.01 | 82.60 | 9.24 |
| 12 | 1.99 | −5.18 | −160.3 |
4. Conclusion
Based on Plackett-Burman design method, the work found that 4 (dye concentration, yeast extract concentration, inoculum size, and incubation time) out of the 11 factors investigated were the most effective factors on RBBR biodegradation in soil. However, only three variables (dye concentration, yeast extract concentration, and incubation time) were selected for further analysis since they have the least p values. Therefore, the Plackett-Burman design used in this work provided an efficient and rapid method for screening and selecting ingredients with a minimal number of experiments.
Competing Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
This research work was supported by Fundamental Research Grant Scheme FRGS/SG05(01)/1065/2013(11).




